Abstract
The European honey bee, Apis mellifera L., is a valuable agricultural and commercial resource noted for producing honey and providing crop pollination services, as well as an important model social insect used to study memory and learning, aging, and more. Here we describe a detailed protocol for the dissection of the dorsal abdominal wall of a bee in order to visualize its dorsal vessel, which serves the role of the heart in the insect. A successful dissection will expose a functional heart that, under the proper conditions, can maintain a steady heartbeat for an extended period of time. This allows the investigator to manipulate heart rate through the application of cardiomodulatory compounds to the dorsal vessel. By using either a digital microscope or a microscope equipped with a digital camera, the investigator can make video recordings of the dorsal vessel before and after treatment with test compounds. The videos can then be scored at a time convenient to the user in order to determine changes in heart rate, as well as changes in the pattern of heartbeats, following treatment. The advantages of this protocol are that it is relatively inexpensive to set up, easy to learn, requires little space or equipment, and takes very little time to conduct.
Keywords: Physiology, Issue 118, honey bee, insect, invertebrate, circulation, dissection, dorsal vessel, heart, heart rate, cardioregulatory
Introduction
The overall goal of this methodology is to allow the investigator to quickly and easily observe and quantify the effect that a pharmacological agent has on the heart rate of honey bees. Bees, like other insects, have an open circulatory system that disseminates hemolymph, the insect equivalent of blood, throughout the body cavity, known as the hemocoel. The circulation of hemolymph is essential for the transport of nutrients, immune factors, waste products, as well neurohormones and other signaling molecules1. Circulation is facilitated by the dorsal vessel, which extends along the dorsal midline of the insect, as well as accessory pulsatile organs. The dorsal vessel is divided into two functionally distinct sections, designated the heart in the abdomen and the aorta in the thorax and head. Propagated contractions in the heart pump hemolymph towards the thorax and head, while accessory pulsatile organs ensure hemolymph flow to the extremities.
Insect cardiac function can be observed using a variety of methods, depending upon the size, physiology, or life stage of the insect. A common approach for observing heart rate in larvae or smaller insects is the use of intravital imaging2. This method is less useful in adult bees, however, as it can be difficult to clearly view the dorsal vessel through the abdominal wall. An established approach for recording heart rate in a variety of insects, including bees, is the use of contact thermography, which utilizes thermistors applied to the exterior of the insect to detect cardiac pulsations3,4. Heart rate in adult bees has also been recorded using an electrophysiological technique to measure an electrical impedance signal4,5. This technique requires the insertion of electrodes into the animal next to the heart and the use of an impedance converter to record heartbeats4. Similarly, electrocardiograms have been used to detect electrical signals produced by the heart and combined with video recording of the bee to observe changes in cardiac activity6. A distinct advantage to these approaches is that heart rate is assessed in an intact, living bee, rather than in a dissected specimen, which helps to ensure the availability of the full range of physiological responses in the subject. The challenges of these approaches include accounting for immobilization or anesthetization of the subject, the need to limit outside variables and stimuli that might alter heart rate, as well as determining an appropriate delivery method when testing pharmacological agents.
Another approach that has been used for studying bee cardiac activity is to partially dissect the insect in order to expose the heart, then measure dorsal vessel contractions using a force displacement transducer7. In this protocol, the heart is continually bathed with running physiological saline and test compounds can be dissolved in this solution for application to the subject7. A significant difference between this method and those previously described is that the ventral nerve cord is removed, eliminating the role that the central nervous system has been shown to play in modulating heart rate5. The result is that the baseline heartbeat, which is usually quite erratic, stabilizes at a much lower frequency and amplitude than is typically observed in a living insect5,7. What all of these methods have in common is that they require highly specialized and often expensive equipment, in addition to a certain level of expertise, in order to be conducted. Perhaps the greatest disadvantage is that none of these approaches are particularly well suited to experiments that involve testing a large number of subjects, such as screening a library of potentially cardiomodulatory compounds.
The greatest strength of the approach described here is its simplicity. The protocol is relatively easy to master, the setup requires little space, and only a minimal financial input is necessary. The method requires little more than some bees, a few surgical instruments, an isotonic solution, and either a digital microscope or a traditional microscope with a digital camera. Bees are dissected to visualize the dorsal vessel and digital videos are used to record heart rate before and after treatment with pharmacological agents. Although video recording is not actually necessary to observe changes in heart rate, it will greatly increase throughput (i.e., the number of subjects that can be processed in a given amount of time). The investigator can maximize efficiency by recording a large number of videos at once and then later scoring these videos at a more convenient time. Another advantage of this approach is that videos allow the investigator to start over, should the scoring process be interrupted, and make it easier for the viewer to be blinded to the treatment in order to reduce bias.
Protocol
1. Collection and Preparation of Test Subjects
Collect the appropriate number of bees from the colony. NOTE: The number needed depends upon not only the size and scope of the experiment, but also the skill of the investigator. For example, if there are 2 treatment groups with a desired sample size of 10 bees per group, a reasonably skilled investigator might collect at least 30 bees to account for unsuccessful dissections and end up with 20 useful videos to score.
- Minimize the amount of time that passes between collection and dissection. NOTE: Although bees can be housed in the lab for days prior to dissection, the success rate of dissections (i.e., the likelihood of maintaining a stable heart rate in a dissected dorsal vessel) has been observed to decrease relative to the amount of time that bees are housed away from the colony.
- Provide bees with a source of water and food while housed in the lab. For example, at a minimum, provide access to a 50% (w/v) solution of sucrose in water (this is sufficient for durations of less than 6 hr). For longer periods, provide bees access to honey.
- House bees in the lab overnight at a temperature of approximately 32 °C and 60-80% relative humidity to reduce stress and avoid dehydration.
- Prior to dissection, anesthetize bees briefly to aid in handling. NOTE: This can decrease the success rate of dissections and reduce throughput.
- Chill the bees either by placing them on ice or into a refrigerator for just long enough to reduce movement in order to aid in handling.
- Alternatively, briefly expose bees to CO2 in order to aid in handling. NOTE: Extended exposure to cold can reduce the success rate of dissections. Extended or repeated exposure to CO2 can also reduce the success rate of dissections.
2. Dissection of Dorsal Abdominal Wall
NOTE: Bees should be alive at the time of dissection.
Using forceps and/or microdissection scissors, remove legs and wings to facilitate dissection of the abdomen. Keep a small beaker or similar container filled with distilled water nearby for the purpose of rinsing instruments between dissections.
While restraining the bee with forceps, utilize the microdissection scissors to cut laterally along the dorsal abdominal wall between the first and second tergites (see Figure 1).
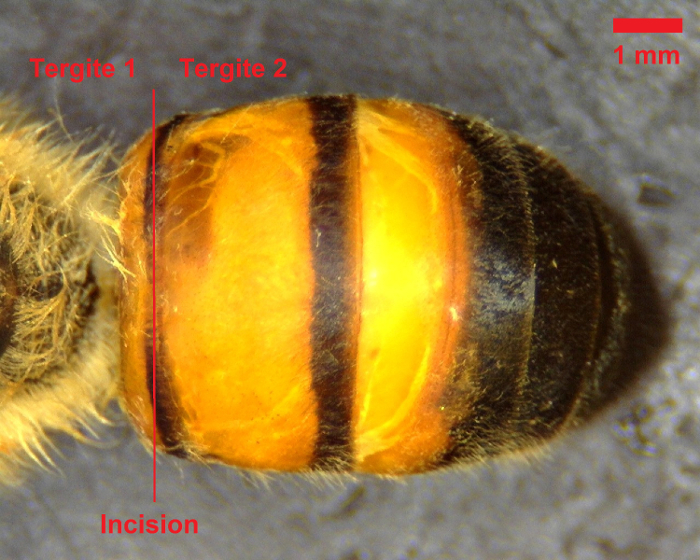 Figure 1: Dorsal view of bee abdomen. The initial incision should be made between the first and second tergites, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Figure 1: Dorsal view of bee abdomen. The initial incision should be made between the first and second tergites, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
While lightly gripping the posterior edge of the second tergite with the forceps, cut longitudinally along each side of the bee from the initial incision to the stinger (see Figure 2). Use caution when cutting to avoid puncturing the gastrointestinal tract.
 Figure 2: Lateral view of bee abdomen. The second and third incisions should be made along either side of the abdomen, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Figure 2: Lateral view of bee abdomen. The second and third incisions should be made along either side of the abdomen, as denoted by the red line. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Exchange the scissors for a second set of fine forceps and utilize them to carefully separate the dorsal abdominal wall from the rest of the abdomen. Gently remove the stinger and any portion of the gastrointestinal tract that remains attached to the dorsal abdominal wall. Avoid rupturing the gut, as the contents can coat the abdominal wall and impede visualization of the dorsal vessel.
 Figure 3: View of the dorsal vessel. Once the gut and stinger have been removed, the dorsal vessel is visible along the midline of the dissected dorsal abdominal wall. Scale bar = 1 mm. Please click here to view a larger version of this figure.
Figure 3: View of the dorsal vessel. Once the gut and stinger have been removed, the dorsal vessel is visible along the midline of the dissected dorsal abdominal wall. Scale bar = 1 mm. Please click here to view a larger version of this figure.
- Arrange the dorsal abdominal wall in the desired orientation beneath the camera so that the dorsal vessel is visible (see Figure 3). Utilize the microdissection scissors to trim away any excess abdominal wall that impedes visualization of the dorsal vessel. The shape of the dorsal abdominal wall should resemble a shallow cup or bowl when properly situated.
- Since the dorsal vessel does not extend into the hindmost abdominal segment of the bee, remove the final tergite in order to improve visualization of the dorsal vessel.
Utilizing an adjustable volume micropipette, cover the dorsal vessel with 10 µl of an isotonic solution to maintain physiological conditions and facilitate a steady heartbeat. NOTE: The recommended solution is quarter strength Ringer's solution (0.120 g/L calcium chloride, 0.105 g/L potassium chloride, 0.050 g/L sodium bicarbonate, and 2.250 g/L sodium chloride), which has been found to facilitate a stable, continuous heartbeat.
3. Observation and Modulation of Heart Rate
Allow the dorsal vessel to sit undisturbed until a stable, continuous heartbeat is achieved (usually within 300 sec). NOTE: Heartbeat is visualized as rhythmic contractions of the dorsal vessel. Initially, there may appear to be no heartbeat, especially if the bee was anesthetized, but the heart will usually resume beating after resting in isotonic solution for several minutes and can continue beating for hours, provided it remains bathed in solution.
- Measure the heart rate in terms of the number of beats per min (BPM).
- Record the number of contractions observed during a 60 sec period. Use a hand tally counter and a timer to facilitate this process.
- Measure the change in heart rate by recording the observed BPM before and after treatment with a cardiomodulatory compound. NOTE: Although the time required to observe an effect on heart rate may vary depending upon the compound being tested, changes in heart rate can typically be observed within minutes.
- Determine the baseline heart rate immediately prior to the addition of any test compound. NOTE: Post-treatment heart rate can usually be determined after 90 to 120 sec.
- Prepare potential cardiomodulators (e.g., octopamine) by dissolving the compound in the same isotonic solution used to bathe the dorsal vessel.
- Add the test compounds to the solution surrounding the dorsal vessel by utilizing a micropipetter.
For greater accuracy and higher throughput, make a video recording of each test subject and then use the videos to score heart rate at a later time. NOTE: This allows a single investigator to stagger dissections in order to facilitate almost continuous production of videos. When recording videos, the minimum recommended length is approximately 240 sec with any test compound being added at the 60 sec mark. This ensures that the investigator has a 60 sec window for scoring baseline heart rate and then another 60 sec window for scoring post-treatment heart rate 120 sec after treatment.
Representative Results
Since many of the pharmacologically active compounds that might be tested using this protocol are not soluble in water, it is necessary to have a reliable solvent that will allow test compounds to be delivered via the isotonic solution used to bathe the dorsal vessel. Dimethyl sulfoxide (DMSO) is a solvent that is commonly used as a vehicle for delivering experimental drugs and other compounds in animals8, and it has been used successfully for this purpose in studies examining the effect of pesticides on honey bee cardiac activity9. Consequently, DMSO was selected as a potential vehicle for delivering test compounds to the dorsal vessel, which required that it first be tested for cardiomodulatory activity. In this protocol, the dissected dorsal vessel is initially bathed in 10 µl of quarter strength Ringer's solution and then heart rate is determined prior to adding test compounds. Test compounds are delivered at a known concentration in 10 µl of DMSO dissolved in quarter strength Ringer's solution and then the dorsal vessel is observed for subsequent changes in heart rate.
Prior to testing any compounds, a concentration range of DMSO was tested for any effect on heart rate, relative to quarter strength Ringer's solution with 0% DMSO. DMSO was dissolved at 0.2%, 2%, 10%, and 20% v/v in quarter strength Ringer's solution in order to test a final concentration of 0.1%, 1%, 5%, and 10% when added in equal volume to the solution already bathing the dorsal vessel. This approach was used, as opposed to adding DMSO at the desired concentration in the initial 10 µl used to bathe the dorsal vessel, because not every dissection yields a stable heartbeat. If DMSO has an adverse effect on heart rate, this effect might not be distinguishable from a failed dissection. Heart rate was assessed immediately prior to adding DMSO, and then again 120 sec later. Figure 4 shows the results of this experiment, demonstrating that 0.1% and 1% DMSO in solution had no significant effect on heart rate, relative to the quarter strength Ringer's solution without DMSO, whereas 5% and 10% DMSO significantly decreased heart rate.
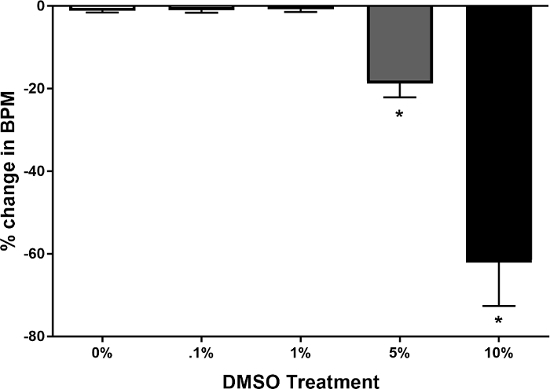 Figure 4: Effect of DMSO on bee heart rate. Graph shows the percentage change in basal heart rate, as measured in beats per minute. Treatment of honey bee dorsal vessel with 0.1% or 1% DMSO v/v in quarter strength Ringer's solution does not significantly increase or decrease heart rate, relative to quarter strength Ringer's solution without DMSO. Treatment with 5% or 10% DMSO in solution causes a significant decrease in heart rate. Results expressed as mean ± SEM (n = 10). *P <0.05 vs 0% DMSO control (Kruskal-Wallis test with Dunn's multiple comparisons test). Please click here to view a larger version of this figure.
Figure 4: Effect of DMSO on bee heart rate. Graph shows the percentage change in basal heart rate, as measured in beats per minute. Treatment of honey bee dorsal vessel with 0.1% or 1% DMSO v/v in quarter strength Ringer's solution does not significantly increase or decrease heart rate, relative to quarter strength Ringer's solution without DMSO. Treatment with 5% or 10% DMSO in solution causes a significant decrease in heart rate. Results expressed as mean ± SEM (n = 10). *P <0.05 vs 0% DMSO control (Kruskal-Wallis test with Dunn's multiple comparisons test). Please click here to view a larger version of this figure.
In order for this assay to be relevant, it must be possible to observe changes in heart rate following treatment with cardiomodulatory compounds. The biogenic amine octopamine is known to act as a neuromodulator in invertebrates10 and has been shown to act as a cardioaccelerant in both fruit flies11 and honey bees7. Octopamine was tested against vehicle (1% DMSO in quarter strength Ringer's solution) as described above at concentrations of 100 nM, 10 µM, and 100 µM. Figure 5 shows the results of this experiment, demonstrating that 10 µM and 100 µM concentrations of octopamine significantly increased heart rate.
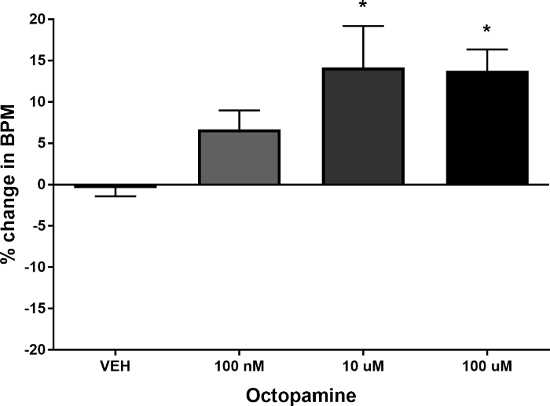 Figure 5:Effect of octopamine on bee heart rate. Graph shows the percentage change in basal heart rate, as measured in beats per minute. Treatment of honey bee dorsal vessel with 100 nM octopamine does not significantly increase or decrease heart rate relative to vehicle (1% DMSO v/v in quarter strength Ringer's solution). Treatment with 10 µM and 100 µM octopamine significantly increased heart rate relative to vehicle. Results expressed as mean ± SEM (n = 10). *P <0.05 vs vehicle control (Kruskal-Wallis test with Dunn's multiple comparisons test). Please click here to view a larger version of this figure.
Figure 5:Effect of octopamine on bee heart rate. Graph shows the percentage change in basal heart rate, as measured in beats per minute. Treatment of honey bee dorsal vessel with 100 nM octopamine does not significantly increase or decrease heart rate relative to vehicle (1% DMSO v/v in quarter strength Ringer's solution). Treatment with 10 µM and 100 µM octopamine significantly increased heart rate relative to vehicle. Results expressed as mean ± SEM (n = 10). *P <0.05 vs vehicle control (Kruskal-Wallis test with Dunn's multiple comparisons test). Please click here to view a larger version of this figure.
Discussion
The protocol presented here provides a simple and effective approach to testing pharmacological compounds for their effects on honey bee heart rate. As observed in prior experiments that either transect the ventral nerve cord of a living insect5 or dissect out the ventral nerve cord when exposing the dorsal vessel7, the loss of central nervous system regulation results in a stable, low frequency heartbeat. The low frequency of beats allows the investigator to visually assess heart rate without having to rely on delicate or expensive instrumentation. As has been demonstrated, the solvent DMSO can be used in low concentrations to dissolve pharmacological agents for delivery via the isotonic solution used to bath the dorsal vessel. Furthermore, heart rate can be accelerated in this method using the invertebrate neuromodulator octopamine, which has been previously shown to act as a cardioaccelerant in bees7. Although this protocol was designed in order to measure changes in heart rate, it is possible to use it to observe other, more qualitative characteristics of heartbeat as well, such as changes in the pattern of contractions. Regardless of the end point in question, the protocol relies primarily upon the ability of the investigator to perform a clean, rapid dissection. The most common challenge that that an investigator will face when using this protocol is the failed dissection, which results in a dissected abdominal wall that is not useful either because there is no heartbeat, or because the dorsal vessel is not visible.
The dorsal vessel can be easily obscured as the result of a poor dissection. Puncturing or tearing the gut can result in gut contents spilling out onto the abdominal wall, typically in the form of yellow/brown excrement that mixes with the vehicle and obscures the dorsal vessel. Although the abdominal wall can be rinsed with an isotonic solution to remove debris, doing so can increase the chances of damaging the dorsal vessel. With practice, this can be easily avoided; however, if this remains an issue, an alternative is to remove the gut prior to dissection. This can be accomplished by first removing the stinger and then pulling the abdominal gastrointestinal tract out through the same hole. One noticeable difference between this approach and the dissection as described in the protocol is that removing the stinger and gut first will typically leave the dorsal abdominal wall covered by a translucent membrane that can also obscure the dorsal vessel. When dissecting the abdomen as described in the protocol, this membrane will usually be removed along with the gut. If the membrane, or some portion of it, is obstructing view, it can be removed carefully with fine forceps.
It is not uncommon for the dorsal vessel to display no sign of a heartbeat immediately following dissection. Bathing it in an isotonic solution and allowing it to rest for several minutes will usually result in a steady heartbeat resuming. A continued lack of a heartbeat can be the result of a number of different issues, including damage to the dorsal vessel caused by a clumsy dissection, excessive anesthesia, premature death of the bee, or a slow dissection. The most common cause, however, would seem to be related to the energy reserves of the bee prior to dissection. Bees that have been housed in the laboratory overnight, even when provided access to food and water, are far more likely to result in a failed dissection than bees just collected from the colony. Age may also be a factor, as the success rate appears to be greater with nurse bees than with foragers. This has not been tested in a controlled study, however, and would be an interesting line of inquiry to pursue.
Overall, the goal of this protocol is to provide a simple, inexpensive approach to assessing the effect of potential cardiomodulators on bee heart rate. This protocol could be adapted to observe differences in cardiac function as a result of other types of treatments or factors, or for use with other insects. The greatest limitation of the protocol is that the dorsal vessel is not being observed in a living, intact insect. It is likely that some potentially cardiomodulatory compounds will not show an effect on heart rate if they function at the level of the central nervous system. In this case, an electrophysiological approach is likely necessary. The greatest strength of this protocol is its simplicity, as it costs little, requires little equipment, can be undertaken with little or no specialized training, and can be conducted quickly once mastered. As such, it can be a valuable tool for applications ranging from exploratory pilot work to large scale drug screening studies, and can also serve as a useful teaching tool in high school or university classrooms.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors thank Drs. Jeffrey Bloomquist and Daniel Swale for their technical comments and suggestions. This project was partially funded by the Department of Entomology and the College of Agriculture and Life Sciences at Virginia Tech.
References
- Klowden MJ. Circulatory Systems. Physiological Systems in Insects, 3rd Edition. 3rd. 2013. pp. 365–413.
- League GP, Onuh OC, Hillyer JF. Comparative structural and functional analysis of the larval and adult dorsal vessel and its role in hemolymph circulation in the mosquito Anopheles gambiae. J Exp Biol. 2015;218(Pt 3):370–380. doi: 10.1242/jeb.114942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserthal LT. Oscillating Hemolymph Circulation in the Butterfly Papilio-Machaon L Revealed by Contact Thermography and Photocell Measurements. J Comp Physiol. 1980;139(2):145–163. [Google Scholar]
- Wasserthal LT. Interaction of circulation and tracheal ventilation in holometabolous insects. Adv Insect Physiol. 1996;26:297–351. [Google Scholar]
- Schwab ER, Chilson RA, Eddleman CD. Heartbeat Rate Modulation Mediated by the Ventral Nerve Cord in the Honey-Bee, Apis-Mellifera. J Comp Physiol B-Biochem Syst Environ Physiol. 1991;161(6):602–610. [Google Scholar]
- Kaiser W, Weber T, Otto D, Miroschnikow A. Oxygen supply of the heart and electrocardiogram potentials with reversed polarity in sleeping and resting honey bees. Apidologie. 2014;45(1):73–87. [Google Scholar]
- Papaefthimiou C, Theophilidis G. Octopamine--a single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): Acceleration vs. inhibition. J Insect Physiol. 2011;57(2):316–325. doi: 10.1016/j.jinsphys.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Castro CA, Hogan JB, Benson KA, Shehata CW, Landauer MR. Behavioral-Effects of Vehicles - Dmso, Ethanol, Tween-20, Tween-80, and Emulphor-620. Pharmacol Biochem Behav. 1995;50(4):521–526. doi: 10.1016/0091-3057(94)00331-9. [DOI] [PubMed] [Google Scholar]
- Papaefthimiou C, Papachristoforou A, Theophilidis G. Biphasic responses of the honeybee heart to nanomolar concentrations of amitraz. Pestic Biochem Phys. 2013;107(1):132–137. doi: 10.1016/j.pestbp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Roeder T. Octopamine in invertebrates. Prog Neurobiol. 1999;59(5):533–561. doi: 10.1016/s0301-0082(99)00016-7. [DOI] [PubMed] [Google Scholar]
- Johnson E, Ringo J, Dowse H. Modulation of Drosophila heartbeat by neurotransmitters. J Comp Physiol B. 1997;167(2):89–97. doi: 10.1007/s003600050051. [DOI] [PubMed] [Google Scholar]


