Abstract
In case of apnea, arterial partial pressure of oxygen (pO2) decreases, while partial pressure of carbon dioxide (pCO2) increases. To avoid damage to hypoxia sensitive organs such as the brain, compensatory circulatory mechanisms help to maintain an adequate oxygen supply. This is mainly achieved by increased cerebral blood flow. Intermittent hypoxia is a commonly seen phenomenon in patients with obstructive sleep apnea. Acute airway obstruction can also result in hypoxia and hypercapnia. Until now, no adequate model has been established to simulate these dynamics in humans. Previous investigations focusing on human hypoxia used inhaled hypoxic gas mixtures. However, the resulting hypoxia was combined with hyperventilation and is therefore more representative of high altitude environments than of apnea. Furthermore, the transferability of previously performed animal experiments to humans is limited and the pathophysiological background of apnea induced physiological changes is poorly understood. In this study, healthy human apneic divers were utilized to mimic clinically relevant hypoxia and hypercapnia during apnea. Additionally, pulse-oximetry and Near Infrared Spectroscopy (NIRS) were used to evaluate changes in cerebral and peripheral oxygen saturation before, during, and after apnea.
Keywords: Medicine, Issue 118, hypoxia, apnea, NIRS, brain, emergency, rSO2, SpO2
Introduction
Clinically relevant acute hypoxia and concomitant hypercapnia is mostly seen in patients with obstructive sleep apnea syndrome (OSAS), acute airway obstruction or during cardiopulmonary resuscitation. Major limitations in the field of OSAS and other hypoxemic conditions include the limited transferable knowledge about the pathophysiology derived from animal studies and that human models are non-existent 1. To mimic hypoxia in humans, hypoxic gas mixtures have so far been used 2-7. However, these conditions are more representative of high altitude surroundings than of clinical situations where hypoxia, in general, is accompanied by hypercapnia. To monitor tissue oxygenation during cardiac arrest and resuscitation, animal studies have been performed 8 to investigate physiological compensatory mechanisms.
Apneic divers are healthy athletes capable of depressing the breathing impulse that is evoked by low arterial oxygen saturation 9 and an increased pCO2 10,11. We investigated apneic divers in order to mimic clinical situations of acute hypoxia and concomitant hypercapnia 12. This model can be used to evaluate clinical setups, improve the pathophysiological understanding of patients with OSAS or pathological breathing disorders, and reveal new possibilities for studying a potential counter balancing mechanism in cases of apnea. Furthermore, different techniques to detect hypoxia in humans can be tested for feasibility and accuracy in the case of dynamic hypoxia that is present in emergency situations (i.e., airway obstructions, laryngospasm or cannot intubate, cannot ventilate situations) or to simulate intermittent hypoxia in patients with OSAS.
Noninvasive techniques to detect hypoxia in humans are limited. Peripheral pulse oximetry (SpO2) is an approved tool in pre-hospital and hospital settings to detect hypoxia 13. The method is based on light absorption of hemoglobin. However, SpO2 measurement is limited to peripheral arterial oxygenation and cannot be used in cases of pulseless electrical activity (PEA) or centralized minimal circulation 14. In contrast, Near-Infrared Spectroscopy can be used to evaluate cerebral tissue oxygen saturation (rSO2) in real-time during PEA, during hemorrhagic shock or following subarachnoid hemorrhage 15-19. Its use is constantly growing 20 and methodological studies have revealed a positive correlation between SpO2 and rSO2 3,4.
In this study, we provide a model to simulate clinically relevant hypoxia in humans and present a step-by-step methodology to compare peripheral pulse oximetry and NIRS in case of de- and re-saturation. By analyzing physiological data in case of apnea, our understanding of counter balancing mechanisms can be improved.
Protocol
Ethics statement All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments. The design of this study was approved by the local ethics committee of the University Hospital of Bonn, Germany.
NOTE: Ensure that subjects are in good and healthy condition, free of any anti-hypertensive medicine and at least 24 hours free of catecholamine inducing agents like caffeine or equal substances.
1. Preparation of the Test Subject
Clean the skin of the forehead with 70% alcohol to degrease the skin prior to NIRS electrode positioning.
Place the NIRS electrode on the right forehead above the eyebrow and to the right of the midsagittal sulcus (locus frontopolar 2) to measure cerebral (=central) tissue oxygenation.
Evaluate the stability of the signal. The rSO2-signal should be constant (± 3%) for at least 5 min.
For measuring peripheral tissue oxygenation with NIRS (NIRStissue-electrode), place one electrode above the middle of the musculus quadriceps femoris (alternatively on the forearm). Do not place the electrode above a venous plexus or an artery.
Place ECG-electrodes on the hair free chest. The ECG leads are marked with different letters. Place "R" on the sternocostal head of pectoralis major right, "L" on the sternocostal head of pectoralis major left, "C" on the fifth intercostal space middle of the medioclavicular line, "F" on the left lower rib edge, "N" on the right lower rib edge.
Measure peripheral pulse oximetry (SpO2) on a fingertip on the same extremity and side where the NIRStissue-electrode is placed.
Measure noninvasive blood pressure (NIBP) by using a blood pressure cuff. Use the contralateral extremity that allows peripheral pulse oximetry to be measured. In order to get a high time-resolution in blood pressure results, choose a one-minute interval for measuring. Choose NIBP by touching the screen and selecting "settings".
- At least 20 min before the apnea, establish an intravenous line into the medial cubital vein of the right or left arm to draw blood samples at individual time points during and after apnea.
- Clean the skin with 70% alcohol.
- Use a tourniquet to help the veins become more prominent.
- Use skin-disinfection to avoid infections and insert the needle through the skin.
- Reduce the insertion angle after blood flashback at the catheter hub. Push the catheter into the vein.
- Remove the needle and flush catheter with sterile saline (NaCl 0.9%).
2. Data Collection
- Calibrate the internal clock of all monitors in order to synchronize measurements for later processing.
- Click the bottom-right clock icon on the desktop, and tap "change date and time settings" in the pop-up window.
- Press the Settings menu button on the NIRS devise and change date and time via the menu.
To store physiological data for offline analysis, insert the monitor device into the docking station and connect it to the computer via the network cable. Ensure that the IP address and subnet mask of the docking station is correct in the network settings in order to get a connection. Contact device provider in order to get this information.
Use a monitor device specific software to save measurements on the computer. Click "start" to begin recordings and save the results after the end of the measurement. Note: In some devices, data has to be saved live during the measurement. Note: For trouble-shooting take care of the following steps: If the variability of NIRStissue signals is too high, re-evaluate the position of the electrode (avoid bigger venous plexus or arteries directly under the electrodes). High variability of NIRScerebral signals can also be an indirect marker for hyperventilation of divers to reduce partial CO2. Instruct the subject to breath slower and with lower tidal-volumes and re-evaluate the signal. Subjects are allowed to take 3 deep inspirations before final apnea. Avoid including this period into the evaluation of baseline values. The first 30 sec after maximal inspiration are characterized by variable values. Do not use them for analysis.
3. Apnea
Have the subjects rest for at least 15 min in a lying position to avoid stress induced changes in blood circulation due to vasoconstriction. Have subjects breathe normally to avoid influences of hyperventilation caused vasoconstriction. Limit the breathing frequency to ≤ 15 breaths/min.
Draw blood samples for baseline analysis. Discard the first 5 ml of drawn blood to avoid measurement uncertainty. Flush the catheter after each venous blood collection with sterile saline to prevent clotting.
Ensure that monitor values are invisible to subjects to avoid visual influences to their apneic performance.
Check each device for functionality and signal quality. Ensure that electrodes cannot be removed by involuntary movements of the test subject at the end of apnea.
Conclude with clear agreements. Give a countdown of the last 2 min verbally. Subjects should breathe normally during this preparation time. Prior to the final breath 3 deep inspirations are allowed. Ask the subject to indicate the last inhalation by finger sign. Apnea should be performed as long as possible. Note: The end of the final breath indicates the start of apnea. The end of apnea is defined as the first inspiration after apnea.
Mark important events (i.e., beginning and end of apnea) electronically to avoid inaccuracies in further time analysis by pressing the "Event Mark Button" on the NIRS device. Note: Movements of the chest and stomach induced by involuntary diaphragm activities are common in the second half of apnea and indicate the struggle phase.
Draw blood samples at different time-points depending on the aim of the study.
Centrifuge blood samples at 1,500 x g for 10 min. Take the supernatant and store it at -80 °C for future analysis.
4. Processing Data
- Processing data from the monitor device:
- Open the saved file on the computer and press "start" to analyze data.
- Click "review" to get access to the trend monitor and select "options" and then "tools" in the MENU submask. Time interval can be changed via "trend interval" if necessary.
- Select the mask "trends" and save. Open file "trends" in a spreadsheet program for further processing.
- Processing data from NIRS device:
- Open the software on the computer and connect the NIRS device via WIFI.
- Transfer the data from the NIRS device to the computer.
- Save the data in CSV-format.
- Open file in a spreadsheet program for further processing.
5. Analyze Values
Create a spreadsheet with both datasets to compare the values. Identify a time interval of at least 30 sec where NIRS-values and SpO2 are constant (± 3%). Take an average of these values to define a baseline-level. Note: Heart rate is known to change considerably prior to apnea. In order to conduct further analysis, a baseline heart rate is defined at a time point 30 sec after initiation of apnea.
Find the start point of monotonic decrease in rSO2 and SpO2 during apnea by looking for a decrease of values > 2% compared to baseline-levels. This time point is defined as "begin of desaturation".
Identify the start point of rSO2 and SpO2 increase at the end of apnea as a monotonic increase of values after termination of apnea. This point is defined as "begin of re-saturation".
Calculate the time difference between "start of apnea" and "begin of desaturation" and the time differences between "end of apnea" and "begin of re-saturation" for NIRScerebral, NIRStissue and SpO2. Save each difference in seconds on a separate spreadsheet.
Optional: Calculate heart rate variability of each participant during the second and the last minute of apnea. This may reveal information about the sympathetic/parasympathetic balance during this stressful phase.
6. Statistical Processing
Compare the time differences between "beginning of desaturation" of SpO2, NIRScerebral, and NIRStissue values. Test for Gaussian distribution of the measurement differences (e.g., using Shapiro-Wilk normality test for sample sizes smaller than 50).
If the distribution of the measurement differences is significantly different from normal distribution, use Wilcoxon signed rank test. If normal distribution can be assumed, consider using paired t-test.
Representative Results
Figure 1 displays simultaneous recordings of SpO2 and NIRS values (NIRScerebral and NIRStissue) during apnea in one patient. Total apnea time was 363 sec. Following apnea NIRS and SpO2 values remained stable for approximately 140 sec. A decrease in SpO2 was detected after 204 sec by peripheral SpO2 whereas a decrease of NIRScerebral was detected after 238 sec. The lowest measured SpO2 following apnea was 58% and lowest measured NIRScerebral was 46%. At the end of apnea NIRScerebral increased after a time delay of 12 sec whereas SpO2 increased after a time delay of 30 sec.
In a recent study of ten apneic divers we showed a significant decrease in NIRScerebral values from 71% (range 85 - 55) to 54% (range 74 - 24) 12. Median SpO2 decreased from 98% (range 100 - 98) to 81% (range 94 - 67). Figure 2 displays the mean time delays between the beginning of apnea and decrease in NIRScerebral versus SpO2 values of these ten divers. Oxygen saturation measured by NIRScerebral decreased significantly later than oxygen saturation on the fingertip measured by SpO2 [175 sec; SD = 50 sec versus 134 sec; SD = 29 sec; (t(9) = 2.865, p = 0.019, r2 = 0.477)]. This can be taken as a sign for elevated cerebral blood flow and preferential oxygen supply of cerebral tissue during apnea.
After restart of respiration (Figure 2c), values of NIRScerebral increased significantly earlier than SpO2 values [10 sec; SD = 4 sec versus 21 sec; SD = 4 sec (t(9) = 7.703, p < 0.001, r2 = 0.868)]. Figures 2b and d display de- and re-saturation measured on the fingertip (SpO2) and above the musculus quadriceps femoris (NIRStissue) during apnea. NIRStissue values decreased significantly earlier than SpO2 values [39 s; SD = 13 sec versus a delay of 125 sec; SD = 36 sec (t(6) = 4.869, p = 0.003, r2 = 0.798)]. This time delay might show that peripheral vasoconstriction leads to a decrease in tissue oxygenation, even before a decrease in arterial oxygen saturation — visualized by SpO2 — is measureable. There was no difference in time delay after restart of respiration between NIRStissue and SpO2 [NIRStissue 30 sec; SD = 16 sec versus SpO2 27 sec; SD = 7 sec (t(6) = 0.631, p = 0.551, r2 = 0.062)]. This indicates, that the observed time delay is not caused by the different devices themselves.
To compare de- and re-saturation during individual apnea durations, we normalized SpO2-, NIRStissue- and NIRScerebral-baseline values to 100% (Figure 3). To compare individual apnea duration, total apnea duration of each subject was also set to 100%. 12
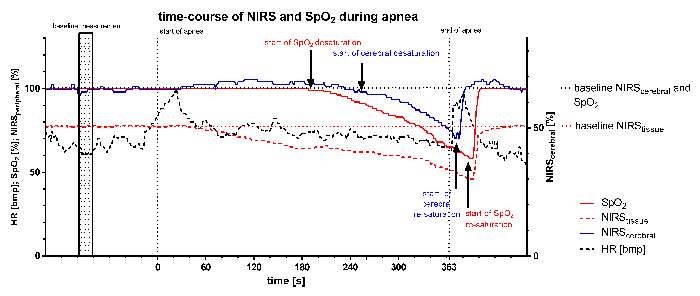 Figure 1: Time-course of NIRS, SpO2, and Heart Rate (HR) during Apnea. Raw data of one participant is displayed. Total apnea-time was 363 sec. Subject exhibited an earlier decrease in SpO2 than in cerebral rSO2. Please click here to view a larger version of this figure.
Figure 1: Time-course of NIRS, SpO2, and Heart Rate (HR) during Apnea. Raw data of one participant is displayed. Total apnea-time was 363 sec. Subject exhibited an earlier decrease in SpO2 than in cerebral rSO2. Please click here to view a larger version of this figure.
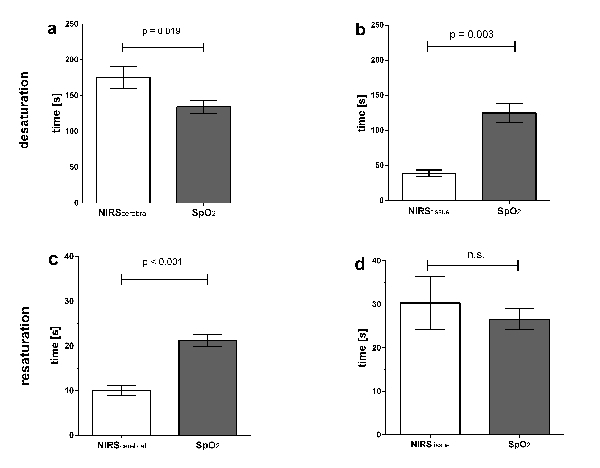 Figure 2: Time Delays during Apnea and Restart of Respiration.a) Mean time delay between beginning of apnea and decrease of NIRScerebral versus SpO2 values; b) Mean time delay between beginning of apnea and decrease of NIRStissue
versus SpO2 values; c) Mean time delay between restart of respiration and an increase of NIRScerebral
versus SpO2 values; d) Mean time delay between restart of respiration and an increase of NIRStissue
versus SpO2 values. Error bars indicate standard error of the mean. Data and figure from Eichhorn et al. 2015 12. Please click here to view a larger version of this figure.
Figure 2: Time Delays during Apnea and Restart of Respiration.a) Mean time delay between beginning of apnea and decrease of NIRScerebral versus SpO2 values; b) Mean time delay between beginning of apnea and decrease of NIRStissue
versus SpO2 values; c) Mean time delay between restart of respiration and an increase of NIRScerebral
versus SpO2 values; d) Mean time delay between restart of respiration and an increase of NIRStissue
versus SpO2 values. Error bars indicate standard error of the mean. Data and figure from Eichhorn et al. 2015 12. Please click here to view a larger version of this figure.
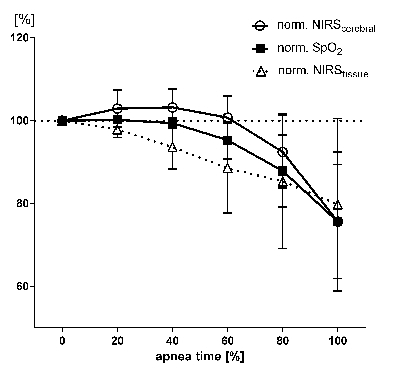 Figure 3: Temporal Progression of Normalized SpO2, NIRScerebral and NIRStissue Values: To equilibrate individual variations in apnea time, all apnea times were standardized to 100%. Thus the variations in the three plotted parameters are assigned to the relative apnea times. Baseline values measured prior to apnea were defined as 100%. Error bars indicate standard error of the mean. Data and figure from Eichhorn et al. 2015 12. Please click here to view a larger version of this figure.
Figure 3: Temporal Progression of Normalized SpO2, NIRScerebral and NIRStissue Values: To equilibrate individual variations in apnea time, all apnea times were standardized to 100%. Thus the variations in the three plotted parameters are assigned to the relative apnea times. Baseline values measured prior to apnea were defined as 100%. Error bars indicate standard error of the mean. Data and figure from Eichhorn et al. 2015 12. Please click here to view a larger version of this figure.
Discussion
The total apnea time is mainly caused by lung size and oxygen consumption per minute and influenced by an individuals' ability to withstand the breathing reflex caused by increasing pCO2 or decreasing pO2. Apnea divers are trained to maximize their breath-hold duration and are used to doing so in maximal inspiration. Therefore, the time until hypoxia is detectable differs between individuals and depends on the subject's physical condition and training status and might even vary by their daily state and willingness to withstand the breathing reflex. The subject's stress levels can be reduced by detailed education of protocol steps and a calm ambient environment.
There are many factors that influence total apnea time, which means that the testing environment should be standardized in order to get results that are reliable and repeatable. If researchers are interested in studying the catecholamine increase or sympathetic nerve activity, substances influencing both (i.e., caffeine, nicotine, food like bananas, nuts, or any medical substances like monoamine oxidase (MAO) inhibitors, etc.) should be avoided. Also the intravenous line should be established at least 20 min before apnea. A subjects' stress level will mainly influence catecholamine-levels and could falsify researchers' results of blood analysis. In general, researchers should create baseline levels of each subject to normalize the results because of the large inter-individual differences.
Non-invasive measurements of tissue oxygenation by NIRS technology uses semi-quantitative changes in oxygenated and deoxygenated hemoglobin 21. The use of NIRS is constantly growing 20 and it can detect saturation of cerebral and peripheral tissue, independent of pulsatile blood flow. NIRS values depend on the amount of venous and arterial vessels placed under the NIRS-electrodes. NIRS values can therefore differ significantly depending on the amount of venous versus arterial vessels under the electrode. Also, placement and contact pressure will influence the reliability of values. Values should be checked for stability before starting measurement. If NIRS signals vary during baseline measurements, replace the electrodes or check for total skin contact. For interpretation of the NIRS results, relative de- or increase of values compared to baseline values should be used (not absolute).
Due to the physical burden of a maximal breath-hold, the number of apneas per subject is limited. The preparation protocols should be equal for each subject and all devices should be double-checked before they are used. Do not modify the protocol in one cohort. Standardized setups are mandatory to create results that are reproducible. Although hyperventilation before maximal breath hold lowers arterial CO2 levels and delays the breathing stimulus, it also affects cerebral autoregulation and vasomotor reactivity22. Active hyperventilation should be avoided to minimize disruptive effects by the subject.
The overall goal of this model is to simulate hypoxia in humans by breath hold. Therefore, additional measurement devices can be established to get more detailed information about blood pressure (i.e., invasive blood pressure measurement) or sympathetic nerve activity. Blood pressure measurements can be used to estimate the burden of prolonged apnea to the vessel system. ECG signals can be used to calculate beat-to-beat variability in R-R interval or to detect cardiac arrhythmia. Furthermore, cortisol-levels in saliva or catecholamine-levels 29 in blood-samples can be measured at different time points during and after apnea. The kinetics of these values opens up a number of possible study opportunities. Still, a reliable detection of hypoxia is necessary to ensure hypoxic conditions caused by apnea. Values measured by different devices but in the same apneic session can be compared directly. Time differences (for instance, until blood pressure increase, desaturation starts, etc.) from different individuals should be normalized to total apnea time.
The respiratory reflex is one of the strongest stimulus of the human body. Acute hypoxia and hypercapnia is therefore only seen in patients with pathologies (i.e., OSA, emergency situations, laryngospasm, CPR, etc.). Mostly unforeseen, hypoxia is difficult to detect, always influenced by a triggering event and difficult to evaluate because of a subjects' comorbidities. Although total apnea time of divers and patients undergoing hypoxia should not be compared because of the completely different starting conditions, human compensatory mechanisms to avoid damage to the brain in case of hypoxia are identical 23-28. An extended voluntary breath-hold also empties the body's oxygen-storage and increases a subject's pCO2 29. Apneic divers were shown to generate reliable results during simulation of dynamic hypoxia in humans 12. We measured a minimum cerebral saturation only slightly higher than values seen in patients during cardiac arrest (42.2 ± 10.7% 15 and 37.2 ± 17.0% 14). This indicates that our model is able to mimic clinically relevant hypoxia. Although hypoxia causes serious health problems, the underling physiological mechanisms are yet not completely understood 1 and till now no relevant clinical human model existed to simulate acute hypoxia in humans. Using healthy apneic divers as a clinical relevant model to simulate hypoxia and hypercapnia in humans holds large potential for future investigations. This model allows scientists to study the compensatory mechanism to avoid hypoxic damage in a reproducible human model. It allows a clinically relevant simulation of hypoxic emergency situations such as laryngospasm or "cannot ventilate - cannot intubate". It might be used to prove the feasibility of new invasive or non-invasive tools for measuring human hypoxia. Furthermore, this model may help to understand the correlation of increased endogenous catecholamines and their impact on cardiac function (i.e., heart rate variability, cardiac output, etc.). By using different and new devices to observe hypoxia in apneic divers new parameters may be explored and may extend our understanding of hypoxia in the future.
Disclosures
The authors have nothing to disclose.
Acknowledgments
Special thanks to all volunteers who participated in the original study. The work of L. Eichhorn was supported through a scholarship of the Else-Kröner-Fresenius Foundation. The authors would like to thank Springer, Part of Springer Science+Business Media, for copyright clearance (License Number 3894660871180) and the kind permission of reusing previously published data.
References
- Drager LF, Polotsky VY, O'Donnell CP, Cravo SL, Lorenzi-Filho G, Machado BH. Translational approaches to understanding metabolic dysfunction and cardiovascular consequences of obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2015;309(7):1101–1111. doi: 10.1152/ajpheart.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N, Trivedi NK, Clack SL, Shah M, Shah PP, Barker S. Impact of hypoxemia on the performance of cerebral oximeter in volunteer subjects. J Neurosurg Anesthesiol. 2000;12(3):201–209. doi: 10.1097/00008506-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Ricci M, Lombardi P, et al. Near-infrared spectroscopy to monitor cerebral oxygen saturation in single-ventricle physiology. J Thorac Cardiovasc Surg. 2006;131(2):395–402. doi: 10.1016/j.jtcvs.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Kusaka T, Isobe K, et al. Quantification of cerebral oxygenation by full-spectrum near-infrared spectroscopy using a two-point method. Comp Biochem Physiol A Mol Integr Physiol. 2002;132(1):121–132. doi: 10.1016/s1095-6433(01)00538-4. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Iwasaki K, Ogawa Y, Shibata S. Oxygen administration, cerebral blood flow velocity, and dynamic cerebral autoregulation. Aviat Space Environ Med. 2007;78(12):1121–1127. doi: 10.3357/asem.2177.2007. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8(2):175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- Sanborn MR, Edsell ME, et al. Cerebral hemodynamics at altitude: effects of hyperventilation and acclimatization on cerebral blood flow and oxygenation. Wilderness Environ Med. 2015;26(2):133–141. doi: 10.1016/j.wem.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Reynolds JC, Salcido D, et al. Tissue oximetry by near-infrared spectroscopy in a porcine model of out-of-hospital cardiac arrest and resuscitation. Resuscitation. 2013;84(6):843–847. doi: 10.1016/j.resuscitation.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Andersson JPA, Evaggelidis L. Arterial oxygen saturation and diving response during dynamic apneas in breath-hold divers. Scand J Med Sci Sports. 2009;19(1):87–91. doi: 10.1111/j.1600-0838.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Friis S, Pedersen RB, Lykkeboe G. Influence of lung volume, glossopharyngeal inhalation and P(ET) O2 and P(ET) CO2 on apnea performance in trained breath-hold divers. Eur J Appl Physiol. 2006;97(2):158–164. doi: 10.1007/s00421-006-0156-2. [DOI] [PubMed] [Google Scholar]
- Ferretti G. Extreme human breath-hold diving. Eur J Appl Physiol. 2001;84(4):254–271. doi: 10.1007/s004210000377. [DOI] [PubMed] [Google Scholar]
- Eichhorn L, Erdfelder F, et al. Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J Clin Monit Comput. 2015;29(6):749–757. doi: 10.1007/s10877-015-9662-2. [DOI] [PubMed] [Google Scholar]
- Eichhorn JH. Pulse oximetry as a standard of practice in anesthesia. Anesthesiology. 1993;78(3):423–426. [PubMed] [Google Scholar]
- Schewe J-C, Thudium MO, et al. Monitoring of cerebral oxygen saturation during resuscitation in out-of-hospital cardiac arrest: a feasibility study in a physician staffed emergency medical system. Scand J Trauma Resusc Emerg Med. 2014;22:58. doi: 10.1186/s13049-014-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn A, Nasir A, Malik H, D'Orazi F, Parnia S. A pilot study examining the role of regional cerebral oxygen saturation monitoring as a marker of return of spontaneous circulation in shockable (VF/VT) and non-shockable (PEA/Asystole) causes of cardiac arrest. Resuscitation. 2013;84(12):1713–1716. doi: 10.1016/j.resuscitation.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107(4):563–569. doi: 10.1097/01.anes.0000281894.69422.ff. [DOI] [PubMed] [Google Scholar]
- Beilman GJ, Groehler KE, Lazaron V, Ortner JP. Near-infrared spectroscopy measurement of regional tissue oxyhemoglobin saturation during hemorrhagic shock. Shock. 1999;12(3):196–200. doi: 10.1097/00024382-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Rhee P, Langdale L, Mock C, Gentilello LM. Near-infrared spectroscopy: continuous measurement of cytochrome oxidation during hemorrhagic shock. Crit Care Med. 1997;25(1):166–170. doi: 10.1097/00003246-199701000-00030. [DOI] [PubMed] [Google Scholar]
- Zweifel C, Castellani G, et al. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41(9):1963–1968. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- Scheeren TWL, Schober P, Schwarte LA. Monitoring tissue oxygenation by near infrared spectroscopy (NIRS): background and current applications. J Clin Monit Comput. 2012;26(4):279–287. doi: 10.1007/s10877-012-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11(4):213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- Aaslid R. Cerebral autoregulation and vasomotor reactivity. Front Neurol Neurosci. 2006;21:216–228. doi: 10.1159/000092434. [DOI] [PubMed] [Google Scholar]
- Palada I, Obad A, Bakovic D, Valic Z, Ivancev V, Dujic Z. Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir Physiol Neurobiol. 2007;157(2-3):374–381. doi: 10.1016/j.resp.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Heusser K, Dzamonja G, et al. Cardiovascular regulation during apnea in elite divers. Hypertension. 2009;53(4):719–724. doi: 10.1161/HYPERTENSIONAHA.108.127530. [DOI] [PubMed] [Google Scholar]
- Joulia F, Lemaitre F, Fontanari P, Mille ML, Barthelemy P. Circulatory effects of apnoea in elite breath-hold divers. Acta Physiol (Oxf) 2009;197(1):75–82. doi: 10.1111/j.1748-1716.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Costalat G, Coquart J, Castres I, Tourny C, Lemaitre F. Hemodynamic adjustments during breath-holding in trained divers. Eur J Appl Physiol. 2013;113(10):2523–2529. doi: 10.1007/s00421-013-2690-z. [DOI] [PubMed] [Google Scholar]
- Busch DR, Lynch JM, et al. Cerebral Blood Flow Response to Hypercapnia in Children with Obstructive Sleep Apnea Syndrome. Sleep. 2016;39(1):209–216. doi: 10.5665/sleep.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex R, Bhave G, et al. An investigation of simultaneous variations in cerebral blood flow velocity and arterial blood pressure during sleep apnea. Conf Proc IEEE Eng Med Biol Soc. 2012. pp. 5634–5637. [DOI] [PubMed]
- Eichhorn L, Erdfelder F, et al. Influence of Apnea-induced Hypoxia on Catecholamine Release and Cardiovascular Dynamics. Int J Sports Med. 2016. [DOI] [PubMed]


