Abstract
Multifunctional nanoplatforms combining versatile therapeutic modalities with a variety of imaging options have the potential to diagnose, monitor, and treat brain diseases. The promise of nanotechnology can only be realized by the simultaneous development of innovative brain-targeting delivery vehicles capable of penetrating the blood–brain barrier without compromising its structural integrity.
Graphical abstract
Comprehensive understanding of the most complex organ in the body, the brain, requires a grand initiative like BRAIN (Brain Research through Advancing Innovative Neurotechnologies). It is envisioned that integrated multidisciplinary efforts will result in innovative neurotechnologies that provide insight into neural circuit functions and also create new avenues in diagnostic and therapeutic approaches for brain diseases. The prevalence of neurodegenerative diseases is escalating with no good treatment options mainly because of the failure of drugs to cross the impenetrable blood–brain barrier (BBB). Herein, we focus on the fast-emerging versatile nanotechnologies, which offer limitless opportunities to address the daunting issue of delivering treatment modalities for neuropathologies across the BBB.
The BBB is a highly specialized, multicellular, and dynamic interface between the central nervous system and the blood capillaries. It restricts the paracellular transport of substances via passive dissemination into the brain and tightly regulates influx and efflux transport to provide a safe haven for the brain.1 Because of its highly selective nature, the BBB constitutes the greatest impediment for delivering drugs via blood circulation to treat brain disorders. Although penetrating the BBB is a formidable challenge, researchers have exploited its architecture, which harbors a variety of transporters, and its anionic nature by utilizing nanoplatforms to bypass the BBB and deliver versatile therapeutics into the brain. The enormous potential of nanoplatforms is well suited for a variety of applications in central nervous system (CNS) pathologies. These applications range from state-of-the-art imaging modalities2 to exquisitely sensitive biomarker detection3–5 and novel strategies to deliver drugs across the BBB.6 Diverse arrays of nanoformulations encapsulating drugs in organic and inorganic nanoparticles, liposomes, and micelles have been developed.7 The strategy comprises conjugating nanoparticles with various ligands or antibodies against several carrier and transporter proteins of the BBB for brain-targeted delivery. Furthermore, the negatively charged BBB has also been exploited through adsorptive-mediated endocytosis by positively charged delivery systems. Examples include glutathione transporters, receptors for transferrin, insulin, diphtheria toxin, nicotinic acetylcholine, and cell-penetrating peptides.7,8 There are several drawbacks to each of these approaches; none of these transporters/receptors are selective for the brain, and most of the peptides and antibodies are highly unstable and immunogenic.
The blood–brain barrier is a highly specialized, multicellular, and dynamic interface between the central nervous system and the blood capillaries.
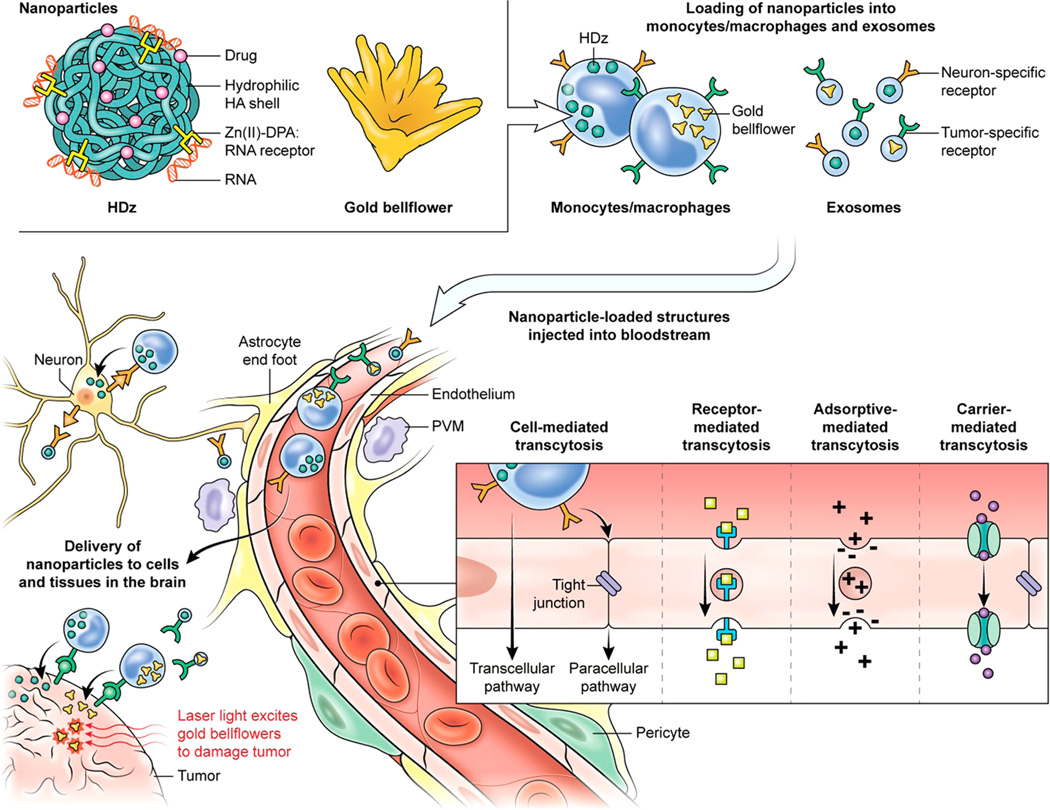
Besides these diverse nanoplatforms exploiting the carrier-, receptor-, and adsorptive-mediated mechanisms for penetrating the BBB, other options of cell-mediated transcytosis are increasingly being explored (Figure 1). The brain is considered to be an immunologically privileged site, which is tightly controlled by the BBB, although circulating immunocytes, such as macrophages and distinct subsets of lymphocytes, use specialized mechanisms to cross the BBB without disrupting its structural integrity.9,10 Stem cells also penetrate the BBB. In particular, mesenchymal stem cells display tropism toward brain tumors in animal models and therefore can serve as delivery vehicles for brain tumors. However, the mechanism of their transmigration through the BBB is poorly understood, and the potential for teratogenesis remains a valid concern.11,12
Figure 1.
The blood–brain barrier (BBB): cell-mediated transcytosis model and delivery of nanostructures. Multicellular composition and transport mechanisms: brain capillary endothelial cells with unfenestrated tight junctions are primarily responsible for maintaining the integrity of the BBB. Additional components of the BBB are astrocytes, pericytes, neurons, and perivascular macrophages (PVM). Shown are major transport mechanisms. The essential nutrients are imported via carrier-mediated transport, whereas receptor- and adsorptive-mediated transcytoses are utilized to transport hormones, peptides, and other macromolecules. Nanoformulations can be imported into the brain using any of the three transport mechanisms. Another mechanism of cell-mediated transcytosis is normally used by monocytes/macrophages and probably by exosomes. Examples are shown of hyaluronic acid nanoparticles (HDz)22 carrying a variety of cargos and gold bell flowers for photothermal therapy24 into macrophages and exosomes, which can be decorated with neuron-specific or tumor-specific ligands. Macrophages enter the brain parenchyma via paracellular and transcellular pathways. Both macrophages and exosomes are known to be capable of delivering cargo directly into the cytosol of the target cells independent of endosomes.
Among various cell types, macrophages appear to be the natural choice as cellular/biological vehicles for the delivery of nanoparticles to treat CNS pathologies for a variety of reasons. First, there appears to be routine trafficking of macrophages through the BBB in homeostasis, even more so during neuroinflammatory conditions, with a high turnover rate of 30% in 90 days.13 Second, the molecular mechanisms of migration of macrophages into the brain are well understood; under normal physiological conditions, monocytes/macrophages traverse the BBB via highly regulated processes—paracellular diapedesis, but also through the less well-defined transcellular diapedesis9,13—without compromising the integrity of the BBB. Third, macrophages are attracted to and infiltrate into the brain during inflammation and tumor development, making them a good choice as delivery vehicles carrying therapeutic nanoparticles for the treatment of neurodegenerative diseases as well as glial tumors. Finally, because of their natural function of phagocytosis of foreign bodies, macrophages are well-suited for entrapping a variety of nanoplatforms. Thus, macrophages lend themselves to being “Trojan horses” to carry innovative nanoformulations of therapeutic as well as diagnostic and imaging agents, which are otherwise restricted by their inability to cross the BBB.
Another type of biological vehicle that is gaining credence for a variety of applications, especially in nanomedicine, is exosomes.14 These extracellular nanovesicles range between 30 and 120 nm in size and are present in biological fluids in both physiological and pathological conditions. Exosomes are involved in intercellular trafficking of cell-specific cargo including genetic material, proteins, and peptides. The surface architecture of exosomes, consisting of complex lipids and membrane proteins, enables efficient fusion with the target cells, thereby delivering the cargo into the recipient cells. The ability to transport cellular contents between both neighboring and distant cells underscores the therapeutic applications of exosomes as delivery vehicles for drugs and a variety of biological molecules.14
A proof of principle for these cellular delivery vehicles has been provided by using neuron-targeting, siRNA-transporting exosomes, and nanoparticle-carrying macrophages to deliver therapeutics for neurodegenerative diseases as well as brain tumors.15–17 Both exosomes and macrophages have numerous advantages as delivery vehicles to the brain including (a) no immunogenicity or biotoxicity; (b) delivery of the cargo directly into the cytoplasm by fusing with the cell membrane and thus bypassing the endosomal pathway; (c) the ability to carry a variety of imaging, diagnostic, and therapeutic modalities simultaneously, thus serving as true theranostic carriers; and (d) the possibility of long-term introduction for chronic neurodegenerative diseases without inducing immunogenicity. Research areas to focus on in the future may include decorating exosomes and macrophages with various brain-targeting ligands to improve trafficking to the brain, and encapsulating unique diagnostic and therapeutic cargos.
Mesenchymal stem cells display tropism toward brain tumors in animal models and therefore can serve as delivery vehicles for brain tumors.
Brain Targeting
Incorporation of a neuron-specific peptide into the outer surface of exosomes has been shown to facilitate their trafficking into the brain, probably through retrograde neuronal transport.15 In principle, the same strategy can be used to enhance the trafficking of macrophages through the BBB. Although macrophages have a natural ability to traverse the BBB, a large fraction of exogenously injected macrophages are taken up by the liver, spleen, and kidneys, with only a small percentage reaching the brain. The feasibility of decorating both macrophages and exosomes via chemical conjugation or simple adsorption of various neuron- and glioblastoma-specific peptides, aptamers, and antibodies should be explored. Examples of brain-targeting ligands include neuron-specific rabies virus glycopeptide18 tet-1 peptide with a strong binding affinity for neurons19 and EGFRvIII-specific antibodies for glioblastomas.20
Both exosomes and macrophages have numerous advantages as delivery vehicles to the brain.
Theranostic Cargos
Iron oxide magnetic nanoparticles have previously been used as contrast agents for imaging, drug carriers, and thermotherapy-induced cell ablation of glioblastomas.21 Recently, a variety of multifunctional nanoplatforms have been developed that combine versatile therapeutic modalities (chemotherapeutic drugs, antibodies, aptamers, siRNAs, and miRNAs) with multiple imaging options (optical, positron imaging tomography, and magnetic resonance) that are capable of photoactivatable diagnostics and therapeutics for treating various diseases. Most of these nanoplatforms cannot be used for neurodegenerative diseases or brain cancers because of their inability to penetrate through the BBB. However, encapsulation of these nanoformulations in BBB-traversing cellular/biological vehicles modified as indicated above with neuron/glial cell-specific ligands would allow the delivery of the theranostic cargo into the brain.
Examples of theranostic platforms with potential applications in treating glioblastomas and neurodegenerative diseases like Alzheimer's and Parkinson's are (a) the nontoxic, biodegradable nanoparticles that can be loaded with a combination of siRNAs, miRNAs, enzymes, and drugs;22,23 and (b) the novel structures of gold nanoparticles with unique features of highly efficient photoacoustic imaging and photodynamic/ photothermal therapy.24,25 These nanoformulations with proven in vivo efficacy, when entrapped within brain-targeting endogenous cellular/biological vehicles, represent promising drug, gene, and/or enzyme delivery tools for brain diseases (Figure 1).
OUTLOOK AND FUTURE CHALLENGES
In the future, rigorous efforts should be dedicated to improving our understanding of the delivery of nanoplatforms to the brain using biological vehicles. For example, better insight into the signaling pathways that facilitate trafficking of monocytes/macrophages to the brain, as well as of the functionally distinct subclasses26,27 with deleterious and beneficial effects, would help researchers to select the most appropriate subset for brain delivery. Similarly, in the case of exosomes, challenges include improving the isolation and purification procedures from an appropriate source and cognizance of the potentially harmful material they might carry. Other issues to consider include the following: (a) biotoxicity/biodegradability concerns for nanoparticles, (b) the effect of size and shape of nanoformulations on loading efficiency in macrophages/exosomes and better insight into their release from these delivery vehicles, (c) protection of theranostic cargos against degradation inside the macrophages/exosomes, (d) efficiency of labeling of macrophages/exosomes with brain-targeting ligands and its effect on their trafficking to the brain and other major organs, and (e) minimizing the toxicity of the cargo that ends up in other organs, perhaps by surface labeling of the nanoformulations with brain-targeting ligands, thus curtailing their uptake by nonbrain tissues when released from the delivery vehicles.
The path to developing innovative nanoplatforms and delivery vehicles capable of crossing the impenetrable BBB for diagnosis and therapy is indeed arduous. However, the payoffs for such an endeavor are enormous; it represents a critically needed tool outlined in the ambitious arsenal being pioneered by the BRAIN initiative.
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
REFERENCES AND NOTES
- 1.Obermeier B, Daneman R, Ransohoff M. Development, Maintenance and Disruption of the Blood–Brain Barrier. Nat. Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viola KL, Sbarboro J, Sureka R, De M, Bicca MA, Wang J, Vasavada S, Satpathy S, Wu S, Joshi H, et al. Towards Non-Invasive Diagnostic Imaging of Early-Stage Alzheimer's Disease. Nat. Nanotechnol. 2015;10:91–98. doi: 10.1038/nnano.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu D, Yang J, Wang H-F, Wang Z, Huang X, Wang Z, Niu G, Walker AR, Chen X. Glucose Oxidase-Catalyzed Growth of Gold Nanoparticles Enables Quantitative Detection of Attomolar Cancer Biomarkers. Anal. Chem. 2014;86:5800–5806. doi: 10.1021/ac500478g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rana S, Le NDB, Mout R, Saha K, Tonga GY, Bain RES, Miranda OR, Rotello CM, Rotello VM. A Multichannel Nanosensor for Instantaneous Readout of Cancer Drug Mechanisms. Nat. Nanotechnol. 2015;10:65–69. doi: 10.1038/nnano.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosaka PM, Pini V, Ruz JJ, da Silva RA, Gonzalez MU, Ramos D, Calleja M, Tamayo J. Detection of Cancer Biomarkers in Serum Using a Hybrid Mechanical and Optoplasmonic Nanosensor. Nat. Nanotechnol. 2014;9:1047–1053. doi: 10.1038/nnano.2014.250. [DOI] [PubMed] [Google Scholar]
- 6.Pardridge WMJ. Drug Transport Across the Blood–Brain Barrier. J. Cereb. Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Liu L. Modern Methods for Delivery of Drugs Across the Blood –Brain Barrier. Adv. Drug Delivery Rev. 2012;64:640–665. doi: 10.1016/j.addr.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Kievit FM, Zhang M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Adv. Mater. 2011;23:H217–H247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelhardt B, Ransohoff RM. Capture, Crawl, Cross: The T Cell Code To Breach the Blood–Brain Barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Corraliza I. Recruiting Specialized Macrophages Across the Borders To Restore Brain Functions. Front. Cell. Neurosci. 2014;8:262. doi: 10.3389/fncel.2014.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman M, Hoh B, Kohler N, Dunbar EM, Murad GJ. The Future of Glioma Treatment: Stem Cells, Nanotechnology and Personalized Medicine. Future Oncol. 2012;8:1149–1156. doi: 10.2217/fon.12.111. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Eckert MA, Riazifar H, Kang DK, Agalliu D, Zhao W. From Blood to the Brain: Can Systemically Transplanted Mesenchymal Stem Cells Cross the Blood–Brain Barrier? Stem Cells Int. 2013;2013:435093. doi: 10.1155/2013/435093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivey NS, Maclean AG, Lackner AA. Acquired Immunodeficiency Syndrome and the Blood–Brain Barrier. J. NeuroVirol. 2009;15:111–122. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A Comprehensive Overview of Exosomes as Drug Delivery Vehicles – Endogenous Nanocarriers for Targeted Cancer Therapy. Biochim. Biophys. Acta Rev. Cancer. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 16.Haney MJ, Zhao Y, Harrison EB, Mahajan B, Ahmed S, He Z, Suresh P, Hingtgen SD, Klyachko NL, Mosley RL, et al. Specific Transfection of Inflamed Brain by Macrophages: A New Therapeutic Strategy for Neurodegenerative Diseases. PLoS One. 2013;8:e61852. doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Choi MR, Bardhan R, Stanton-Maxey KJ, Badve S, Nakshatri H, Stantz KM, Cao N, Halas NJ, Clare SE. Delivery of Nanoparticles to Brain Metastases of Breast Cancer Using a Cellular Trojan Horse. Cancer Nanotechnol. 2012;3:47–54. doi: 10.1007/s12645-012-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, Carter EE, Barber RD, Baban DF, Kingsman SM, et al. Rabies Virus Glycoprotein Pseudotyping of Lentiviral Vectors Enables Retrograde Axonal Transport and Access to the Nervous System after Peripheral Delivery. Hum. Mol. Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 19.Liu JK, Teng Q, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, Boulis NM. A Novel Peptide Defined through Phage Display for Therapeutic Protein and Vector Neuronal Targeting. Neurobiol. Dis. 2005;19:407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Hegi ME, Rajakannu P, Weller M. Epidermal Growth Factor Receptor: A Re-emerging Target in Glioblastoma. Curr. Opin. Neurol. 2012;25:774–779. doi: 10.1097/WCO.0b013e328359b0bc. [DOI] [PubMed] [Google Scholar]
- 21.Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Orawa H, Budach V, Jordan A. Efficacy of Intratumoral Thermotherapy using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Gliobastoma Multiforme. J. Neuro-Oncol. 2011;103:317–324. doi: 10.1007/s11060-010-0389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi KY, Silvestre OF, Huang X, Min KH, Howard GP, Hida N, Jin AJ, Carvajal N, Lee SW, Hong JI, et al. Versatile RNA Interference Platform for Systemic Delivery of RNAs. ACS Nano. 2014;8:4559–4570. doi: 10.1021/nn500085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong Y, Love KT, Dorking JR, Sirirungruang S, Zhang Y, Chen D, Bogorad RL, Yin H, Chen Y, Vegas AJ, et al. Lipopetide Nanoparticles for Potent and Selective siRNA Delivery in Rodents and Nonhuman Primates. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang P, Rong P, Lin J, Li W, Yan X, Zhang MG, Nie L, Niu G, Lu J, Wang W, et al. Triphase Interface Synthesis of Plasmonic Gold Bellflowers as Near-Infrared Light Mediated Acoustic and Thermal Theranostics. J. Am. Chem. Soc. 2014;136:8307–8313. doi: 10.1021/ja503115n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Black KC, Luehmann H, Li W, Zhang Y, Cai X, Wan D, Liu SY, Li M, Kim P, et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. ACS Nano. 2013;7:2068–2077. doi: 10.1021/nn304332s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS Myeloid Cells and Their Role in Neurodegeneration. Nat. Neurosci. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- 27.Shechter R, Schwartz M. Harnessing Monocyte-Derived Macrophages To Control Central Nervous System Pathologies: No Longer 'If' but 'How'. J. Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]