Abstract
Gastrulation is the first set of morphologically dynamic events that occur during the embryonic development of multicellular animals such as Drosophila. This morphological alteration is also recognized as epithelial to mesenchymal transition (EMT). Dysregulation of EMT is associated with fibrosis and cancer metastasis. There is emerging evidence that EMT is controlled by a number of molecular mechanisms. As such, many key genes that control apical constriction are also known to be important factors in the EMT observed in cancer metastasis. Like EMT during Drosophila gastrulation, epithelial cells can be induced to change their shape and be reprogrammed to redirect cell fate towards various other cell types. Here we provide a robust imaging method of Drosophila gastrulation to assay the initiation of morphogenetic cellular movements and cell fate identification during this stage of embryonic development. Using this method, we identify cell rearrangement at the time of gastrulation and demonstrate the importance of apical constriction during gastrulation using GFP labeled DE-cadherin.
Keywords: Developmental Biology, Issue 118, Drosophila, live imaging, gastrulation, DE-cadherin, epithelial to mesenchymal transition (EMT)
Introduction
Gastrulation is the first set of morphologically dynamic events that occur during embryonic development of multicellular animals such as Drosophila1,2. Interestingly, emerging evidence suggests that this process is regulated through the interplay between mechanical and molecular mechanisms3. Moreover, the epithelial to mesenchymal transition (EMT), which is a crucial process in gastrulation, is also implicated in human disease processes such as cancer metastasis4-8. As such, many genes that control apical constriction are also known to be key factors in the EMT observed in cancer metastasis9. Thus, apical constriction at the time of gastrulation is an excellent model to investigate the aforementioned regulatory mechanisms and to enhance our understanding of cancer metastasis. The advantage of this technique is that we can observe cell movement at the time of gastrulation in real-time and therefore, we will be able to screen genes involved in gastrulation as well as cancer metastasis.
Although relatively unknown, cell-to-cell adhesion is thought to play a central role in apical constriction1. Drosophila genetics is well suited for single cell level investigations exploring regulatory molecular mechanisms. This model will enable us to uncover the importance of apical constriction during gastrulation. Moreover, this method can be used to screen genes involved in cancer metastasis. Capturing live images of Drosophila gastrulation has further enabled us to understand in greater detail the molecular mechanisms governing tissue rearrangement. Herein, we provide a comprehensive description of a simple method to achieve this.
Protocol
NOTE: The transgenic flies used in this study include the following: DE-cad::GFP 10.
1. Preparation of Apple Plate
Prepare a mixture of 12.5 g agar, 125 mL 100% commercially available apple juice, 12.5 g glucose, and 375 mL H2O. Heat the mixture in a microwave and pour it into a 3 cm cell culture dish. Store the mixture at 4 °C for future use.
After preparing the apple plate, add a thin layer of kneaded yeast paste on top of it to allow the flies to lay egg.
2. Embryo Preparation and Live Imaging Protocol
Place approximately 50 flies (25 males and 25 females) in a bottle to mate and subsequently lay eggs overnight on an apple plate covered in kneaded yeast. Set the apple juice plate at the mouth of the bottle.Keep the bottle upside down in an incubator. Wrap the plastic Drosophila stock bottles with aluminum foil to prompt the flies to lay more eggs.
The next morning, replace the old apple juice plate with a new one.
Let the flies lay eggs for 3 to 4 h by wrapping the bottle with aluminum foil.
Three to four hours later, collect the embryos in an embryo strainer from the apple juice plate using a brush or cotton bad and wash them with PBS. Wash the embryos 2 to 3 more times with PBS in 12-well culture dishes.
Dechorionate the embryos with 50% bleach for 5 min in 12-well culture dishes.
Following the dechorionation process, wash the embryos 2 to 3 more times with PBS.
Transfer the embryos to a 3 cm cell culture dish containing PBS and select stage 5 embryos under the microscope based on the level of transparency at their borders. Pipette the staged embryos using a 200 µL pipette tip.
Next, place two selected embryos on a glass coverslip, and remove excess PBS with finely twisted tissue paper. Orient the embryos dorsal side up on the coverslip using a finely twisted tissue paper. After that, attach the embryos to the glass coverslip with silicon grease using a fine needle and twisted tissue paper.
Add a small drop of halocarbon oil 700 on the embryos and place the coverslip containing the embryos upside down on the indented slide, leaving some space at the bottom of the slide.
Examine the embryos using a confocal imaging system, an Argon/488 laser, and an oil-immersion objective (63X). NOTE: Identifying the embryo at the appropriate developmental stage is important to capture images of the gastrulation event. Under these conditions, observe almost all embryos develop normally up to at least stage 14. The analyzed embryo can be cultured further to develop as an adult.
Representative Results
Here, we show the gastrulation events of the Drosophila embryo and a general overview of the embryo preparation procedure (Figure 1). Cell membranes are labeled using DE-cadherin::GFP and live imaging of cell movements is performed at the time of gastrulation in Drosophila (Figure 2). Since DE-cadherin GFP flies allow us to visualize cell adherence junctions, we are able to trace apical cell shape and movements using this system. More importantly, this system also allows us to trace endogenous DE-cadherin expression patterns.
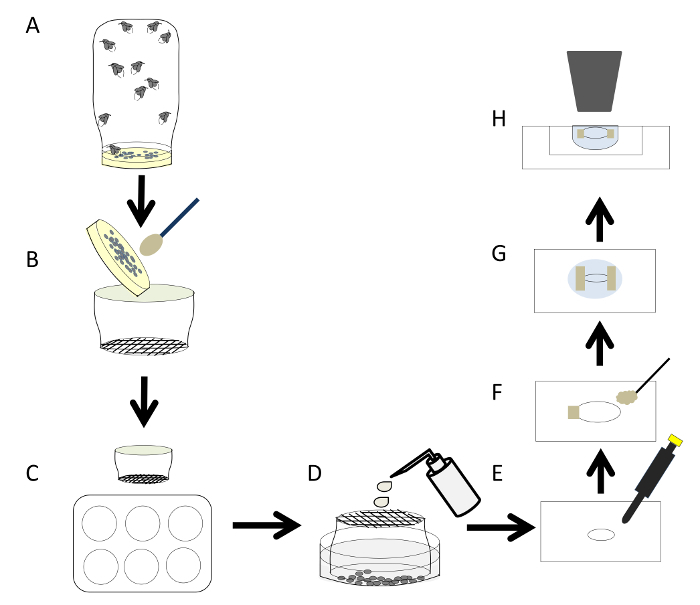 Figure 1: General Overview of the Embryo Preparation Procedure. A) Set up flies for egg laying. B) Collect embryos and place them in an embryo strainer. C) After washing the embryos with PBS, dechorionate them immediately. D) Transfer the embryos to a 3 cm cell culture dish using PBS. E) Pipet the embryos that are at the correct developmental stage and place them on a coverslip. F) Attach the embryos to the coverslip using vacuum grease. G) Next add a drop of halocarbon oil onto the coverslip. H) Finally place the coverslip upside down on the indented slide. The embryos are now ready for time-lapse imaging. Please click here to view a larger version of this figure.
Figure 1: General Overview of the Embryo Preparation Procedure. A) Set up flies for egg laying. B) Collect embryos and place them in an embryo strainer. C) After washing the embryos with PBS, dechorionate them immediately. D) Transfer the embryos to a 3 cm cell culture dish using PBS. E) Pipet the embryos that are at the correct developmental stage and place them on a coverslip. F) Attach the embryos to the coverslip using vacuum grease. G) Next add a drop of halocarbon oil onto the coverslip. H) Finally place the coverslip upside down on the indented slide. The embryos are now ready for time-lapse imaging. Please click here to view a larger version of this figure.
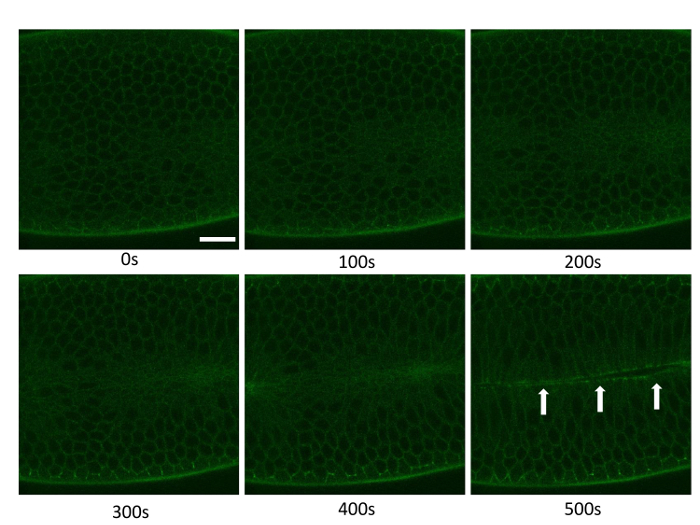 Figure 2: The Result of an Experimental Procedure utilizing DE-cad::GFP-based Live Imaging During Gastrulation. Cell membranes were labeled using DE-cadherin::GFP and live imaging of cell movement was then captured for approximately 500 s during gastrulation of Drosophila. Apical cell perimeters were visualized using the GFP fluorescence of DE-Cad in order to trace the apical areas of individual cells using a representative set of time-lapse images; Scale bar = 20 µm. The arrowheads show where apical constriction and invagination occur. Please click here to view a larger version of this figure.
Figure 2: The Result of an Experimental Procedure utilizing DE-cad::GFP-based Live Imaging During Gastrulation. Cell membranes were labeled using DE-cadherin::GFP and live imaging of cell movement was then captured for approximately 500 s during gastrulation of Drosophila. Apical cell perimeters were visualized using the GFP fluorescence of DE-Cad in order to trace the apical areas of individual cells using a representative set of time-lapse images; Scale bar = 20 µm. The arrowheads show where apical constriction and invagination occur. Please click here to view a larger version of this figure.
Discussion
Although we have previously reported a similar procedure to capture live images of the gastrulation process in Drosophilla1, the method we describe here is detailed and easy to trace endogenous cadherin expression and thus is quite useful for genetic screening of key factors involved in gastrulation. To maximize success with this imaging procedure, it is essential to use an indented slide. Mechanical pressure sometimes causes embryonic death. Therefore, it is also important to handle the embryos as gently as possible since any mechanical stress could disrupt the process of embryonic gastrulation. Using the proper amount of grease during embryo fixation will further improve the results of the live imaging procedure.
Ensuring that both the dechorionation and selection of the embryos are performed quickly is a key factor in achieving success with this protocol. Using a fine needle to glue the embryos before completely drying is also vital to maximizing results with this procedure. After fixing the embryo, GFP labeled DE-cadherin is examined with a confocal microscope. During live imaging experiments, images were taken every 10 s for less than 30 min1. Under these conditions, almost all embryos develop normally up to at least stage 14. The laser power should also be optimized. It is also important to perform the embryo treatment properly to reduce the potential autofluorescence from the vitelline membrane.
Gastrulation is a dynamic event that occurs during stage 6 of the developmental process. This stage of development is directly controlled by inherited genetic patterning. Using our established Drosophila gastrulation live imaging procedure with DE-cadherin GFP transgenic lines, we will be able to further understand the molecular mechanisms underlying Drosophila gastrulation as well as the epithelial-to-mesenchymal transition involved in cancer metastasis.
Disclosures
The authors have nothing to disclose.
Acknowledgments
This study was supported by the Astellas Foundation for Research on Metabolic Disorders (HT), Takeda Science Foundation (HT), and MEXT-Supported Program for the Strategic Research Foundation at Private Universities (HT).
References
- Haruta T, Warrior R, Yonemura S, Oda H. The proximal half of the Drosophila E-cadherin extracellular region is dispensable for many cadherin-dependent events but required for ventral furrow formation. Genes Cells. 2010;15(3):193–208. doi: 10.1111/j.1365-2443.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- Oda H, Takeichi M. Evolution: structural and functional diversity of cadherin at the adherens junction. J. Cell Biol. 2011;193(7):1137–1146. doi: 10.1083/jcb.201008173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sanchez ME, Serman F, Ahmadi P, Farge E. Mechanical induction in embryonic development and tumor growth integrative cues through molecular to multicellular interplay and evolutionary perspectives. Methods Cell Biol. 2010;98:295–321. doi: 10.1016/S0091-679X(10)98012-6. [DOI] [PubMed] [Google Scholar]
- Alderton GK. Metastasis: Epithelial to mesenchymal and back again. Nat. Rev. Cancer. 2013;13(1):3. doi: 10.1038/nrc3428. [DOI] [PubMed] [Google Scholar]
- Fabregat I, Malfettone A, Soukupova J. New Insights into the Crossroads between EMT and Stemness in the Context of Cancer. J. Clin. Med. 2016;5(3) doi: 10.3390/jcm5030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol. Cancer. 2016;15 doi: 10.1186/s12943-016-0502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AQ, Ding Y, Li CL, Yang Y, Yan SR, Li DS. TALEN-induced disruption of Nanog expression results in reduced proliferation, invasiveness and migration, increased chemosensitivity and reversal of EMT in HepG2 cells. Oncol. Rep. 2016;35(3):1657–1663. doi: 10.3892/or.2015.4483. [DOI] [PubMed] [Google Scholar]
- Zheng X, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhou W, Dong W, Watson AM, Hong Y. Directed, efficient, and versatile modifications of the Drosophila genome by genomic engineering. Proc. Natl. Acad. Sci. U.S.A. 2009;106(20):8284–8289. doi: 10.1073/pnas.0900641106. [DOI] [PMC free article] [PubMed] [Google Scholar]


