Abstract
Background
Unresponsive to most clinical therapies, triple-negative breast cancer (TNBC) is the dominant biological cause of population-based racioethnic disparities in breast cancer mortality in the United States. We report the chemotherapeutic vulnerability of TNBC cells and stem cell-derived tumors to Vernonia amygdalina aqueous leaf extracts (VA extracts). VA extracts arrest cell proliferation and induce apoptosis in vitro and inhibit growth of implanted tumors and show chemo-preventive efficacy in vivo.
Materials and Methods
HRAS cells and MDA-MB-468 cells were subcutaneously implanted into nude mice with or without pretreatment with VA extracts before chemotherapeutic treatment with VA extracts and/or paclitaxel to evaluate their ability to inhibit tumor growth.
Results
The most significant reduction in tumor volume was observed in the MDA-MB-468 cell-induced tumors following VA extract pre-treatment compared to those from HRAS cell implantation.
Conclusion
VA extracts induce apoptosis, exhibit additive effects, inhibit tumor growth and display chemo-preventive actions against TNBCs.
Keywords: Vernonia amygdalina, aqueous extracts, triple-negative breast cancer, chemo-preventive, chemotherapeutic, anticancer
According to the 2014 American Cancer Society Cancer Facts & Figures, breast cancer (BC) is the most frequently diagnosed cancer in women and ranks second after lung cancer as the leading cause of cancer-related deaths of women in the U.S. Though death rates for BC have steadily decreased in women since 1989, representing improvements in early detection, treatment and, possibly, decreased incidence, these uplifting statistics are not equally shared across the major biological subgroups of this clinically heterogeneous disease. In fact, different molecular classes of BC, including luminal A, luminal B, normal-like, erbB2+ (mostly HER2-enriched and ER−), and basal-like, respond differently to neoadjuvant anthracycline-based chemotherapy (1–3). Luminal/HER2E groups have realized great therapeutic success, unlike the basal-like, often referred to as triple-negative breast cancers (TNBCs) that have the poorest prognosis of all types of BC. Patients with TNBC have increased pathologic complete response (pCR) rates compared with non-TNBC, whereas those with higher pCR rates have excellent survival regardless of receptor status. However, TNBC patients with residual disease after neoadjuvant chemotherapy have significantly worse survival compared to non-TNBC patients, particularly in the first three years (4–6). Therefore, there is an urgent need to develop novel treatments for either pre-surgical neoadjuvant therapy or in combination with standard therapies to improve TNBC treatment outcomes.
Susan G. Komen’s, “The Who, What, Where, When and Sometimes, Why”, TNBC document and TNBC Foundations’ Guides To Understanding TNBC and To Understanding Treatment Decisions report approximately 20% of all BCs in the U.S. are TNBCs. These often aggressive TNBCs are diagnosed more in women who are younger, African-American, and/or BRCA positive (7–9). TNBCs are poorly differentiated and 70 to 90% of TNBCs are basal-like, receiving signals from more activated kinase pathways than other types of cancer (8). Basal-like status does not factor into treatment, but lacking expression of estrogen, progesterone and HER2/neu receptors, tremendously impacts treatment decisions. Currently, there are no effective anti-TNBC approved targeted treatment regimens available (9). Adjuvant or neoadjuvant chemotherapy to prevent metastasis is the traditional standard of care for TNBC, however, confounding factors, such as selection pressure induced by nonspecific chemotherapy drugs, excessive release of growth factors intended for wound healing, resistance to treatment drugs and harsh side-effects associated with chemotherapy, have led researchers to search for more natural chemotherapeutic agents (10–11). Numerous results from cell culture model experiments show that the use of complementary and alternative medicine and some herbal products have potential for use as chemo-preventive and chemotherapeutic agents for certain types of cancers (12–16). The aqueous extracts of Vernonia amygdalina, a bitter leaf plant grown in Nigeria, has been shown to be more than 1,400 times more efficacious as an anticancer agent than some other plant extracts reported (17–18). Reportedly, aqueous extracts of Vernonia amygdalina elicit a plethora of effects with exposure inducing antibacterial, amebicidal, antioxidant, hypoglycemic/antidiabetic, oxytocic, hepatoprotective, serum lipid modulatory, gastric secretory, analgesic, and phytotoxic actions (19). Earlier investigators have shown that purified fractions of the chloroform extract of Vernonia amygdalina elicited anticancer effects in human carcinoma of the nasopharynx (20). We have provided compelling evidence that edible Vernonia amygdalina’s aqueous leaf extracts (VA extracts) reduce cell proliferation of estrogen receptor-positive (ER+), estrogen receptor-negative (ER−), and triple-negative human breast carcinoma cells, as well as PC-3 androgen independent prostate adenocarcinoma cells, a broader range than has been described with other anticancer agents; promoting apoptosis in BC and prostate cancer cells with no effect on normal human peripheral blood mononuclear cells (21–24). The prerequisites for induction of apoptosis may vary among different cell lines (25–29). Since TNBC is a disease consisting of many heterogeneous, undifferentiated cell types, including very high mammary cancer stem cell (MCSC) content, we now provide data of VA extracts’ growth inhibitory and apoptotic efficacy in a number of cell lines within a larger panel of triple-negative human breast cancerous cells. We also show that treatment with VA extracts decreased MCSC proliferation in vitro, and reduced initiation and progression of tumors in vivo, surpassing Taxol’s efficacy. We believe that validation of our implantable tumor model, as well as the results and broad conclusions presented in this work, will be directly relevant to better understanding the importance of prevention strategies and novel combination therapies, including VA extracts, for insidious forms of BC. Use of this anti-BC phytoceutical by oncologists and other cancer health care providers, either alone or to accompany prescribed conventional therapies, will potentially improve the survival rates of patients with all forms of BC, including TNBC.
Materials and Methods
Materials
A panel of cells with basal-like morphology and a panel with mesenchymal and luminal morphology were purchased from American Type Culture Collection, ATCC (Manassas, VA, USA). P-glycoprotein kit, phosphate-buffered saline (PBS), trypsin-EDTA, and antibiotic solution were purchased from Fisher Scientific (Houston, TX, USA). All other chemicals were obtained from Sigma (St. Louis, MO, USA).
Aqueous Vernonia amygdalina extract preparation
Pesticide-free Vernonia amygdalina leaves, acquired from Benin City, Nigeria, were rinsed with cold, distilled water. After rinsing, the leaves were spread out evenly on galvanized-wire screens with the edges bent upward 2 inches on all sides. Dried leaves were soaked in 6 l of ddH2O (1:20, w/w) overnight at 4°C before gently being crushed into a mixture. The mixture was filtered through 0.45 μm filtration units for sterilization after filtration through clean white gauges to remove the particulate matter. The resulting sample solution was lyophilized to a dry powder (30 g) on a Savant SC210A SpeedVac Concentrator from Thermo Fisher Scientific (Carlsbad, CA, USA), transferred into a 50-ml centrifugation tube and stored at −20°C for bioactivity assays.
Cell culture of multiple cell lines and mammary cancer stem cells
The HMLEHRASV12 cell line (HRAS cells), obtained after transforming human mammary epithelial (HMLE) cells with HRAS T24 oncogene, was collected in 2011 from Robert Weinberg (Whitehead Institute, Cambridge, MA, USA), and cultured in DMEM-F12 medium (ATCC) containing 10% fetal bovine serum (FBS) from ATCC. The human breast cancer cell lines MDA-MB-231, HCC1806 and MDA-MB-468 were obtained from ATCC in 2015. ATCC uses the Promega PowerPlex 1.2 system and the Applied Biosystems Genotyper 2.0 software for amplicon analysis. We have not performed any further testing in our lab. MDA-MB-231 and MDA-MB-468 cells were propagated in Leibovitz L-15 medium (ATCC) containing 10% FBS. HCC 1806 cells were propagated in RPMI-1640 media (ATCC) supplemented with 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were incubated at 37°C in a humidified atmosphere containing 5% CO2, with the exception of the MDA-MB-231 and MDA-MB-468 cells that were grown in the Leibovitz L-15 medium formulation at 37°C in a free gas exchange with 100% atmospheric air.
Cell viability assay
To determine the effects of VA extracts, breast cancer cell lines were treated with VA extracts (100 μg/ml) for 18 h, after which their viability was assessed by cell counts and Trypan blue exclusion assays. The efficacy of VA extracts was compared to that of paclitaxel (Tax) wherein cells were treated with Tax (100 nM). In order to determine possible VA extracts/Tax synergistic effects, cells were treated with a combination of VA extracts and Tax (100 μg/ml and 100 nM, respectively). Cells were seeded in 100-mm plates, incubated and allowed to become 80% confluent before 18 h treatment with VA extracts and/or Tax. Cells were harvested and viability was determined by the Trypan blue exclusion method (Thermo Fisher Scientific) on a hemacytometer. Briefly, 10 μl of a 0.5% solution of the dye was added to 100 μl of treated cells (1.0×105 cells/ml). The suspension was then applied to a hemocytometer. Both viable (transparent) and non-viable (blue) cells were counted. A minimum of 200 cells were counted for each data point in a total of eight microscopic fields. The viability of treated cells was expressed as percent compared to control.
DNA synthesis determination
DNA synthesis was determined by [3H] thymidine incorporation assays. Treated or untreated cells were incubated for 18 h at 37°C, followed by addition of twenty microliters (2 μCi/2 ml) of [3H] thymidine/35 mm well and incubation for 6 h. All incubations were terminated by aspirating the RPMI-1640 medium and doing triplicate washes with 2 ml of cold PBS to remove residual [3H] thymidine. Two milliliters of 10% cold trichloroacetic acid (TCA) was added to each well and incubated at 4°C for 10 min for cell fixation. Following fixation, the cells were washed three times with ddH20 and solubilized by incubation for 30 min with 0.5 M NaOH (2 ml/35 mm) at 37°C. Upon solubilization, 1 ml of cell solution was added and vigorously mixed with 5 ml of scintillation cocktail. A beta scintillation counter was used to determine radioactivity.
Comet assay
Kits and reagents were obtained from Trevigen Inc., Gaithersburg, MD, and Comet assays were carried out using methods described by Collins (30) with modifications (31). VA extracts genotoxicity was analyzed with all precautions taken to avoid the UV light effect on DNA. Treated and untreated TNBC cells were incubated for 18 h in an atmosphere containing 5% CO2 at 37°C. After incubation, the cells were centrifuged, washed with calcium and magnesium-free PBS, and re-suspended in 100 μl PBS. Fifty milliliters of the cells suspension and 500 μl of melted LM agarose were mixed in a 2-ml tube, and 75 μl was pipetted onto a pre-warmed comet slide. The side of the pipette tip was used to completely spread agarose/cells over the sample area. The slides were placed flat in the dark at 4°C for 10 min to allow solidification and then immersed in pre-chilled lysis solution at 4°C for 40 min. Slides were removed from lysis solution, tapped, and immersed in alkaline solution for 40 minutes at room temperature (RT) in the dark. Slides were washed twice for 5 min with Tris-Borate-EDTA (TBE) followed by electrophoresis at low voltage (300 mA, 25 V, 4°C) for 20 min. The slides were placed in 70% ethanol for 5 min, removed, tapped, and air-dried overnight. The slides were stained with SYBR Green stain designed for the comet assay and allowed to air dry at RT for 6 h. SYBR Green-stained comet slides were viewed with an Olympus fluorescence microscope from Olympus Scientific Solutions Americas (Center Valley, PA, USA) and analyzed using LAI’s Comet Assay Analysis System software (Loats Associates, Inc. Westminster, MD, USA).
P-glycoprotein activity assay
Reagents were prepared according to manufacturer’s protocol. Then, 20 μl of PgP-Glo assay buffer was added to wells labeled “no treatment” (NT). Twenty microliters of 0.25 mM Na3VO4 dissolved in PgP-Glo assay buffer was added to the wells labeled Na3VO4 on the 96-well plate. Next, 20 μl of 0.5 mM Verapamil in PgP-Glo assay buffer was added to the wells labeled “Ver” and 20 μl of 2.5 X concentrated test compounds was added to the experimental test compound wells. Then, 20 μl of diluted PgP membranes was added to each well and incubated at 37°C for 5 min while floating in a 37°C water bath. Reactions were initiated by adding 10 μl of 25 mM Mg ATP to all wells, and mixing briefly by gentle tapping before placing in the 37°C incubator for 40 min. Fifty microliters of ATP detection reagent was added to each well after removing the plate from the heat source. The plate was mixed briefly and incubated at RT for 20 min to allow luminescent signal to develop. Luminescence was read on a plate-reading luminometer (Promega Corporation, Madison, WI, USA).
Caspase assay
Cells were lysed in buffer (10 mm Tris-HCl (pH 8.0), 1% Triton X-100, 0.32 m sucrose, 5 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 2 mm DTT) for 30 min at 4°C. The lysate (3 μg) was used for the caspase-3 assay using the substrate N-Acetyl-Asp-Glu-Val-Asp-7-amido-4-Methylcoumarin (Ac-DEVD-AMC). Active caspase-3 cleaves the substrate Ac-DEVD-AMC after the aspartic acid residue and before the AMC group. The released AMC becomes fluorescent, and the fluorescence was quantified using a fluorometer (VersaFluor; Bio-Rad, Hercules, CA, USA) with excitation at 380 nm and emission at 440 nm.
Western blot analysis
Cells were lysed in buffer containing 50 mm 4-2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.5), 1 mm dithiothreitol (DTT), 150 mm NaCl, 1 mm EDTA, 0.1% Tween 20, 10% glycerol, 10 mm β-glycerophosphate, 1 mm NaF, 0.1 mm orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 0.1 mm phenylmethylsulfonyl fluoride at 4°C for 30 min. Lysates (30 μg) were resolved electrophoretically on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and electrotransferred to polyvinylidene difluoride membranes (Bio-Rad) using a tank blot procedure (Bio-Rad Mini Protean 11). The membranes were incubated in 1:1000 dilution of anti-caspase-3 or Bax antibody from Amersham Biosciences Corporation (Piscataway, NJ, USA). The washed filter was incubated with 1:1,000 dilution of horseradish peroxidase-linked F(ab) fragment secondary antibody (Amersham Corp.) for 1 h. Immunoreactive bands were visualized by enhanced chemiluminescence using a detection system purchased from Amersham Corp.
Statistical analysis
The experimental replicates within individual experiments were averaged and expressed as mean±standard deviation (SD). The comparisons between means were determined by Dunnett’s test, and unpaired Student’s t-test with 2 tailed p-values reported, employing the GraphPad statistical software package from GraphPad Software, Inc. (La Jolla, CA, USA). Each experiment was replicated three times with comparable results. Mean data values of p≤0.05 were determined to be statistically significant.
Mammosphere formation
Mammosphere growth assays were performed as follows. Cell lines were trypsinized and suspended in serum-free mammosphere media (MSM) comprised of DMEM: Ham’s F-12 (1:1) supplemented with 10 mM HEPES, 5 μg/ml insulin, 20 ng/ml epidermal growth factor (EGF) and 10 ng/ml basic fibroblast growth factor (FGF). Cells were counted and 50,000 cells/well were plated in Corning® Costar® 3471 ultra-low attachment 6-well plates (Sigma Aldrich, St. Louis, MO, USA).
Xenograft formation
Aged five- to eight-week-old, female Hsd:Athymic Nude-Foxn1nu, nude mice were purchased from Harlan Laboratories Inc. (Placentia, CA, USA). HMLEH-RAS, MDA-MB-231 or MDA-MB-468 cells (5×106 cells) suspended in 100 μl matrigel were subcutaneously transplanted into nude mice (10 mice per cell line) and the resultant tumor growth was monitored. Nude mice were grouped as follows: Gp 1-control, inoculated with Hras cells only, no chemotherapeutic treatment; Gp 2-starting VA extracts treatment (10 mg/kg) simultaneously with injection of Hras cells, followed by continued 16-day VA extracts treatment; Gp 3-replicate of Gp 2; Gp 4-pretreatment with VA extracts 10 days before injection of Hras cells, followed by 16-day VA extracts treatment; Gp 5-intraperitoneal inoculation of Tax (10 mg/kg) simultaneously with injection of Hras cells; and Gp 6-subcutaneous inoculation of VA extracts (5 mg/kg) plus intraperitoneal injection of Tax (5 mg/kg) simultaneously with injection of Hras cells. We compared anti-tumor growth initiation and progression efficacy of VA extracts with that of either Tax alone or a combination of VA extracts and Tax.
Measurement of tumor volume
Tumor volume was calculated as ½ (length × width2). Calibrated calipers were used to measure the length, width and thickness of the xenografts in all our experimental groups twice a week, starting 2 weeks following implantation of MCSCs in the nude mice. Body weights were monitored weekly along with tumor measurement. When the tumors reached 1.5 cm in length, the animals were euthanized with CO2, and a complete necropsy was performed to collect the tumors and to determine any toxicity effects of the VA extracts or the other test drug. Volumes of the tumors were presented as mean±SD, minimum and maximum. The distribution of the variable (tumor volume) was transformed if not normally distributed.
Sample size and power analysis
The sample size was based on the following parameters: alpha=0.05, beta=0.20 (power of 0.8). Using the analysis of variance test with fixed effects in which only the means of each treatment group and a variance calculation (an average variance within the treatment groups is used), 5 animals per group were needed to test whether there is a significant difference between the groups in tumor size. The International Business Machines Corporation (IBM) SPSS Statistics software package (IBM, Armonk, NY, USA) was used for statistical analysis with the data expressed as mean±standard error (SE). Analysis of variance (ANOVA) test with the Fisher’s least significant difference (LSD) test was used to assess the significant difference in tumor volume between the groups based upon treatment, indicating statistically significant difference. Nude mice experiments were performed in accordance with Institutional Animal Care and Use Committee (IACUC) procedures and guidelines at Charles Drew University of Medicine and Science with approval from the IACUC at Jackson State University.
Results
VA extracts reduce viability of triple-negative breast cancerous cells in vitro
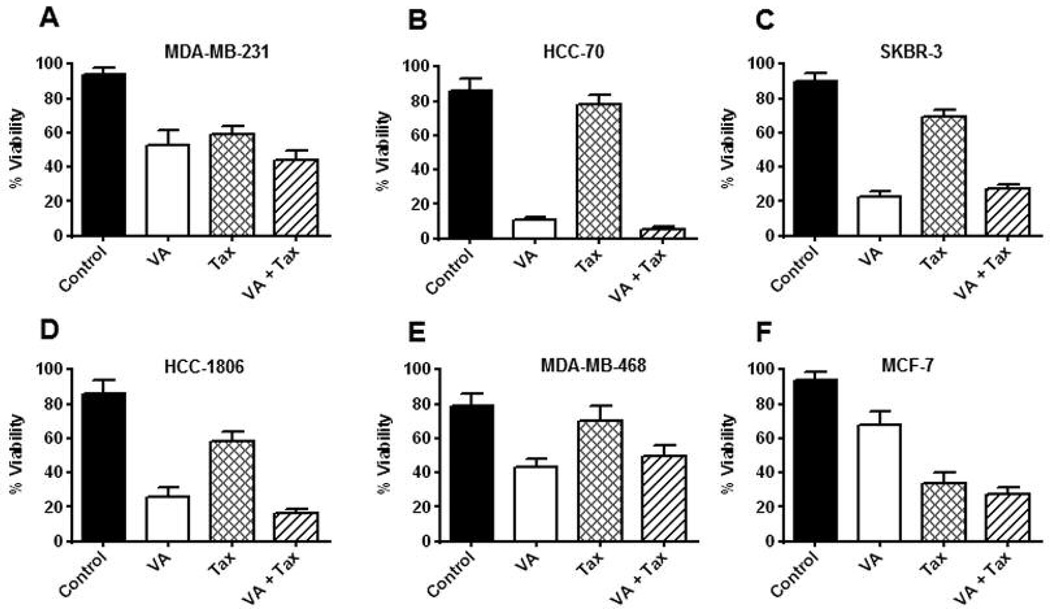
The effects of VA extracts (100 μg/ml, 18 h) on growth of breast cancer cell lines were assessed by cell counts and Trypan blue exclusion assays. VA extracts were effective in reducing viability of MDA-MB-231 cells (40%), HCC-70 cells (70%), SKBR-3 (65%), HCC-1806 cells (60%), MDA-MB-468 cells (40%) and MCF-7 cells (25%). Compared to VA extracts, Tax (100 nM) was able to reduce viability of MDA-MB-231 cells (40%), HCC-70 cells (10%), SKBR-3 (25%), HCC-1806 cells (20%), MDA-MB-468 cells (10%) and MCF-7 cells (65%). A slight additive effect in reduction of viability of MDA-MB-231 cells (5% more), HCC-70 cells (3% more), HCC-1806 cells (10% more) and MCF-7 cells (10% more) was seen when cells were treated with VA extracts in combination with Tax (100 μg/ml and 100 nM, respectively). The data suggest that VA extracts were more effective than Tax in reducing viability of TNBC cell lines but showed, however, only moderate cytotoxicity in MCF-7 cells wherein Tax was more effective (Figure 1A, B, C, D, E and F).
Figure 1.
Increased sensitivity of six cell lines to VA extracts. VA extracts decrease cell viability in TNBC cells. Cells were cultured for 18 h with VA extracts (0.1 mg/ml) and/or Tax (100 nM) and cell viability was determined. Percentage of viable A: MDA-MB-231, B: HCC-70, C: SK-BR-3, D: HCC 1806, E: MDA-MB-468 and F: and MCF-7 cells was calculated after performing Trypan blue exclusion with cell counting. Each data point represents the mean±SD of three independent experiments done in triplicates (n=9).
VA extracts inhibit DNA synthesis and induce DNA damage in triple-negative breast cancer cell lines
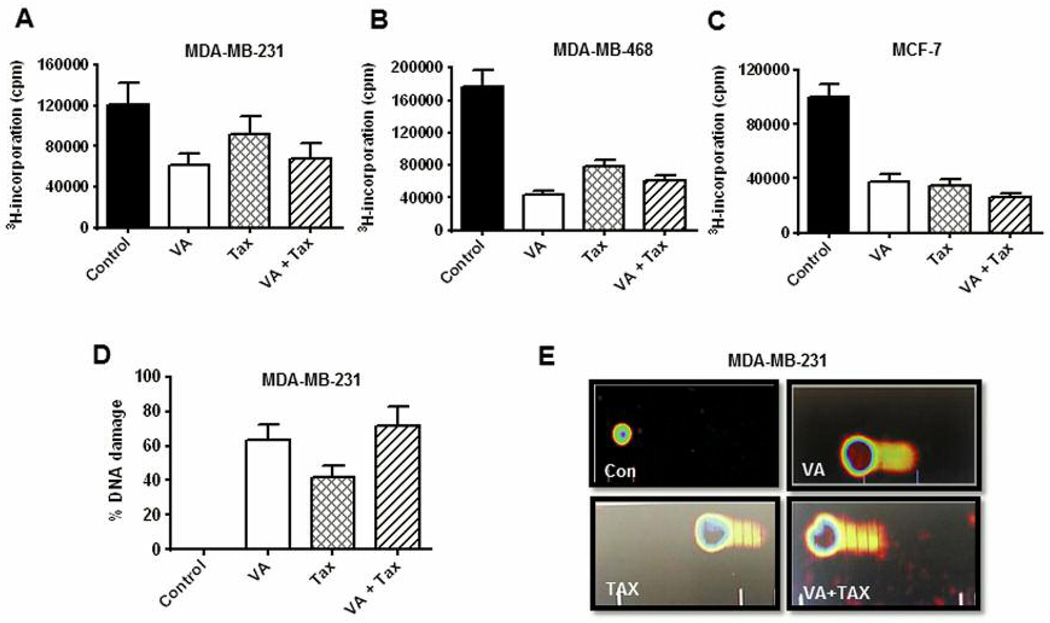
The effects of VA extracts on DNA synthesis were evaluated in TNBC cells. VA extracts inhibited DNA synthesis in MDA-MB-231 cells (50%), MDA-MB-468 cells (75%) and MCF-7 cells (60%). Tax treatment resulted in more moderate inhibition of DNA synthesis in MDA-MB-231 cells (20%) and more pronounced in MDA-MB-468 cells (50%) and MCF-7 cells (70%). Neither an additive effect nor synergy was observed in either TNBC cell line following combined treatment with VA extracts and Tax, although a slight additive effect was seen in MCF-7 cells (5% more). The results showed that VA extracts were more efficacious inhibitors of DNA synthesis than Tax (Figure 2A, B and C). DNA damage assessment using the comet assay showed a significant percentage of VA extracts-induced DNA damage in MDA-MB-231 cells (62%), 40% DNA damage from Tax treatment and 70% damage for combined treatment with VA extracts and Tax (Figure 2D). Representative comet cell images of untreated (control) and VA extracts and/or Tax using SYBR Green stain showed significant increases in the mean values of comet tail length, tail moment, arm tail and percentages of DNA cleavage of MDA-MD-231 cells over control for all three treatment regimens. Slight increases in damage were resultant of an additive effect from combined treatment with VA extracts and Tax (Figure 2E).
Figure 2.
Effect of VA extracts treatment on DNA synthesis and DNA damage. Cells were treated with VA extracts alone or in combination with Tax for 18 h before adding 1 μCi/ml of [3H] thymidine for 6 h. [3H] thymidine uptake was determined for A: MDA-MB-231, B: MDA-MB-468 and C: MCF-7 cells using methods described under Materials and Methods. Each data point represents the mean±SD of three independent experiments with three replicates per dose. D: Representative DNA damage assessed using the comet assay for MDA-MB-231 cells, E: Representative SYBR Green comet assay images of untreated and VA extracts-treated, Tax-treated and VA extracts/Tax-treated MDA-MB-231 cells.
VA extracts increase P-glycoprotein (PgP) ATPase activity
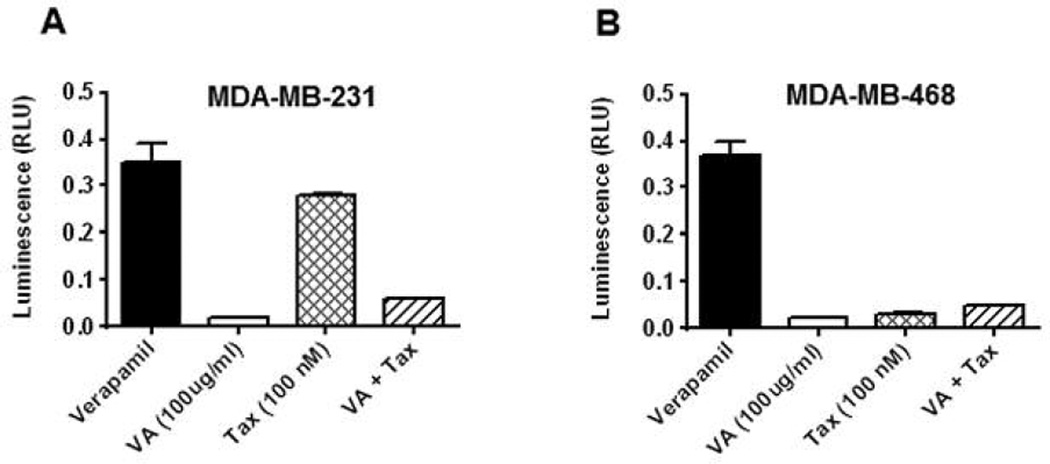
Suppression of PgP activity may improve cancerous cell sensitivity and also treatment outcome (23, 32). To evaluate the effects of VA extracts on PgP ATPase activity, assays were conducted. Treatments using test chemotherapeutic agents yielded luminescence readings that were measured against sodium orthovanadate (Na3VO4)-treated samples relative light unit (RLU)(Na3VO4). Differences between average luminescent signals from (RLU)(Na3VO4) and relative light unit test compounds (RLUTC) were used to determine PgP ATPase activity in the presence of a test compounds. Neither cell line showed statistically relevance effects of VA extracts on the ATPase activity of PgP compared to basal level control. In sharp contrast, Tax treatment in MDA-MB-231 cells resulted in an average three-fold increase in the ATPase activity of PgP compared to control (Figure 3A). Combined treatment with VA extracts/Tax dramatically averted the Tax-induced MDA-MB-231 cell sensitivity. No such changes were observed in any of the other TNBC cell lines, nor MCF-7 cells (Figure 3B).
Figure 3.
Effects of VA extracts and/or Tax treatment on stimulation of P-glycoprotein ATPase activity. Treatments using VA and/or Tax at the concentrations indicated in the Materials and Methods section in MDA-MB-231 (A) and MDA-MB-468 (B) cells yielded luminescence readings, which were measured against Na3VO4-treated samples. Differences between luminescence signals from verapamil and test compounds were used to determine P-glycoprotein ATPase activity in the presence of test compounds.
VA extracts treatment increases Caspase-3 activation
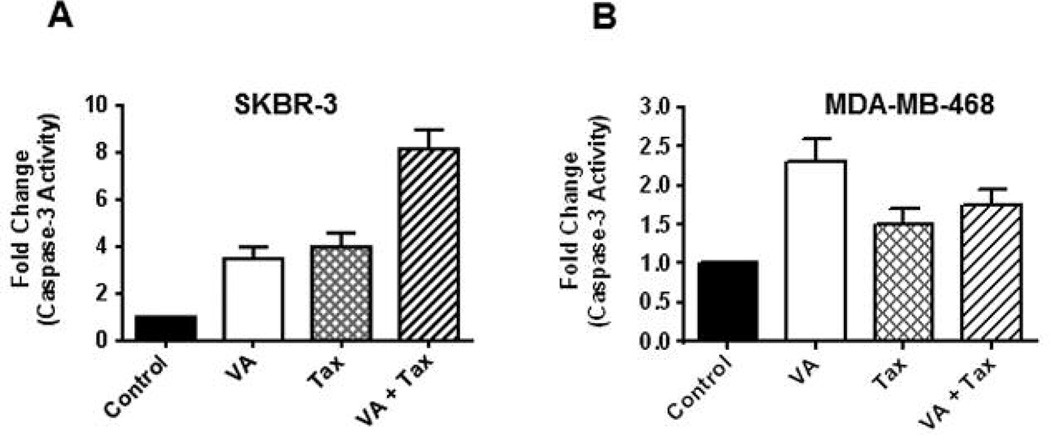
Caspase-3 is an established effector caspase whose activation commits cells to apoptosis by cleavage of important intracellular substrates, such as DFF-45 and PARP (25, 33). The activation profiles of caspase-3 upon exposure to VA extracts and/or Tax were measured using the substrate Ac-DEVD-AMC. After exposure of the cells to VA extracts (100 μg/ml), activation of caspase-3 activity was detected after 18 h in SKBR-3 cells (3-fold) and in MDA-MB-468 cells (1.5-fold). Increases in caspase-3 activation were detected in SKBR-3 cells (3.5-fold) and in MDA-MB-468 cells (0.5-fold) following exposed to Tax (100 nM) for 18 h. The most notable increase in caspase-3 activation was observed following combined treatment with VA extracts and Tax in SKBR-3 cells with an 8-fold increase in caspase-3 activity (Figure 4A). A slight change was observed in MDA-MB-468 cells (1-fold) following combined treatment with the two chemotherapeutic agents (Figure 4B).
Figure 4.
Apoptosis induced by VA extracts. Cell apoptosis in SKBR-3 (A) and MDA-MB-468 (B) cells was assessed using caspase-3 fluorometric assay to quantitatively measure the presence of apoptosis using fluorometric standard units (FSU) following treatment of cells with VA extracts and/or Tax.
VA extracts treatment decreases mitochondrial membrane integrity
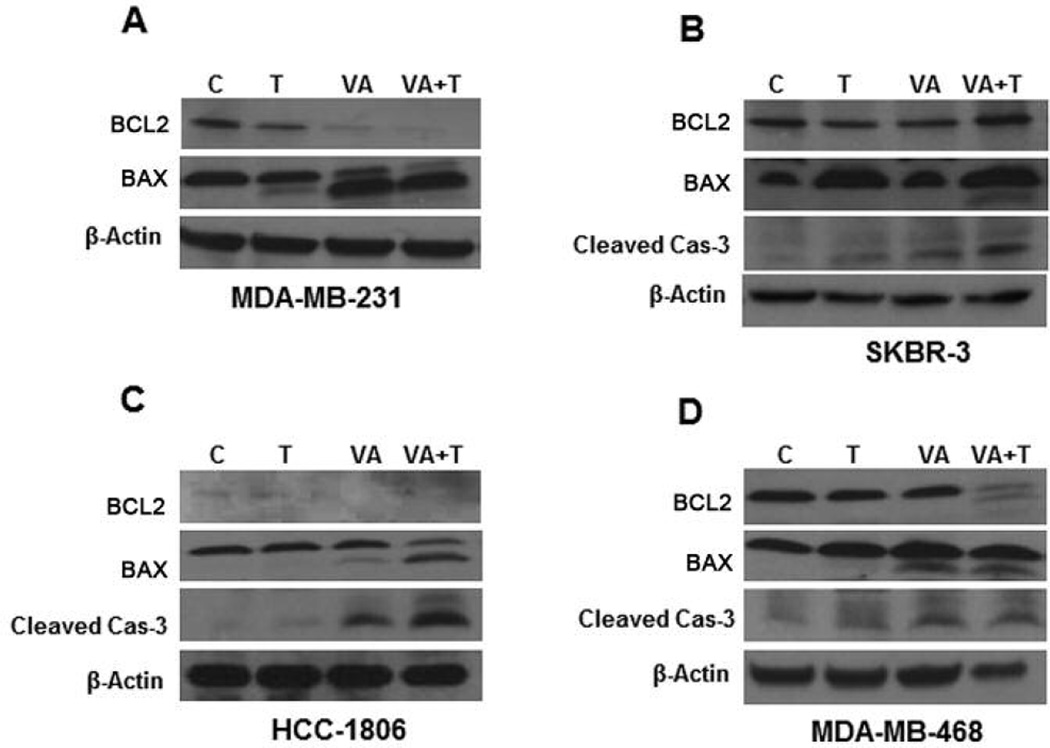
Since we were able to detect increased caspase-3 activity with VA extracts treatment, we sought to determine whether the integrity of the mitochondrial membrane was compromised following VA extracts treatment. It has been reported that apoptosis is stimulated by the insertion of BAX, a cytosolic protein, from the cytosol into the mitochondrial membrane (25, 33). We examined the levels of anti-apoptotic protein BCL-2 and pro-apoptotic protein BAX. It is known that the ratio of BCL-2/BAX controls the integrity of the mitochondrial membrane. VA extracts were effective in decreasing BCL-2 in MDA-MB-231 cells (Figure 5A). VA extracts induced BAX cleavage prominently in MDA-MB-231 cells, slightly in HCC-1806 cells, and significantly in MDA-MB-468 cells (Figure 5A, C and D). Tax treatment alone had no effect on either BCL-2 or BAX levels, however, following combined treatments with VA extracts and Tax, for 18 h, BCL-2 levels were increased and BAX cleavage was induced in MDA-MB-231, HCC-1806, SKBR-3 and MDA-MB-468 cells (Figure 5A, B, C and D).
Figure 5.
Western blots to analyze the presence of key proteins involved in cell apoptosis for MDA-MB-231 cells of white origin (A), SK-BR-3 cells (B), HCC1806 (C) and MDA-MB-468 cells of African-American origin (D) were run. Cleavage of a pro-apoptotic molecule BAX, which activates caspase-3, is implicated as the mechanism of VA extracts to induce apoptosis, compared to Tax.
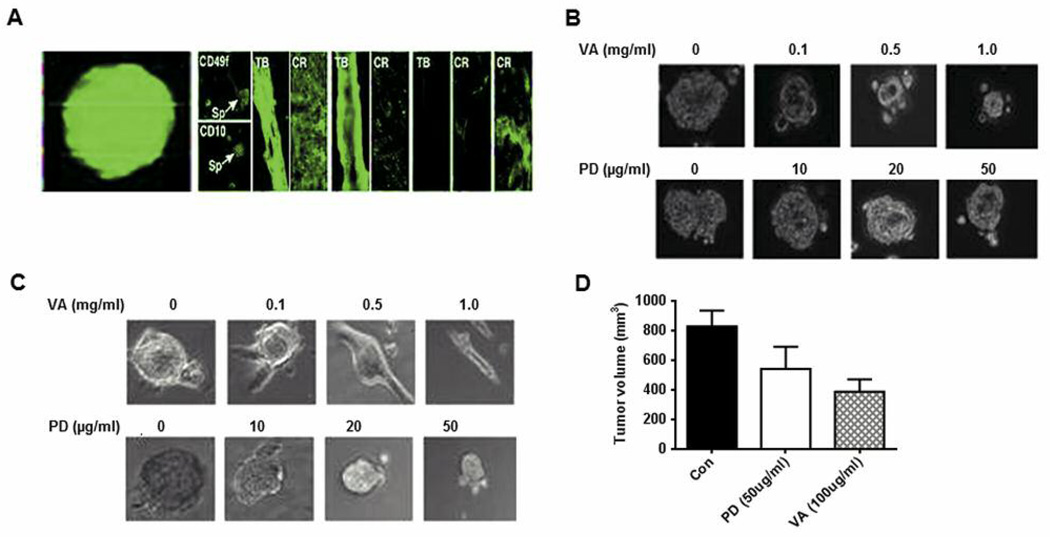
VA extracts reduce pERK1/2. It is known that MCSCs play an important role in breast tumor initiation and progression
We next examined the efficacy of VA extracts in reducing MCSC populations in breast cancer cell lines. MCSCs were enriched from HMLEHRASV12 (Hras) cells as mammospheres that were propagated on low attachment plates under defined conditions. The clonal nature of the mammospheres is shown by growing them from Hras cells that are overexpressing enhanced green fluorescence protein (eGFP) (Figure 6A, right panel). Staining showed that mammospheres expressed CD49 and CD10 MCSC markers (Figure 6A, middle panel). Further, when mammospheres were plated on high-attachment plates, they underwent differentiation to various lineages (Figure 6A, left panel). VA extracts activated pERK1/2 that is nuclear and stable in MCSCs. Activated extracellular signal-regulated kinase (ERK) can turn on the genes needed for cell cycle progression. Since ERK1/2 is one of the key targets of VA extracts (18), we examined its effect on the proliferation of MCSCs and found that VA extracts are more effective in reducing the level of pERK1/2 and MCSC proliferation than MEK1/2 inhibitor, PD98059. Compared to PD98059, we found that VA extracts were more effective than this known mitogen-activated protein (MAP) kinase inhibitor in reducing the size of mammospheres (Figure 6B). VA extracts were also more effective than PD98059 in inducing differentiation of the spheres (Figure 6C). Additionally, VA extracts (50%) were more effective than PD98059 (25%) in reducing tumor volume (Figure 6D).
Figure 6.
MCF10A and MCF10AHRAS cells from spheres that can be induced to differentiate into tubular and epithelial-like structures. A: Left panel: 10A and 10AHRAS cells were exposed to suboptimal concentrations of lentivirus expressing eGFP and then grown at clonal dilutions under sphere-forming conditions. After 48 h, micrographs were collected under fluorescence (left) and phase-contrast (right) conditions. Right panel: Spheres were transferred to high-attachment plates and cultured in the presence of serum. After 7 days, the cells were fixed and stained for various differentiation markers. Spheres (Sp), Tubules (TB) and cobblestone-like regions (CR) could be identified. Structures were immunostained for stem-like (CD49f, CD10), myoepithelial (CK 14, SMA) or luminal (CK18, ESA) markers. These structures were analyzed under fluorescence illumination after staining with Alexa Fluor 488 anti-mouse or anti-rabbit FITC conjugated secondary antibody. B: MCF10AHRAS mammospheres treated with VA extracts or PD98059 (PD) at various concentrations for 48 h after which the diameter of the spheres was analyzed under the microscope. C: MCF10AHRAS mammospheres were grown for 6 days after which they were plated on high-attachment plates in the presence of high serum and simultaneously treated with various concentrations of VA extracts or PD. After 48 h of treatment the cells were fixed and analyzed. D: Graphical data of tumor volumes from implanted mammospheres are reported as mean±SD.
VA extracts reduce xenograft growth in vivo
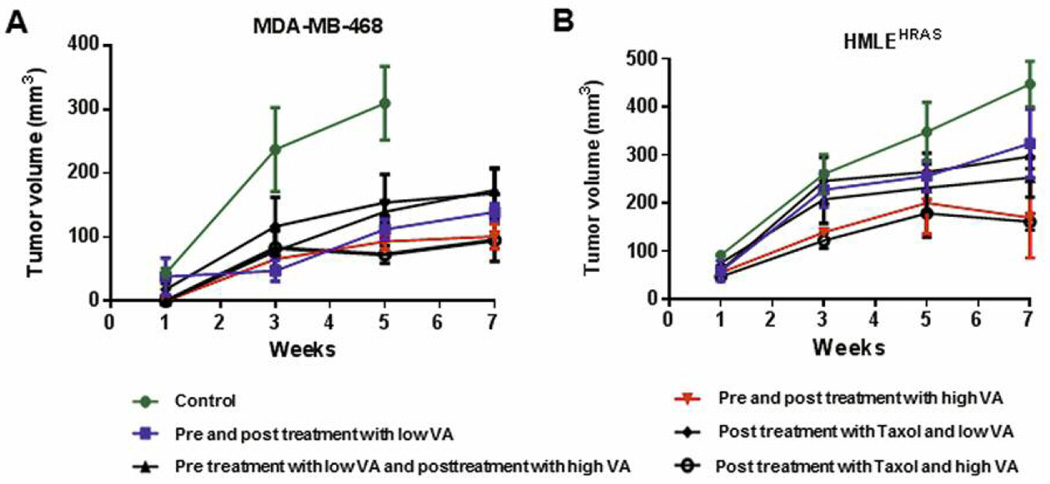
We next examined the effects of VA extracts on growth of tumors from breast cancer cell lines in our nude mice model. Tumor volumes in various treated groups were compared to control. There was a significant reduction in tumor volume in all animal groups pretreated with VA extracts followed by continued treatment with VA extracts following subcutaneous implantation of breast cancer cells. The most prominent reductions in tumor volume were seen in animals that were pretreated with VA extracts (20 μg/ml) 10 days before subcutaneous implantation of MDA-MB-468 cells (75%), HCC 1806 cells (50%) and HRAS cells (60%) followed by 16-day VA extracts (20 μg/ml) treatment (Figure 7A and B).
Figure 7.
Therapeutic and chemo-preventive efficacies of VA extracts in a nude mice model. Animals (female, 5–6 weeks) were untreated or pretreated with VA extracts (20 mg/kg, high-dose or 10 mg/kg, low-dose) subcutaneously each day for ten days before a single subcutaneous injection of MDA-MB-468 cells (A) or Hras cells (B). The treatment groups were characterized and the protocols for measurements of tumor volumes are described in the Materials and Methods section. VA extracts can significant inhibit TNBC-derived tumor growth. Although there was a lag in each treatment group, pre-treatment with VA was the most efficacious, implicating VA extracts as chemo-preventive agents.
Discussion
Regimens that combine treatment drugs to target a variety of growth pathways have worked well in some patients in whom, if the TNBC responds well, the chance of survival is higher. However, aggressive TNBCs with higher MCSC content are frequently diagnosed in pre-menopausal African-American (AA) women who, when compared to White women, they suffer worse outcomes to chemotherapy. Additionally, these aggressive tumors are inherently resistant to most available therapies, resulting in increased incidence of relapse and recurrence; treatment with commonly used anti-BC therapies is oftentimes accompanied by harsh side-effects, with no specific molecular targets or chemotherapeutic vulnerabilities being identified. Therefore, there remains a critical need to identify effective treatment regimens against TNBCs.
Although conventional chemotherapy and radiation therapies are effective against some forms of BC, these modalities fail to spare normal cells to a high degree, and researchers are unsure as to whether they work at all in TNBCs (4–6). Approximately, 70% of basal-like cancers are TNBCs. These tumors are typically devoid of ER, PR and HER2, thus respond strikingly different to treatment agents than luminal/HER2E subtypes. Conflicting trial data has emerged related to benefits of using taxanes and platinum-based analogues or anti-angiogenic therapies against TNBCs (2). Basal-like and erbB2+ subtypes of BC were shown to be more sensitive to Tax-doxorubicin combined adjuvant therapy than the luminal and normal-like cancers, determined by pCR (3). In neoadjuvant clinical trials, women with hereditary BRCA 1 mutations have been shown to have high cisplatin sensitivity, though cisplatin is not commonly used for BC treatment (4–5). A particularly poor outcome is seen among the two hormone receptor-negative subtypes (basal-like and HER2+/ER−) compared with the hormone receptor-high luminal group (3). It is expected that Tax can inhibit ER+ BC. Nevertheless, the high mortality rates for women presenting with aggressive TNBCs urgently require development of adequate treatment strategies and approaches for drug development or the discovery of new targets or markers that could aid in chemo-prevention and viable treatment options for such patients (34).
Contentions exist between conventional medicine practitioners and clinicians who are proponents of use of therapies, such as VA extracts, aqueous herbal extracts. The main contention is that conventional drugs are standardized and chemically defined with the structures and quantities of the active ingredients known. Therefore, it is relatively easy to determine therapeutic doses. Though VA extracts elicit demonstrated medicinal benefits, they are extracts, not a purified active agent(s). Because of increasing use and demand, failsafe measures, quality control procedures and authentication and adulteration detection protocols have been developed to enhance safety of medicinal products from botanical VA extracts (35). Additionally, aqueous and organic extractions were utilized to obtain the fingerprint analysis and to study active fractions from VA extracts (24). These anticancer components are highly extractable by polar protic solvents, while further separation efforts to narrow the fractions to only a few of the most active molecules are underway. Edible VA extracts-derived edoTIDEplus herbal supplements, which promote either general health and well-being, breast and prostate health or immune system health, all minimizing harsh side-effects, have been marketed (17, 24). In lieu of looming disparities in clinical outcomes for TNBC patients, these dietary supplements-validated by emerging scientific evidence of their anticancer activities and safety-make attractive choices for cancer patients to improve their prognosis or quality of life. Still, we and our collaborators are currently working on developing VA extracts, as well as purified, more potent VA extracts-derived compounds, for subsequent testing and eventual clinical trials to test the pronounced anticancer activity of VA extracts and its products against BC, including TNBCs in humans.
In summary, we have generated extensive in vitro and in vivo data indicating that VA extracts have therapeutic potential alone or in combination with known anti-BC agents. We have shown that VA extracts (i) are novel inhibitors of the ERK signaling pathway; (ii) induce apoptosis by cleavage of a pro-apoptotic molecule BAX, which activates caspase-3 and caspase-9 in triple-negative breast cancerous cell lines of AA and White origins; (iii) decrease MCSC proliferation, as well as mammosphere initiating ability in vitro; and (iv) reduce the initiation and progression of MCSC-induced xenografts in vivo (in nude mice). These findings, demonstrating VA extracts’ anti-cancer activities and ability to synergize with Tax, coupled with its chemo-preventive actions and our published data spanning over a decade, provide hope for better cancer treatment, absence of severe side-effects, and improved survival rates for patient with BC, even TNBCs. This supports VA extract’s potential as powerful treatment for TNBC, a health problem that disproportionately affects AA women and reinforces the idea that VA extracts deserve increased attention for further development as a phytoceutical, anticancer drug entity. Knowledge from these studies could lead to developing new interventions and early prevention strategies to treat and prevent recurrence of TNBC, a disease type against which there are currently no approved targeted effective treatment therapies available.
Acknowledgments
The research reported in this publication was supported in part by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIH) under Award Number P20MD006899 and Grant Number 8 G12 MD007581-15 through the RCMI-Center for Environmental Health at Jackson State University (JSU) and the National Center for Research Resources (Grant Number 5 G12 RR 013459-15); and Grant number SC1CA165865 from the NIH National Cancer Institute. Additional partial support was provided by the National Science Foundation (NSF) under Award Number 1008708 through Transforming the Climate and Advancing STEM Women at JSU, an HBCU in the South (JSUAdvance). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NSF. We thank Dr. Ernest B. Izevbigie, Vice Chancellor, Benson Idahosa University, PMB 1100, Benin City, Edo State, Nigeria, for generously providing the Vernonia amygdalina leaves used in this study. Also, we express our appreciation to Dr. Rajan Singh from CDU and the David Geffen School of Medicine at UCLA for providing suggestions, assistance with the study concept and his technical support.
Footnotes
This article is freely accessible online.
References
- 1.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borrensen-Dale AL, Brown PO, Botstein D. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 4.Liedtke C, Mazouni C, Hess KR, Andre’ F, Tordai A, Mejia JA, Symmans WF, Gonzalez-Angulo AM, Hennessy B, Green M, Cristofanilli M, Hortobagyi GN, Pusztai L. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 5.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong C, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy to neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrski T, Huzarski T, Dent R, Marczyk E, Jasiowka M, Gronwald J, Jakubowicz J, Cybulski C, Wisniowski R, Godlewski D, Lubinski J, Narod SA. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res Treat. 2014;147(2):401–405. doi: 10.1007/s10549-014-3100-x. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed May 5, 2016]. http://ww5.komen.org/BreastCancer/TripleNegativeBreastCancer.html. [Google Scholar]
- 8.Hartsock MA. Guide to understanding triple-negative breast cancer, Living Beyond Breast Cancer. (3rd) 2013 https://www.tnbcfoundation.org/guide-to-understanding-triple-negative-breast-cancer/. webmaster@lbbc.org.
- 9.Conner KM, Guglielmino JE. Guide to understanding treatment decisions, Living Beyond Breast Cancer. (3rd) 2014 http://www.lbbc.org/get-support/print/guides-to-understanding/guide-understanding-treatment-decisions. webmaster@lbbc.org.
- 10.Cragg GM, Beutler JA, Jones WP. [Accessed May 5, 2016];The American Society of Phamacognosy: 50 years of progress in natural products research, website Amer Soc Pharmacog 1959–2009. http://www.pharmacognosy.us/wordpress/wp-content/uploads/Front_Matter.pdf.
- 11. [Accessed May 5, 2016];Success stories: Taxol. website NCI. http://dtp.nci.nih.gov/timeline/flash/success_stories/S2_taxol.htm.
- 12.Singletary K. Natural products and cancer chemoprevention. J Nutr. 2000;130:465–466. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- 13.Richardson MA. Biopharmacologic and herbal therapies for cancer: research update from NCCAM. J Nutr. 2001;131(11):3037S–3040S. doi: 10.1093/jn/131.11.3037S. [DOI] [PubMed] [Google Scholar]
- 14.Wargovich MJ, Wood C, Hollis DM, Zander ME. Herbals, cancer prevention and health. J Nutr. 2001;131(11):3034S–3036S. doi: 10.1093/jn/131.11.3034S. [DOI] [PubMed] [Google Scholar]
- 15.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 16.Uno M, Kokuryo T, Yokoyama Y, Senga T, Nagino M. α-Bisabolol inhibits invasiveness and motility in pancreatic cancer through KISS1R activation. Anticancer Res. 2016;36:583–590. [PubMed] [Google Scholar]
- 17.Izevbigie EB. Discovery of water soluble anticancer agents (Edotides) from vegetables found in Benin City, Nigeria. Exp Bio and Med. 2003;228:293–298. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- 18.Izevbigie EB, Bryant JL, Walker A. A novel natural inhibitor of extracellular signal-regulated kinases and human breast cancer cell growth. Exp Bio and Med. 2004;229:163–169. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- 19.Ijeh II, Ejike CECC. Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J Medicinal Plant Res. 2011;5(7):1051–1061. [Google Scholar]
- 20.Kupchan SM, Hemingway RJ, Karim A, Werner D. Tumor inhibitors 47: vernodalin and vernomygdin, two new cytotoxic sesquiterpene lactones from Vernonia amygdalina Del. J Org Chem. 1969;34(12):3908–3911. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 21.Howard CB, Stevens J, Izevbigie EB, Walker A, McDaniel O. Time and dose-dependent modulation of phase 1 and phase 2 gene product expression in response to treatment of MCF-7 cells with a natural anticancer agent. J Cell Mol Biol. 2003;49(7):1057–1065. [PubMed] [Google Scholar]
- 22.Howard CB, Cameron K, Gresham L, Zhang Y, Izevbigie E. Environmental carcinogens and sensitization of paclitaxel and vincristine by Vernonia amygdalina extracts. African Environ Perspectives. 2011;1:103–114. [Google Scholar]
- 23.Cameron KS, Howard CB, Izevbigie EB, Hill BJ, Tchounwou P. Sensitivity and mechanisms of taxol-resistant prostate adenocarcinoma cells to Vernonia amygdalina extract. Exp Toxicol Pathol. 2013;65:759–765. doi: 10.1016/j.etp.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard CB, Johnson WK, Pervin S, Izevbigie EB. Recent perspectives on the anticancer properties of aqueous extracts of Nigerian Vernonia amygdalina. Botanics: Targets and Ther. 2015;5:65–76. doi: 10.2147/BTAT.S62984. http://dx.doi.org/10.2147/BTAT.S62984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pervin S, Singh R, Chaudhuri G. Nitric-Oxide-induced Bax integration into the michondrial membrane commits MDA-MB-468 cells to apoptosis: essential role of Akt1. Cancer Research. 2003;63:5470–5479. [PubMed] [Google Scholar]
- 26.Keene S, Azuelos C, Majumdar SK. Sensitivity evaluation of two human breast cancer cell lines to tamoxifen through apoptosis induction. Open J Apop. 2014;3:70–77. http://dx.doi.org/10.4236/ojapo.2014.34008. [Google Scholar]
- 27.Liang H, Fu Z, Jiang X, Wang N, Wang F, Wang X, Zhang S, Wang Y, Yan X, Guan W, Zhang C-Y, Zen K, Zhang Y, Chen X, Zhou G. miR-16 promotes the apoptosis of human cancer cells by targeting FEAT. BMC Cancer. 2015;15:448. doi: 10.1186/s12885-015-1458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alhazzazi TA, Kamarajan P, Xu Y, AI T, Chen L, Verdin E, Kapila YL. A Novel Dirtuin-3 Inhibitor, LC-0296, inhibits cell survival and proliferation, and promotes apoptosis of head and neck cancer cells. Anticancer Res. 2016;36:49–60. [PMC free article] [PubMed] [Google Scholar]
- 29.Park J-S, Lim CJ, Bang Ok-S, Kim NS. Ethanolic extract of Descurainia Sophia seeds sensitizes A549 human lung cancer cells to TRAIL cytotoxicity by upregulating death receptors. BMC Comp Alt Med. 2016;16:115. doi: 10.1186/s12906-016-1094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol. 2004;26:249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 31.Yedjou C, Izevbigie E, Tchounwou P. Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anticancer agent in the management of breast cancer. Intl J Environ Res Pub Health. 2008;5(5):337–341. doi: 10.3390/ijerph5050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Mariscal L, Hernández S, Vega J. Inventions designed to enhance drug delivery across epithelial and endothelial cells through the paracellular pathway. Recent Patents on Drug Delivery & Formulation. 2008;2(2):145–176. doi: 10.2174/187221108784534117. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Pervin S, Chaudhuri G. Caspase-8-mediated BID cleavage and release of mitochondrial cytochrome c during Nomega-hydroxy-L-arginine-induced apoptosis in MDA-MB-468 cells. Antagonistic effects of L-ornithine. J Biol Chem. 2002;277:37630–37636. doi: 10.1074/jbc.M203648200. [DOI] [PubMed] [Google Scholar]
- 34.Hanahan D. The cancer wars 2: Rethinking the war on cancer. Lancet. 2014;383:558–563. doi: 10.1016/S0140-6736(13)62226-6. [DOI] [PubMed] [Google Scholar]
- 35.Gresham LJ, Ross J, Izevbigie EB. Vernonia amygdalina: anticancer activity, authentication, and adulteration detection. Intl J Environ Res Public Health. 2008;5(5):342–348. doi: 10.3390/ijerph5050342. [DOI] [PMC free article] [PubMed] [Google Scholar]