Abstract
Background
AMPAkines augment the function of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors in the brain to increase excitatory outputs. These drugs are known to relieve persistent pain. However, their role in acute pain is unknown. Furthermore, a specific molecular and anatomic target for these novel analgesics remains elusive.
Methods
We studied the analgesic role of an AMPAkine, CX546, in a rat paw incision (PI) model of acute postoperative pain. We measured the effect of AMPAkines on sensory as well as depressive symptoms of pain using mechanical hypersensitivity and forced swim tests. We asked whether AMPA receptors in the nucleus accumbens (NAc), a key node in the brain's reward and pain circuitry, can be a target for AMPAkine analgesia.
Results
Systemic administration of CX546 (n=13), compared with control (n=13), reduced mechanical hypersensitivity (50% withdrawal threshold of 6.05±1.30g (mean±SEM) vs. 0.62±0.13g), and it reduced depressive features of pain by decreasing immobility on the forced swim test in PI-treated rats (89.0±15.5s vs. 156.7±18.5s). Meanwhile, CX546 delivered locally into the NAc provided pain-relieving effects in both PI (50% withdrawal threshold of 6.81±1.91g vs. 0.50±0.03g; control n=6, CX546 n=8) and persistent postoperative pain (spared nerve injury – SNI) models (50% withdrawal threshold of 3.85±1.23g vs. 0.45±0.00g; control n=7, CX546 n=11). Blocking AMPA receptors in the NAc with 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2, 3-dione (NBQX) inhibited these pain-relieving effects (50% withdrawal threshold of 7.18±1.52g vs. 1.59±0.66g; n=8 for PI groups; 10.70±3.45g vs. 1.39±0.88g; n=4 for SNI groups).
Conclusions
AMPAkines relieves postoperative pain by activating AMPA receptors in the NAc.
Introduction
Postoperative pain impairs rehabilitation, and 30% of postoperative patients develop persistent or chronic pain.1 Respiratory depression caused by opioids and other sedatives remains a serious postoperative complication, and common affective pain symptoms such as depressed mood further delay postsurgical recovery2-7. Newer and safer analgesics that can treat both sensory and affective pain symptoms are urgently needed.
Glutamate signaling in the central nervous system (CNS) plays an important role in regulating pain sensitivity as well as mood. α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors are the primary glutamate receptors in the brain.8 AMPA receptor signaling is crucial for the function of nucleus accumbens (NAc), a key region for the regulation of both reward and aversion-driven behaviors.9-11 Human imaging studies reveal that acute and chronic pain activate the NAc,12-14 and signaling through AMPA receptors in the NAc generate pain-induced analgesia in animal studies.15,16 More importantly, recent studies indicate that persistent pain alters AMPA receptor composition and function in the NAc, and that increased AMPA receptor activities can relieve sensory and affective symptoms of postoperative pain.17-19
AMPAkines enhance glutamate transmission by binding to an allosteric site on the AMPA receptor to slow the kinetics of channel deactivation.20,21 AMPAkines have been investigated in schizophrenia, depression, Huntington's and Alzheimer's diseases.20,22-25 Interestingly, recent studies have shown that AMPAkines can stimulate the respiratory drive in the context of hypoventilation caused by opioids or other sedatives, thus making these drugs tantalizing options in the postoperative setting.26-28 A previous study showed AMPAkines can relieve both sensory and affective symptoms of persistent pain.29 However, it is not known whether AMPAkines can also relieve acute postoperative pain. If so, such drugs can be ideal analgesics in the postoperative setting to relieve pain and improve mood and at the same time to enhance the safety profile of sedatives commonly administered during or after surgery. From a mechanistic standpoint, AMPAkines are known to have high affinity for neurons in the NAc and brain stem.30 Given the crucial role AMPA receptors in the brain play in pain regulation, these receptors may form an important target for AMPAkine analgesia.
To investigate the potential analgesic effects of AMPAkines in the postoperative setting, we tested CX546, an established AMPAkine which has been studied in hypoventilation, Rett syndrome, anxiety, and autism,27,31-34 in a classic acute postoperative pain model – paw incision (PI) model.35 We examined whether this AMPAkine is able to relieve both mechanical hypersensitivity and depressive symptoms of pain in this model. We delivered CX546 specifically into the NAc to see if AMPA receptors in the NAc could mediate its pain-relieving effects. Furthermore, we tested the analgesic effects of systemic administration of CX546 after blocking AMPA receptors in the NAc locally with 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX). We confirmed the role of the NAc AMPA receptors in AMPAkine analgesia using a persistent postoperative pain (spared nerve injury –SNI) model. Finally, as glutamate signaling in the NAc may play a role in drug craving and addiction,36-38 we performed conditioned place preference test to show that a short-term use of AMPAkines did not result in craving.
Materials and Methods
Animals
All procedures in this study were approved by the New York University School of Medicine Institutional Animal Care and Use Committee (IACUC) as consistent with the National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (publication number 85-23) to ensure minimal animal use and discomfort. Male Sprague-Dawley rats were purchased from Taconic Farms, Albany, NY and kept at Mispro Biotech Services Facility in Alexandria Center for Life Science, with controlled humidity, room temperature, and 12-h (6:00 AM to 6:00 PM) light-dark cycle. Food and water were available ad libitum. Animals arrived to the animal facility at 250 to 300 grams and were given on average 7 days to adjust to the new environment prior to the onset of any experiments. Rats were randomly assigned to each experimental condition. Blinding was attempted whenever possible for all behavior experiments. The person who performed and analyzed the test was blinded to the condition of the animals.
Animal Surgeries and Procedures
Paw Incisional (PI) procedure
The paw incisional surgery was performed as previously described.18,35,39 Briefly, rats were anaesthetized with Isoflurane anesthesia (1.5 to 2%), and the plantar surface of the right hind paw was sterilized and prepared. A 1.5 cm longitudinal incision was made with a number 10 scalpel, through skin and fascia of the right plantar aspect of the paw. The incision started 0.5 cm from the proximal end of the heel and extended to the middle of the paw. The plantaris muscle was elevated and incised longitudinally. Gentle pressure was applied in order to cease bleeding, and the superficial wound was opposed with three single sutures using 5–0 nylon. The animals were allowed to recover in their home cages. Control rats only received Isoflurane anesthesia.
Spared Nerve Injury (SNI) surgery
The Spared Nerve Injury (SNI) surgery has been previously described in detail.17,40,41 Briefly, under Isoflurane anesthesia (1.5 to 2%), the skin on the lateral surface of the right thigh of the rat was incised and the biceps femoris muscle was dissected in order to expose three branches of the sciatic nerve: sural, common peroneal, and tibial. The common peroneal and tibial nerves were tied with non-absorbent 5.0 silk sutures at the point of trifurcation. The nerves were then cut distal to the knot, and about 3 to 5 mm of the distal ends were removed. In sham surgeries (control), the nerves mentioned above were dissected but not cut. Muscle and skin layers were then sutured closed in distinct layers.
Cannula implantation and intracranial injections
For cannula implantation, as described previously,17,42 rats were anesthetized with isofluorane (1.5 to 2%). Rats were stereotaxically implanted with two 26-gauge guide cannulas (PlasticsOne, Roanoke, VA) bilaterally in the NAc core with coordinates: 1.6 mm anterior to bregma; 2.9 mm lateral to the sagittal suture, tips angled 8° toward the midline, 5.6 mm ventral to skull surface. Cannulas were held in place by dental acrylic and patency was maintained with occlusion stylets. For intracranial injections, solutions were loaded into two 30 cm lengths of PE-50 tubing attached at one end to 10-μl Hamilton syringes filled with distilled water and at the other end to 33-gauge injector cannula, which extended 2.0 mm beyond the implanted guides. Injection of solution then delivered bilaterally 0.5 ul of injection volume over a period of 100 sec. Injector cannulas were kept in place for another 60 sec prior to removal from guides to allow diffusion of solution into the brain. Following the removal of injector cannulas from cannula guides, stylets were replaced, and animals were subject for behavior tests. Behavior tests were done 15 minutes after intracranial injections. Following animal sacrifice, cryogenic brain sections were collected with thickness of 20 um using Microm HM525 Cryostat and analyzed for cannula localization with histological staining; animals with improper cannula placements were excluded from the study.
Drugs
CX546 (Sigma-Aldrich) was suspended in Dimethyl sulfoxide (DMSO) to different concentrations (2.5, 5, 10mg/Kg) for systemic administration; CX546 was resuspended in 0.9% saline to concentrations of 400 uM/ul and 800 uM/ul for intra-NAc infusions in PI and SNI-treated rats. Two, 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2, 3-dione (NBQX), (Tocris Bioscience), was resuspended in 0.9% saline to a concentration of 0.55 nmol/l. CX546 was systemic administered (intraperitoneally) in PI or SNI-treated rats, while same volumes of DMSO were applied in the control group. Systemic administrations were given 4 hours after PI or 14 days after SNI and were followed by behavioral tests. In addition, CX546, NBQX or saline were locally infused into the NAc of PI-treated and SNI-treated rats. For local infusions, 0.5 μl of drug or control was injected in each side of the brain. Intracranial administrations were given at least 7 days after cannula implantation and were followed by behavioral tests. Intra-NAc infusions were given 4 hours, 1, 4 and 7 days after PI or 14 days after SNI.
Animal Behavioral Tests
Mechanical hypersensitivity test
A traditional Dixon up-down method with von Frey filaments was used to measure mechanical hypersensitivity as described previously.41,43,44 In brief, rats were individually placed into plexiglass chambers over a mesh table and acclimated for 20 min before the onset of examination. Beginning with 2.55g, von Frey filaments in a set with logarithmically incremental stiffness (0.45, 0.75, 1.20, 2.55, 4.40, 6.10, 10.50, 15.10 g) were applied vertically to the plantar surface of the right paw, adjacent to the wound of rats after PI. For SNI and sham group, von Frey filaments were applied to the lateral 1/3 of right paws (in the distribution of the sural nerve) of rats after SNI or sham surgery. 50% withdrawal threshold was calculated as described previously.41
Forced swim test (FST)
On the first session of the test, each animal was placed for fifteen minutes into a standard clear Porsolt chamber with water at 25° C filled to 25 cm. Afterwards, the animal was taken out of the chamber, dried and put back in its home cage. 24 hours later, the animal was placed into the Porsolt chamber again under the same conditions for 5 min. Only the second session was videotaped and analyzed. Immobility was defined as a lack of movement of the hind paws lasting greater than 1 second. An independent observer, blinded to the test conditions, examined and graded the total time of immobility for each rat, and the average grade was presented for each animal. FST was conducted 4 hours after PI or 14 days after SNI.
Locomotion activities
Rats were placed in a 0.5 × 0.5m chamber with an overhead camera. Using a video analysis software (ANY-maze), animal movements were tracked during a 30 min test. The total distance traveled was computed for 10 bins of 3 min. Later, videos were visually examined to correlate with automatic results in order to verify tracking accuracy.
Conditioned Place Preference (CPP)
CPP experiments were conducted in a standard three-compartment apparatus (Stoelting co., Wood Dale, IL) consisting of two large compartments of equal size (45 × 40 × 35cm) joined by a tunnel (40 × 9 × 35cm). The movements of rats in each chamber were automatically recorded by a camera and analyzed with the Any-maze software. The CPP protocol was modified from King et al. and included preconditioning, conditioning, and testing phases.45 Preconditioning was performed across 3 days. During preconditioning, all animals were exposed to the environment with full access to all chambers for 30 min each day. On day 3, the movement of each rat was recorded for 15 min and analyzed to verify the absence of any preconditioning chamber preference. Animals spending more than 720s or less than 120s of the total time in any chamber were eliminated from further testing or analysis (approximately 15% of total animals). Following the pre-conditioning phase, the rats underwent conditioning for 4 days with alternating intraperitoneal injection of CX546 (10mg/kg) or control treatment-chamber pairings in the morning and afternoon. During conditioning, rats were placed in the paired chamber without access to the other compartments for 30 min. Drug treatments and chamber pairings were counterbalanced, and at least 4 hours separated morning and afternoon sessions. On the test day, the animals were placed into the neutral chamber and had access to all chambers for a total of 15 min. There were no drug treatments on test days. During these 15 min, animal movements in each of the chambers were recorded, and the time spent in either of the treatment chambers was analyzed by the AnyMaze software. Increased time spent in a chamber associated with drug or control treatment indicates preference for that chamber. This test was repeated with morphine (4mg/kg, subcutaneous injection) instead of CX546 for comparison.
Subcellular fractionation and Western blotting
PI-treated rats were anesthetized with isofluorane (1.5-2%) and decapitated immediately. Brains were quickly removed and nucleus accumbens were collected on ice. Synaptoneurosome fractions were prepared as described previously.46,47 To prepare synaptoneurosome fractions, nucleus accumbens samples were homogenized in ice-cold solution A (0.32 M sucrose, 1 mM NaHCO3, 1 mM MgCl2, 0.5 mM CaCl2, 0.1 mM PMSF and 1x Complete Protease Inhibitors (Roche Applied Science). Homogenates were centrifuged at 4,000 rpm for 10 min. The supernatant was collected and the pellet re-homogenized in solution A and centrifuged again at 3,000 rpm for 10 min. Combined supernatants were subjected to a second centrifugation at 3,000 rpm for 10 min. Supernatants were then spun at 14,000 rpm for 30 minutes. Pellet was resuspended in solution B (0.32 M sucrose, 1 mM NaHCO3) and homogenized. Homogenate was layered on top of a 5 mL 1 M sucrose and 1.2 M sucrose gradient and centrifuged at 30,000 rpm for 2 hours. Purified synaptosomes were collected at the 1 M and 1.2 M sucrose interface, suspended in solution B and centrifuged at 40,000 rpm for 45 min. Synaptosomal pellets were resuspended in 25 mM TRIS with 4% SDS. Fractions were analyzed by Western blot on SDS-PAGE gels as described previously.46,47 The following antibodies were used: GluA1 (1:1,000, Millipore), GluA2 (1:1,000, Millipore) and tubulin (1:30,000, Sigma).
Data analysis and statistics
The results of behavioral experiments were given as mean ± SEM. A two-way ANOVA with repeated measures, followed by post hoc multiple pair-wise comparison Bonferroni test was used to compare the 50% withdrawal threshold of PI vs. control rats and SNI vs. sham rats. For the FST, an unpaired two-tailed Student's t-test was used to compare the performances of PI vs. control groups, CX546 vs. DMSO groups, and CX546 vs. saline groups. For dose–response experiments, a one-way ANOVA was used to compare the analgesia effects of CX546 with DMSO as control. For the experiment testing the duration of analgesic effects, a two-way ANOVA with repeated measures and post hoc multiple pair-wise comparison Bonferroni tests was used to compare mechanical hypersensitivity after CX546 vs. DMSO (control) treatments. A two-way ANOVA with repeated measures, followed by post hoc multiple pair-wise comparison Bonferroni test was also used to compare the 50% withdrawal threshold of CX546 vs. saline local infusion treatments at multiple time points. An unpaired two-tailed Student's t-test was used to compare the 50% withdrawal threshold of NBQX vs. saline in both PI and SNI rats, as well as intracranial CX546 vs. saline treatments in SNI rats. For locomotion, a two-way ANOVA with repeated measures and post hoc multiple pair-wise comparison Bonferroni tests was used to compare the performances of PI vs. control groups, CX546 vs. DMSO in PI rats and CX546 vs. saline in both of PI and SNI rats. For CPP tests, differences in time spent in each chamber before conditioning (pre-conditioning) and after conditioning (test) were analyzed using a two-way ANOVA with repeated measures followed by post hoc Bonferroni tests. An unpaired Student's t test was used to compare the Western blot results. For all tests, a p value <0.05 was considered statistically significant. For intracranial injections, rats with improperly implanted cannulas were excluded from further analysis. Sample sizes were estimated based on previous experience with the same experimental designs.17-19,29 All data were analyzed using GraphPad Prism Version 6 software (GraphPad, La Jolla, CA).
Results
PI produces acute postoperative pain
We applied the PI (Brennan) model to mimic acute post-incisional pain in rats.35,39 Here, we incised the right hind paw and measured mechanical hypersensitivity over the next seven days. As reported previously, mechanical hypersensitivity, which indicates the sensory component of pain, developed quickly after paw incision (<4 h) (Fig. 1A).35,39 This sensory hypersensitivity lasted up to 4 days after incision, and it resolved by the 7th day post-incision18,39 (Fig. 1A). In contrast, control rats that had only undergone Isofluorane anesthesia treatment without surgical incision did not display any mechanical hypersensitivity.
Figure 1. Paw incision (PI) leads to acute postoperative pain and depression-like behaviors in rats.
A. Rats that underwent PI surgery developed mechanical hypersensitivity as early as 4 hours and up to 4 days after surgery, compared with control rats that underwent Isofluorane treatment without surgery. Two-way ANOVA with repeated measures and post hoc Bonferroni multiple comparison tests, control group n=6; PI group n=6, ***p=0.0004 at 4 hr, ****p<0.0001 at postoperative day (POD) 1, ***p=0.0001 at POD2, **p=0.0015 at POD4, and p>0.9999 at POD7. B. PI-treated rats developed depression-like behaviors compared with control rats 4 hours after surgery, as shown by an increase in immobility during the force swim test (FST). Unpaired two-tailed Student's t-test, control group n=5; PI group n=4, **p=0.0081. C. Locomotion was not affected by the PI surgery. Two-way ANOVA, control group n=6; PI group n=6, p=0.6096. Error bars represent standard error mean (SEM).
Studies in various animal models have shown that pain can lead to depression-like behaviors in rats.41,48,49 Decreased motivation is a key feature of depression, especially in the context of pain.2,3,7 Increased immobility on the FST has been used as a standard measure for decreased motivation or behavioral despair in rodents,50 and a number of studies have shown clinically relevant pharmacological validity of this measure.51 We and others have reported previously that increased immobility on FST was found in various rodent models of pain.17,19,41,48,49,52,53 Here, we applied FST in our study to assess depression-like behaviors in the PI model. As expected, we found PI-treated rats, compared with control, displayed increased immobility after paw incision (Fig. 1B, p=0.0081). To exclude the possibility that this increased immobility was caused by a deficit in locomotor abilities resulting from pain or incision, we directly measured locomotion in PI-treated and control groups. We found no statistically significant difference in locomotion between these two group of rats (Fig. 1C, p=0.6096). Thus, results on the FST suggest that some features of depression-like behaviors can accompany acute postoperative pain.
Systemic administration of CX546 relieves sensory hypersensitivity and depression-like behaviors associated with acute postoperative pain
CX546 is a well-established AMPAkine that has been found to oppose sedative-induced respiratory depression, and it has been studied in a variety of CNS diseases.26,27,32,33,54,55 We have previously shown that systemic administration of CX546 can relieve both sensory hypersensitivity and depressive behaviors in response to chronic neuropathic pain and inflammatory pain in rats.56 The pathogenesis and maintenance of acute postoperative and chronic pain, however, are different mechanistically at synaptic and circuit levels.57 Hence, we wanted to test whether CX546 could also relieve acute postoperative pain in the PI model. First, we applied CX546 at different doses to test its effect on mechanical hypersensitivity after PI. Compared with control (DMSO), CX546 improved mechanical hypersensitivity at 5 and 10mg/kg doses (Fig. 2A, p=0.0404 and p<0.0001 respectively), with the 10mg/kg dose providing a greater anti-hypersensitivity effect. Next, we evaluated the timing and duration of the anti-allodynic effect after a single systemic administration. We found that the anti-nociceptive effects of CX546 (10mg/kg) began within one hour after administration (Fig.2B, p<0.0001) and lasted > 4 hours (Fig.2B, p=0.03). This anti-nociceptive effect diminished after 8 hours.
Figure 2. Systemic administration of CX546 relieves mechanical hypersensitivity in paw incision (PI)-treated rats.
A. Intraperitoneal administration of CX546 relieved mechanical hypersensitivity in PI-treated rats at doses of 5mg/kg and 10mg/kg, compared with Dimethyl sulfoxide (DMSO). One-way ANOVA with post hoc Bonferroni multiple comparison tests, DMSO group n=12; CX546 2.5mg/kg group n=7; CX546 5mg/kg group n=7; CX546 10mg/kg group n=6, *p=0.0404, ****p<0.0001. B. A single dose of CX546 (10mg/kg) improved mechanical hypersensitivity in PI-treated rats at 1, 2 and 4 hour after intraperitoneal injection, compared with the DMSO control group. Two-way ANOVA with repeated measures, DMSO group n=13, CX546 group n=13, *p=0.0300, **p=0.0031, ****p<0.0001.
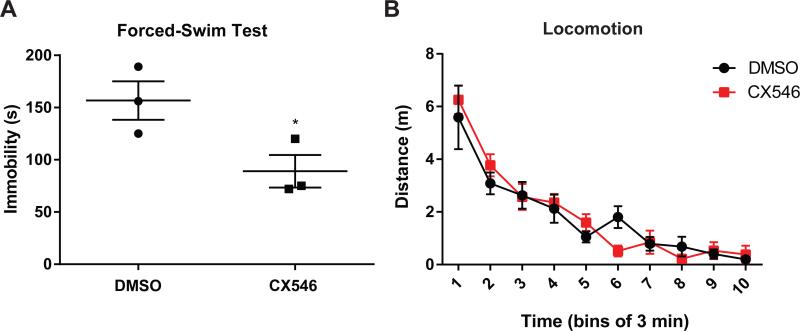
AMPA receptor signaling is known to regulate both sensory and affective components of pain, and we have previously shown that AMPAkines can relieve depressive symptoms of chronic neuropathic pain and inflammatory pain.56 Thus, we used the FST to assess the effects of CX546 on the depressive symptoms associated with acute incisional pain (Fig. 1B). We found that systemic administration of CX546 (10mg/kg) also alleviated depressive symptoms by decreasing immobility in PI-treated rats (Fig. 3A, p=0.0486). Lastly, CX546 did not alter locomotion (Fig. 3B).
Figure 3. Systematic administration of CX546 relieves depression-like behaviors in paw incision (PI)-treated rats.
A. Intraperitoneal administration of CX546 (10mg/kg) relieved depression-like behaviors in PI-treated rats, as shown by a decrease of immobility during the FST, compared with control group. Unpaired two-tailed Student's t-test, Dimethyl sulfoxide (DMSO) group n=3; CX546 group n=3, *p=0.0486. B. Systemic administration of CX546 (10mg/kg) did not change locomotion in PI-treated rats, compared with DMSO injection. Two-way ANOVA with repeated measures, DMSO group n=7; CX546 group n=7, p=0.8364.
AMPAkines target AMPA receptors in the NAc to relieve acute postoperative pain
Recent human imaging and animal studies suggested that glutamate signaling in the nucleus accumbens (NAc), the brain's reward center,9,10 plays a critical role in pain regulation.12-14 This pain-relieving role for NAc is not surprising, as it receives inputs from a number of regions important for pain regulation including the prefrontal cortex (PFC), amygdala and hippocampus,48,49,58-61 and is known to project to the RVM to provide descending inhibition.16 Meanwhile, AMPAkines are known to have high affinity for AMPA receptors in the NAc.30 Thus, AMPA receptors in the NAc are ideal molecular targets for these drugs.
To test the hypothesis that the pain-relieving properties of AMPAkines act through AMPA receptors in the NAc, we injected CX546 locally into the NAc of PI-treated rats (Fig. 4A, 9). Compared with saline control, local infusion of CX546 relieved mechanical hypersensitivity after PI (Fig. 4B). Furthermore, the degree of pain relief (as indicated by improvement in the threshold for mechanical hypersensitivity) provided by the intra-NAc infusion of CX546 is quantitatively similar to the degree of pain relief provided by the systemic infusion of this drug. These results strongly suggest a prominent role of the NAc in mediating the analgesic effect of AMPAkines.
Figure 4. Local infusion of CX546 into the nucleus accumbens (NAc) relieves mechanical hypersensitivity in paw incision (PI)-treated rats.
A. Schematic showing the timeline of pharmacological experiments. B. Intra-NAc infusion of CX546 (400uM/ul) on 4 hours and 1 day after paw incision reduced mechanical hypersensitivity in PI-treated rats. Two-way ANOVA with Bonferroni post-test, Saline group n=6, CX546 group n=8, *p=0.0248, **p=0.0012. C. Intra-NAc infusion of 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2, 3-dione (NBQX) blocked the analgesic effects of systemic administration of CX546 (10mg/kg) in PI-treated rats. Unpaired two-tailed Student's t-test, Saline group n=8; NBQX group n=8, **p=0.0046.
Figure 9. Site of intracranial pharmacological delivery.
A. Schematic showing the placement of intracranial injectors in paw incision (PI)-treated rats. B. Schematic showing the placement of intracranial injectors in spared nerve injury (SNI)-treated rats. C. Histology of a brain slice containing the nucleus accumbens (NAc).
To further confirm the importance of the NAc AMPA receptors in AMPAkine analgesia, we applied NBQX, a highly specific antagonist of AMPA receptors, locally in the NAc after the systemic infusion of AMPAkines (Fig. 4C). Not surprisingly, NBQX, by inhibiting AMPA receptor transmission in the NAc, blocked the anti-nociceptive effect of AMPAkines (Fig. 4C, p=0.0046). It is of course conceivable that NBQX inhibits the NAc core function in descending pain control in parallel to the analgesic pathway provided by CX546. Nevertheless, this data, combined with the data indicating that direct intra-NAc infusion of CX546 confers analgesic effects, provides evidence that AMPA receptors in the NAc are an important molecular target for AMPAKines.
Next, we tested whether AMPA receptors in the NAc can also be modified by AMPAkines to relieve the depressive symptoms of pain. We infused CX546 locally into the NAc of PI-treated rats and performed the FST. We found that local infusion of CX546, compared with saline, resulted in a statistically significant decrease of immobility on the FST (Fig.5A, p=0.033), and the degree of improvement in the immobility index is similar to the degree of improvement after systemic infusion of CX546. In contrast, CX546 in the NAc didn't affect locomotion (Fig.5B). Thus, by potentiating AMPA receptors in the NAc, AMPAkines can relieve acute postoperative pain.
Figure 5. Local infusion of CX546 into the nucleus accumbens (NAc) relieves depression-like behaviors in paw incision (PI)-treated rats.
A. Intra-NAc infusion of CX546 (400uM/ul) relieved depression-like behaviors in PI rats, as shown by a decrease in immobility during the force swim test (FST), compared with control (saline) group. Unpaired two-tailed Student's t-test, Saline group n=10; CX546 group n=10, *p=0.033. B. Intra-NAc infusion of CX546 (400uM/ul) did not change locomotion in PI rats. Two-way ANOVA with repeated measures and post hoc Bonferroni multiple comparison tests, Saline group n=6; CX546 group n=6, p=0.2027.
AMPAkines target AMPA receptors in the NAc to relieve persistent postoperative neuropathic pain
To further confirm the role of NAc AMPA receptors as a pharmacological target for AMPAkines, we sought to repeat our pharmacological experiments in another postoperative model. Persistent neuropathic pain can occur following inadvertent resections of peripheral nerves.62,63 The spared nerve injury (SNI) model is typically used to model chronic neuropathic pain, but it can also be used to model persistent postoperative neuropathic pain, since the nerve injury and pain symptoms (hypersensitivity and hyperalgesia) in this model resemble the kind of injury and resulting neuropathic pain that can sometimes occur after intra-operative nerve resections.62,63 Similar to PI, SNI has also been shown to lead to pain and depression-like behaviors in rats.17 We have previously shown that systemic infusion of CX546 can relieve both mechanical hypersensitivity and depression-like behaviors in this pain model.56 Here we tested whether AMPA receptors in the NAc function as a pharmacological target of AMPAKines in this model of persistent postoperative pain (Fig. 6A).
Figure 6. Local infusion of CX546 into the nucleus accumbens (NAc) decreases mechanical hypersensitivity in rats after spared nerve injury (SNI) procedure.
A. Schematic showing the timeline of pharmacologic experiment in SNI-treated rats. B. SNI-treated rats developed mechanical hypersensitivity up to 14 days after surgery, compared with sham-treated rats. Two-way ANOVA with repeated measures and Bonferroni post-tests, sham group n= 6; SNI group n= 11, ***p=0.0006 for postoperative day (POD) 1, ***p=0.0002 for POD3, **p=0.0042 for POD7, ***p=0.0009 for POD14. C. Intra-NAc infusion of CX546 (800uM/ul) relieved mechanical hypersensitivity in SNI-treated rats. Unpaired two-tailed Student's t-test, Saline group n=7; CX546 group n= 11, *p=0.0437. D. Intra-NAc infusion of 3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2, 3-dione (NBQX) blocked the analgesic effects of systemic administration of CX546 (10mg/kg) in SNI-treated rats. Unpaired two-tailed Student's t-test, Saline group n=4; NBQX group n=4, *p=0.0401.
As shown previously, SNI resulted in persistent mechanical hypersensitivity (Fig. 6B).17,40 When we infused CX546 locally into the NAc of SNI-treated rats, we found that CX546 relieved mechanical hypersensitivity in these rats (Fig. 6C, p=0.0437). Next, we infused NBQX to block these AMPA receptors in the NAc. We found that NBQX blocked the anti-nociceptive effects of systemic administration of CX546 (Fig. 6D, p=0.0401). These results suggest that AMPA receptors in the NAc can be targeted by AMPAkines to relieve persistent postoperative pain. We then used FST to assess whether AMPAkines can potentiate these receptors to treat the depressive symptoms of persistent postoperative pain as well. Indeed, intra-NAc infusion of CX546 decreased immobility on the FST without changing locomotion (Fig. 7A, p=0.0199; 7B, p=0.9782). These data suggest that AMPAkines can target the NAc AMPA receptors to relieve persistent postoperative pain.
Figure 7. Local infusion of CX546 into the nucleus accumbens (NAc) relieves depression-like behaviors in spared nerve injury (SNI)-treated rats.
A. Intra-NAc infusion of CX546 (800uM/ul) diminished depression-like behaviors in SNI-treated rats, as shown by a decrease of immobility during the force swim test (FST). Unpaired two-tailed Student's t-test, Saline group n=8; CX546 group n=7, *p=0.0199. B. Intra-NAc infusion of CX546 (800uM/ul) did not change the locomotion of SNI rats, compared with saline infusion. Two-way ANOVA with repeated measures and Bonferroni post-test, Saline group n=4; CX546 group n=7, p=0.9782.
Short-term use of AMPAkines lacks intrinsic rewarding properties
In addition to its ability to provide descending pain inhibition,12,13,16,17,64-68 the NAc is also known as the brain's reward center. Through its projection to the ventral pallidum and substantia nigra, the NAc can influence the function of ventral anterior, dorsal and lateral thalamus, which in turn projects to the motor cortex as well as parts of the prefrontal cortex. Through this striato-thalamo-cortical circuit, the NAc can regulate behavioral outcomes in the presence of rewards.69 Furthermore, activation of glutamatergic signaling in the NAc has been involved in the rewarding potential of drugs of addiction.36-38 Thus, we analyzed the rewarding potential of AMPAkine administration in rats. We used a classic conditioned place preference (CPP) test to assess drug craving (Fig. 8A). Since we anticipated that the use of AMPAkines would be limited to the acute postoperative period, we used a four-day conditioning protocol in naïve rats. After 4 consecutive days of conditioning, rats did not develop place preference for the chamber associated with AMPAkine treatment (Fig. 8A, p=0.9046). In contrast, 4 days of conditioning resulted in a statistically significantly preference for the chamber associated with morphine, a commonly used analgesic that is known to carry intrinsic rewarding properties (Fig. 8B, p=0.0349). These results suggest that while AMPAkines can augment excitatory AMPA receptor transmission in the NAc, its short-term use does not necessarily have intrinsic rewarding properties in wild type rats. All intracranial drug infusion sites for PI and SNI experiments are shown in Fig. 9.
Figure 8. Short-term use of AMPAkines does not lead to conditioned place preference (CPP).
A. Rats were conditioned for 4 days with alternating CX546 (10mg/kg) and control treatments in the morning and afternoon, and then they were tested on the following day for place preference. Rats did not develop place preference for the chamber paired with AMPAkine treatment. Two-way ANOVA with repeated measures and Bonferroni post-test was used to compare pre-conditioning with test values, n=11, p=0.9046. B. Rats developed place preference for the chamber paired with morphine treatment (subcutaneous injection, 4mg/kg). n=10, p=0.0349.
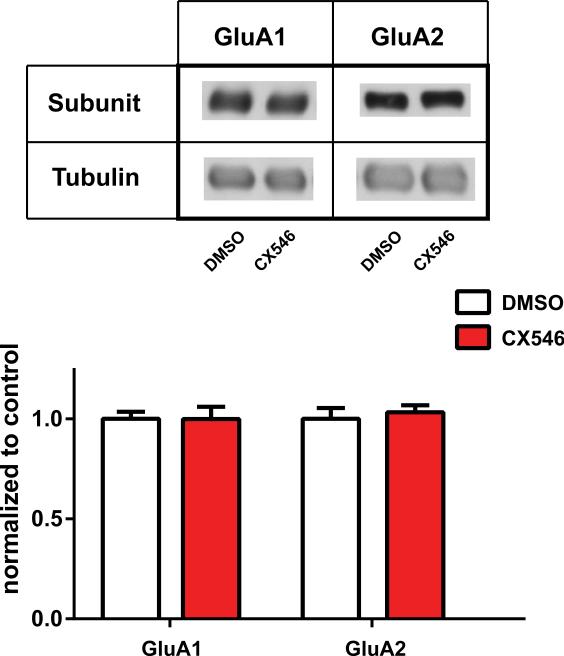
AMPAkines do not alter AMPA receptor subunit levels
Subunit trafficking is important for providing long-term plasticity of excitatory neurotransmission through AMPA receptors.70 A previous study has shown that persistent postoperative pain, but not acute postoperative pain, can increase GluA1 subunit levels at the synapse of the NAc core.18 Here we investigated whether AMPAkines can further increase AMPA receptor trafficking in the NAc. We isolated synaptoneurosome fractions of NAc and measured the GluA1 and GluA2 subunit levels after AMPAkine treatment in PI-operated rats. We did not observe an increase in these subunit levels (Fig. 10, p=0.9972 for GluA1, p=0.6249 for GluA2). These results indicate that AMPAkines do not necessarily alter the synaptic levels of AMPA receptors over time. Thus, the pharmacologic effects of AMPAkines are more likely to be restricted to the biophysical function of the AMPA receptors, rather than on the long-term changes in receptor composition. These biochemical results are consistent with our pharmacological results, demonstrating that the analgesic effects of AMPAkines do not last for a prolonged period of time.
Figure 10. AMPAkines do not increase AMPA receptor trafficking.
CX546 (10mg/kg) did not cause changes in levels of GluA1 or GluA2 subunits in the synaptoneurosome fractions of nucleus accumbens (NAc) on postoperative day (POD) 1 after paw incision (PI). Student's t test, n=12 rats (GluA1), p=0.9972, n=12 rats (GluA2), p=0.6249.
Discussion
In the current study, we have investigated the role of AMPAkines in postoperative pain. We have found that CX546, a well-known AMPAkine, reduces sensory hypersensitivity and depression-like behaviors associated with acute postoperative pain. Furthermore, we have identified AMPA receptors in the NAc as a potential molecular target for these novel analgesics.
New analgesics that do not suppress the respiratory drive remain urgently needed, especially in the postoperative period. The acute postoperative period is also a high-risk time for the development of depressed mood that leads to worse surgical outcome.4,5 AMPAkines slow the kinetics of AMPA receptor deactivation to enhance the inward excitatory synaptic current.20,21 By increasing excitatory inputs in neurons of the pre-Botzinger complex in the medulla, AMPAkines can stimulate the respiratory drive26,27,71-73 to treat or prevent hypoventilation caused by sedatives often used intra- or postoperatively.26,28,74,75 A previous study has shown the effect of AMPAkines in persistent or chronic pain. Our current study demonstrates that these drugs can also effectively relieve the incisional pain commonly associated with surgery. Furthermore, the analgesic dose for CX546 found in our study is comparable to the dose tested in rats to treat respiratory depression,27 and the time course of analgesia after a single administration (4 hours) is similar to the duration of respiratory stimulation provided by these drugs.26,27 Thus, from a pharmacologic and pharmacokinetic standpoint, AMPAkines can relieve postoperative pain, improve mood and stimulate the respiratory drive at the same time. These properties suggest that AMPAkines can be potentially very useful in the acute postoperative period.
In addition to the canonical PAG-RVM-spinal projection,76-78 the NAc can also use AMPA receptor signaling to provide descending pain inhibition through its projection to the RVM.16 Intra-NAc administration of AMPA receptor antagonists, for example, has been shown to specifically disrupt this pain-induced analgesic circuit.15 Previous work has demonstrated, furthermore, that in both acute and chronic pain states, increasing AMPA receptor signaling in the NAc improves sensory hypersensitivity and associated depressive symptoms, whereas antagonism of AMPA receptor activities has the opposite effect.17-19 Interestingly, persistent pain can cause a homeostatic increase in AMPA receptor number and function in the NAc, likely as an innate adaptive response.17,18 Thus, given their role in pain and pain-induced mood changes, AMPA receptors in the NAc are potentially ideal targets for AMPAkines.
Depressed mood has been well described in postoperative pain patients,2-6 and our finding that AMPAkines can treat pain-induced depression in a rat model of acute postoperative pain is consistent with the role of central AMPA receptors in depression79. Other pharmacological examples include ketamine, which has been shown to elevate the levels of GluA1 AMPA receptor subunits in the brain80 and effectively treat pain-induced depression.41 By directly amplifying postsynaptic currents through AMPA receptors, AMPAkines have also been found to treat depression in animal models.81 Our results demonstrate that this antidepressant effect of AMPAkines is preserved in the acute postoperative pain state. An alternative explanation is that AMPAkines, by relieving pain, also relieve depression associated with pain. This is a strong possibility. However, given the pharmacological role of AMPAkines in depression and the importance of AMPA receptors in the NAc in mood disorders,17,20,23,82 AMPAkines could also be expected to potentially relieve sensory and depressive symptoms of pain independently.
Results on withdrawal tests and the FST can be confounded by locomotor deficits. For example, a worse performance on the FST may reflect a decrease in locomotion due to movement-induced pain or neuropathy. Our locomotion tests, however, do not reveal any deficits, compatible with earlier findings.17-19 If rats do not demonstrate locomotor deficits over 30 minutes, they are unlikely to have deficits while swimming for 5 minutes during the FST. Thus, results on the FST likely reflect the phenotype of depression, rather than deficiencies in locomotion. In addition, other studies on AMPAkines have also demonstrated no effects on locomotion.54
A limitation of our study is that we did not sufficiently differentiate the cellular composition within the NAc. The NAc is composed of the core and shell regions anatomically. While core and shell are both primarily composed of GABAergic medium spiny neurons (MSNs), they differ in precise cellular morphology, neurochemistry, afferent and efferent projections.83 Functionally, the core has been proposed to mediate cue-conditioned behavioral activation, such as seeking of rewards or avoidance of noxious stimuli. The shell, meanwhile, is thought to code the salience of a particular behavioral condition.83 Our pharmacological and behavior experiments were conducted in the core because of its established role in nociception and its ability to induce behavioral modification reflected in behavioral despair. However, it is conceivable that some of the drug may have spread to the shell subregion, despite being infused directly in the NAc core and limited in volume. It is therefore possible that the shell subregion may have contributed to the analgesic effects of the AMPAkines in addition to the core subregion. The shell has been known to mediate the affective responsiveness to rewards and stress,84-86 and thus it has been suggested to code the aversive quality of pain.87 Furthermore, MSNs can be subdivided into D1 or D2 neurons based on the specific subtypes of dopamine receptors expressed on their surfaces. D1 and D2 neurons have distinct projections and may be involved in different behavioral modifications. Therefore, future studies are needed to further elucidate the roles of D1 vs. D2 subtypes of MSNs and the role of the NAc shell in AMPAkine analgesia.
AMPA receptor signaling can have both pro-nociceptive and anti-nociceptive roles, depending on the target CNS region. In the PFC, PAG and NAc, augmentation of AMPA receptor signaling results in the activation of descending inhibition through projection to the RVM.16,88-90 In contrast, in neurons of the spinal dorsal horn, ACC and amygdala, AMPA receptor activities can have pro-nociceptive effects. For example, chronic inflammatory pain increases membrane targeting of GluA1 AMPA receptor subunits in the spinal dorsal horn,91-93 leading to the formation of GluA2-lacking receptors to augment pain transmission.94 Similarly, AMPA receptor signaling in the ACC and amygdala has also been suggested to confer hyperalgesia.95-99 An interesting pharmacological property of the AMPAkines is that it has uniquely high affinity for neurons in the NAc and brain stem.30 Thus, it is plausible that AMPAkines, when given systemically, selectively bind to AMPA receptors in the NAc to activate a descending inhibitory circuit. Nevertheless, the NAc is unlikely to be the only target for AMPAkines, and future studies examining other regions in the brain including the PAG and prefrontal cortex are needed to elucidate the full spectrum of analgesic mechanisms for these drugs.
Activation of glutamatergic signaling in the NAc is a determinant of a drug's rewarding potential, particularly in craving and relapse.36-38 Hence, one concern for drugs such as AMPAkines which can activate the glutamatergic signaling in the NAc is whether such drugs can have addictive potential in the clinical setting. Our CPP results suggested that a short-term use of these drugs does not carry intrinsic rewarding or addictive properties. Furthermore, three additional factors lead us to believe that the addictive risk of AMPAkines is minimized in the postoperative setting. First, AMPAkines only exert effects on AMPA receptors that are already open.21,100 Thus, these drugs have an unique use-dependent profile. Studies have shown that activation of the corticostriatal pathway can exert analgesic effects.19 Therefore, it is likely that AMPA receptors in the NAc carry out endogenous pain-inhibiting activities, and that AMPAkines function to potentiate this analgesic effect. In this way, AMPAkines may have behavioral specificity for pain conditions. Secondly, AMPAkines, including CX546, have been studied in clinical trials, but no report of addiction and abuse has been found.27,28,75 Thirdly, drugs of abuse not only increase glutamatergic signaling, but a vast majority of them also activate either the dopamine or opioid signaling pathways. However, AMPAkines do not activate either of these pathways. Thus, we argue for a relatively short-term use of AMPAkines, as we believe that their use in the acute postoperative period can complement opioid analgesia, and at the same time, their respiratory stimulatory activity increases the margin of safety for opioids. Prudent use in the acute postoperative period is less likely to lead to addiction and abuse.
In summary, we show that AMPAkines have novel analgesic properties in rat models of postoperative pain. A combination of analgesic and respiratory stimulatory properties can make AMPAkines ideal drugs for the acute postoperative period.
Acknowledgments
This work was supported by the National Institute for General Medical Sciences (GM102691, GM115384) (Bethesda, MD, USA), the Anesthesia Research Fund of the New York University Department of Anesthesiology (New York, NY, USA), National Natural Science Foundation of China (No. 81172546), and the China Scholarship Council.
Footnotes
Dr. Wang has a patent on the use of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor modulators in pain regulation. The other authors declare no conflict of interests.
Contributor Information
Chen Su, Department of Anesthesiology, The Third Xiangya Hospital, Central South University, Changsha, Hunan Province, China
Hau Yeuh Lin, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University School of Medicine, New York, NY
Runtao Yang, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University School of Medicine, New York, NY
Duo Xu, New York University School of Medicine, New York, NY
Michelle Lee, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University School of Medicine, New York, NY
Natalie Pawlak, New York University, New York, NY.
Monica Norcini, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University School of Medicine, New York, NY
Alexandra Sideris, Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University School of Medicine, New York, NY
Esperanza Recio-Pinto, Department of Anesthesiology, Perioperative Care and Pain Medicine, Department of Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, NY
Dong Huang, Department of Anesthesiology, Institute of Pain Medicine, The Third Xiangya Hospital, Central South University, Changsha 410013, Hunan, China.
Jing Wang, Department of Anesthesiology, Perioperative Care and Pain Medicine, Department of Neuroscience and Physiology, New York University School of Medicine, New York, NY
REFERENCES
- 1.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 2.Dworkin RH, Gitlin MJ. Clinical aspects of depression in chronic pain patients. Clin J Pain. 1991;7:79–94. doi: 10.1097/00002508-199106000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Romano JM, Turner JA. Chronic pain and depression: does the evidence support a relationship? Psychol Bull. 1985;97:18–34. [PubMed] [Google Scholar]
- 4.Rieckmann N, Burg MM, Gerin W, Chaplin WF, Clemow L, Davidson KW. Depression vulnerabilities in patients with different levels of depressive symptoms after acute coronary syndromes. Psychother Psychosom. 2006;75:353–61. doi: 10.1159/000095441. [DOI] [PubMed] [Google Scholar]
- 5.Edwards RR, Haythornthwaite JA, Smith MT, Klick B, Katz JN. Catastrophizing and depressive symptoms as prospective predictors of outcomes following total knee replacement. Pain Res Manag. 2009;14:307–11. doi: 10.1155/2009/273783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott CE, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Joint Surg Br. 2010;92:1253–8. doi: 10.1302/0301-620X.92B9.24394. [DOI] [PubMed] [Google Scholar]
- 7.Miller LR, Cano A. Comorbid chronic pain and depression: who is at risk? J Pain. 2009;10:619–27. doi: 10.1016/j.jpain.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–88. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 9.Fields HL. Understanding how opioids contribute to reward and analgesia. Reg Anesth Pain Med. 2007;32:242–6. doi: 10.1016/j.rapm.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–5. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008;12:866–9. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–60. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, Fields HL, Apkarian AV. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15:1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghalandari-Shamami M, Hassanpour-Ezatti M, Haghparast A. Intra-accumbal NMDA but not AMPA/kainate receptor antagonist attenuates WIN55,212-2 cannabinoid receptor agonist-induced antinociception in the basolateral amygdala in a rat model of acute pain. Pharmacol Biochem Behav. 2011;100:213–9. doi: 10.1016/j.pbb.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. Journal of Neuroscience. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goffer Y, Xu D, Eberle SE, D'Amour J, Lee M, Tukey D, Froemke RC, Ziff EB, Wang J. Calcium-permeable AMPA receptors in the nucleus accumbens regulate depression-like behaviors in the chronic neuropathic pain state. J Neurosci. 2013;33:19034–44. doi: 10.1523/JNEUROSCI.2454-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su C, D'Amour J, Lee M, Lin HY, Manders T, Xu D, Eberle SE, Goffer Y, Zou AH, Rahman M, Ziff E, Froemke RC, Huang D, Wang J. Persistent pain alters AMPA receptor subunit levels in the nucleus accumbens. Mol Brain. 2015;8:46. doi: 10.1186/s13041-015-0140-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Manders TR, Eberle SE, Su C, D'Amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015;35:5247–59. doi: 10.1523/JNEUROSCI.3494-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007;8:583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 21.Lynch G. Glutamate-based therapeutic approaches: ampakines. Curr Opin Pharmacol. 2006;6:82–8. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2005;72:225–34. doi: 10.1016/j.schres.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Zheng YW, Balabhadrapatruni S, Masumura C, Darlington CL, Smith PF. Effects of the Putative Cognitive-Enhancing Ampakine, CX717, on Attention and Object Recognition Memory. Current Alzheimer Research. 2011;8:876–882. doi: 10.2174/156720511798192709. [DOI] [PubMed] [Google Scholar]
- 25.Simmons DA, Rex CS, Palmer L, Pandyarajan V, Fedulov V, Gall CM, Lynch G. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington's disease knockin mice. Proc Natl Acad Sci U S A. 2009;106:4906–11. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology. 2009;110:1364–70. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 27.Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174:1384–91. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 28.Oertel BG, Felden L, Tran PV, Bradshaw MH, Angst MS, Schmidt H, Johnson S, Greer JJ, Geisslinger G, Varney MA, Lotsch J. Selective antagonism of opioid-induced ventilatory depression by an ampakine molecule in humans without loss of opioid analgesia. Clin Pharmacol Ther. 2010;87:204–11. doi: 10.1038/clpt.2009.194. [DOI] [PubMed] [Google Scholar]
- 29.Le AMLM, Su C, Zou A, Wang J. AMP Akines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology. 2014;121:1080–90. doi: 10.1097/ALN.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery KE, Kessler M, Arai AC. Modulation of agonist binding to AMPA receptors by 1-(1,4-benzodioxan-6-ylcarbonyl)piperidine (CX546): differential effects across brain regions and GluA1-4/transmembrane AMPA receptor regulatory protein combinations. J Pharmacol Exp Ther. 2009;331:965–74. doi: 10.1124/jpet.109.158014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagarajan N, Quast C, Boxall AR, Shahid M, Rosenmund C. Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546. Neuropharmacology. 2001;41:650–63. doi: 10.1016/s0028-3908(01)00133-2. [DOI] [PubMed] [Google Scholar]
- 32.Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–7. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silverman JL, Oliver CF, Karras MN, Gastrell PT, Crawley JN. AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology. 2013;64:268–82. doi: 10.1016/j.neuropharm.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Procaccini C, Aitta-aho T, Jaako-Movits K, Zharkovsky A, Panhelainen A, Sprengel R, Linden AM, Korpi ER. Excessive novelty-induced c-Fos expression and altered neurogenesis in the hippocampus of GluA1 knockout mice. Eur J Neurosci. 2011;33:161–74. doi: 10.1111/j.1460-9568.2010.07485.x. [DOI] [PubMed] [Google Scholar]
- 35.Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–501. doi: 10.1016/0304-3959(95)01441-1. [DOI] [PubMed] [Google Scholar]
- 36.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintero GC. Role of nucleus accumbens glutamatergic plasticity in drug addiction. Neuropsychiatr Dis Treat. 2013;9:1499–512. doi: 10.2147/NDT.S45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson A, Mead AN, Stephens DN. Behavioural effects of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate-receptor antagonists and their relevance to substance abuse. Pharmacol Ther. 2000;88:59–76. doi: 10.1016/s0163-7258(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 39.Zahn PK, Brennan TJ. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 1999;90:863–872. doi: 10.1097/00000542-199903000-00030. [DOI] [PubMed] [Google Scholar]
- 40.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–58. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, Zou AH, Blanck TJ, Ziff EB. A single subanesthetic dose of ketamine relieves depression-like behaviors induced by neuropathic pain in rats. Anesthesiology. 2011;115:812–21. doi: 10.1097/ALN.0b013e31822f16ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165:1074–86. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 44.Bourquin AF, Suveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I. Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain. 2006;122:14, e1–14. doi: 10.1016/j.pain.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 45.King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–6. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA. Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics. 2004;3:857–71. doi: 10.1074/mcp.M400045-MCP200. [DOI] [PubMed] [Google Scholar]
- 47.Restituito S, Khatri L, Ninan I, Mathews PM, Liu X, Weinberg RJ, Ziff EB. Synaptic autoregulation by metalloproteases and gamma-secretase. J Neurosci. 2011;31:12083–93. doi: 10.1523/JNEUROSCI.2513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122:2940–54. doi: 10.1172/JCI61884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncalves L, Silva R, Pinto-Ribeiro F, Pego JM, Bessa JM, Pertovaara A, Sousa N, Almeida A. Neuropathic pain is associated with depressive behaviour and induces neuroplasticity in the amygdala of the rat. Exp Neurol. 2008;213:48–56. doi: 10.1016/j.expneurol.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 50.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. European journal of pharmacology. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 51.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki T, Amata M, Sakaue G, Nishimura S, Inoue T, Shibata M, Mashimo T. Experimental neuropathy in mice is associated with delayed behavioral changes related to anxiety and depression. Anesth Analg. 2007;104:1570–7. doi: 10.1213/01.ane.0000261514.19946.66. table of contents. [DOI] [PubMed] [Google Scholar]
- 53.Hu B, Doods H, Treede RD, Ceci A. Depression-like behaviour in rats with mononeuropathy is reduced by the CB2-selective agonist GW405833. Pain. 2009;143:206–12. doi: 10.1016/j.pain.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 54.Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–62. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers GA, Schehr RS, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–9. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- 56.Le AM, Lee M, Su C, Zou A, Wang J. AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology. 2014;121:1080–90. doi: 10.1097/ALN.0000000000000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73:638–52. doi: 10.1016/j.neuron.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar KJ, Wagner G, Koschke M, Boettger S, Boettger MK, Schlosser R, Sauer H. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62:1281–7. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 60.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 61.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–64. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 62.Borsook D, Kussman BD, George E, Becerra LR, Burke DW. Surgically induced neuropathic pain: understanding the perioperative process. Ann Surg. 2013;257:403–12. doi: 10.1097/SLA.0b013e3182701a7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth. 2011;107:25–9. doi: 10.1093/bja/aer116. [DOI] [PubMed] [Google Scholar]
- 64.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–81. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becerra L, Breiter HC, Wise R, Gonzalez RG, Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–46. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- 66.Magnusson JE, Martin RV. Additional evidence for the involvement of the basal ganglia in formalin-induced nociception: the role of the nucleus accumbens. Brain Research. 2002;942:128–132. doi: 10.1016/s0006-8993(02)02489-7. [DOI] [PubMed] [Google Scholar]
- 67.Gear RW, Levine JD. Rostral ventral medulla cholinergic mechanism in pain-induced analgesia. Neurosci Lett. 2009;464:170–2. doi: 10.1016/j.neulet.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu LC, Han JS. Habenula as a relay in the descending pathway from nucleus accumbens to periaqueductal grey subserving antinociception. Int J Neurosci. 1990;54:245–51. doi: 10.3109/00207459008986640. [DOI] [PubMed] [Google Scholar]
- 69.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–86. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- 71.Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–49. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- 73.Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBotzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–25. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greer JJ, Ren J. Ampakine therapy to counter fentanyl-induced respiratory depression. Respir Physiol Neurobiol. 2009;168:153–7. doi: 10.1016/j.resp.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Ren J, Lenal F, Yang M, Ding X, Greer JJ. Coadministration of the AMPAKINE CX717 with propofol reduces respiratory depression and fatal apneas. Anesthesiology. 2013;118:1437–45. doi: 10.1097/ALN.0b013e318291079c. [DOI] [PubMed] [Google Scholar]
- 76.Fields HL, Anderson SD, Clanton CH, Basbaum AI. Nucleus raphe magnus: a common mediator of opiate- and stimulus-produced analgesia. Trans Am Neurol Assoc. 1976;101:208–10. [PubMed] [Google Scholar]
- 77.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 78.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–25. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Skolnick P. AMPA receptors: a target for novel antidepressants? Biol Psychiatry. 2008;63:347–8. doi: 10.1016/j.biopsych.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Tan CH, He X, Yang J, Ong WY. Changes in AMPA subunit expression in the mouse brain after chronic treatment with the antidepressant maprotiline: a link between noradrenergic and glutamatergic function? Exp Brain Res. 2006;170:448–56. doi: 10.1007/s00221-005-0228-2. [DOI] [PubMed] [Google Scholar]
- 83.Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, Meloni EG, Carlezon WA., Jr. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci. 2011;31:3095–103. doi: 10.1523/JNEUROSCI.5973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen YW, Rada PV, Butzler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–66. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–13. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Praag H, Frenk H. The role of glutamate in opiate descending inhibition of nociceptive spinal reflexes. Brain Res. 1990;524:101–5. doi: 10.1016/0006-8993(90)90497-y. [DOI] [PubMed] [Google Scholar]
- 89.Spinella M, Cooper ML, Bodnar RJ. Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain. 1996;64:545–52. doi: 10.1016/0304-3959(95)00192-1. [DOI] [PubMed] [Google Scholar]
- 90.Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 91.Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN, Tao YX. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol Pain. 2008;4:67. doi: 10.1186/1744-8069-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–19. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–70. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- 94.Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–50. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 95.Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu XJ, Su HC. The contribution of TNF-alpha in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett. 2013;541:275–80. doi: 10.1016/j.neulet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–4. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- 97.Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci. 2010;30:5451–64. doi: 10.1523/JNEUROSCI.0225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu H, Wu LJ, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M. Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci. 2008;28:7445–53. doi: 10.1523/JNEUROSCI.1812-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain. 2004;110:112–22. doi: 10.1016/j.pain.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 100.Arai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G. A centrally active drug that modulates AMPA receptor gated currents. Brain Res. 1994;638:343–6. doi: 10.1016/0006-8993(94)90669-6. [DOI] [PubMed] [Google Scholar]