Abstract
Objective
Working memory impairment is well established in psychotic disorders. However, the relative magnitude, diagnostic specificity, familiality pattern, and degree of independence from generalized cognitive deficits across psychotic disorders remain unclear.
Method
Participants from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study included probands with schizophrenia (N=289), psychotic bipolar disorder (N=227), schizoaffective disorder (N=165), their first-degree relatives (N=315, N=259, N=193, respectively), and healthy controls (N=289). All were administered the WMS-III Spatial Span working memory test and the Brief Assessment of Cognition in Schizophrenia (BACS) battery.
Results
All proband groups displayed significant deficits for both forward and backward span compared to controls. However, after covarying for generalized cognitive impairments (BACS composite), all proband groups showed a 74% or greater effect size reduction with only schizoaffective probands showing residual backward span deficits compared to controls. Significant familiality was seen in schizophrenia and bipolar pedigrees. In relatives, both forward and backward span deficits were again attenuated after covarying BACS scores and residual backward span deficits were seen in relatives of schizophrenia patients.
Conclusions
Overall, both probands and relatives showed a similar pattern of robust working memory deficits that were largely attenuated when controlling for generalized cognitive deficits.
Working memory deficits are a core cognitive feature of psychotic disorders (Lee and Park, 2005) (De et al., 2013) (Kelleher et al., 2013a) (Koychev et al., 2012) (Reichenberg and Harvey, 2007). Working memory encompasses a variety of cognitive processes ranging from relatively simple encoding and maintenance to more complex manipulation of stored information. Working memory impairments for basic maintenance and rehearsal both have been reported in schizophrenia patients (Lencz et al., 2003) (Reilly et al., 2006) (Reilly et al., 2007) (Park and Holzman, 1993) and in their relatives (Myles-Worsley and Park, 2002;Glahn et al., 2003) (Saperstein et al., 2006;Kelleher et al., 2013b). Abnormalities have also been observed when more complex processing is required (MacDonald, III et al., 2005) (Cannon et al., 2005) (Tan et al., 2007) (Kim et al., 2004). In recent years working memory impairments have come into focus as a cognitive feature in bipolar disorder with psychosis (Bora et al., 2010) (Glahn et al., 2006) (Brandt et al., 2014), suggesting that impairment in this RDoC domain extends across disorders. Yet, the relative magnitude of impairments across psychotic disorders and the extent to which these impairments are familial (Schulze et al., 2011) (Bora et al., 2008) remains unclear. No studies have directly compared simple and complex working memory processes across psychotic disorders and among their first-degree relatives.
This report was designed to 1) clarify the relative magnitude and diagnostic specificity of spatial working memory impairments (forward and backward span) across psychotic disorders, 2) examine whether forward and/or backward span impairments merely reflect the generalized cognitive impairments associated with psychotic disorders or specific informative cognitive deficits above those predicted by generalized impairments, and 3) assess the degree to which working memory impairments extend to first-degree relatives and estimate their familiality.
Methods
The five-site B-SNIP consortium (Maryland Psychiatric Research Center, University of Chicago/University of Illinois at Chicago, University of Texas – Southwestern, Wayne State University/Harvard University, and the Institute of Living/Yale University) was organized to address questions about diagnostic boundaries and familiality of intermediate phenotypes in psychotic disorders.
Identical inclusion criteria and testing procedures were employed across all sites. Recruitment and clinical assessment procedures have been reported previously (Tamminga et al., 2013). Probands were required to have a diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder with a history of psychosis based on the Structured Clinical Interview for DSM Disorders (SCID) (First et al., 1995). Probands were clinically stable and on stable medication regimens for the month prior to testing. Healthy participants were recruited from the community and were required to have no personal history of a psychotic disorder or recurrent depression and no known immediate family history of these disorders.
All participants had 1) no history of seizures or head injury with loss of consciousness (> 10 minutes), 2) no diagnosis of substance abuse in the prior 30 days or substance dependence in the prior 6-months, 3) negative urine drug screen for common drugs of abuse on the day of testing, 4) no history of systemic medical or neurological disorder likely to impact cognitive abilities, 5) age-corrected Wide Range Achievement Test-IV Reading standard score (SS) > 65, and 6) sufficient fluency in English to complete testing.
Measures
All participants completed the Brief Assessment of Cognition in Schizophrenia (BACS) neuropsychological battery and the WMS-III Spatial Span subtest was included to assess maintenance and manipulation aspects of spatial working memory.
Statistical analyses
Demographic and clinical sample characteristics are presented in Table 1 for probands and Table 2 for first-degree relatives. Consistent with our prior B-SNIP reports (Hill et al., 2013) (Hill et al., 2014), neither antipsychotic dose nor the presence (vs. absence) of current antipsychotics, mood stabilizers or antidepressants were meaningfully related to forward or backward span scores in probands or relatives (r’s <0.22). Thus, neither dosage nor medication status were modeled in the analyses. Our prior B-SNIP report indicated statistically significant group differences for age, race, and sex (Hill et al., 2013). Therefore, age, race, and sex were used as covariates in all analyses. Using a normative based regression approach, hierarchical linear modeling (HLM) was used to test for group differences. Post-hoc comparisons were conducted where indicated by a significant omnibus finding. Primary hypothesis testing were completed using a Hochberg correction (Hochberg, 1988) for multiple comparisons. The previous B-SNIP findings demonstrated significant generalized cognitive deficits across psychotic disorders as measured by the BACS (Hill et al., 2013). In addition to investigating working memory deficits in probands and relatives, this report sought to determine whether performance deficits on the Spatial Span test were independent of the generalized cognitive deficit by examining the impact of adding BACS composite scores as a covariate. This was done to determine the degree to which working memory deficits assessed by span scores were explained by the level of generalized neuropsychological deficit to assess their value as specific tests of cognitive deficit.
Table 1.
Demographic and clinical data for probands with a history of psychosis and healthy controls
| Healthy Controls | Schizophrenia | Schizoaffective | Bipolar w/ Psychosis |
Findings | |||||
|---|---|---|---|---|---|---|---|---|---|
| n=289 | n=289 | n=165 | n=227 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 37.78 | (12.67) | 36.01 | (12.67) | 36.58 | (11.81) | 36.19 | (12.79) | F=1.119 ns |
| Education (years) | 15.1 | (2.58) | 12.75 | (2.25) | 13.06 | (2.22) | 14.18 | (2.37) | F=51.803§ a |
| Wide Range Achievement | 103.01 | (13.77) | 93.79 | (15.42) | 96.68 | (14.76) | 101.35 | (13.76) | F=23.179§ a |
| Test–IV: Reading test (SS) | |||||||||
| n | % | n | % | n | % | n | % | ||
| Sex | |||||||||
| Male | 123 | 42.6% | 197 | 68.2% | 67 | 40.6% | 85 | 37.4% | χ2=64.033§ b,c |
| Female | 166 | 57.4% | 92 | 31.8% | 98 | 59.4% | 142 | 62.6% | |
| Race | |||||||||
| Caucasian | 181 | 62.8% | 135 | 46.7% | 89 | 53.9% | 169 | 74.4% | χ2=55.832§ d |
| Afr.-American | 79 | 27.4% | 137 | 47.4% | 66 | 40.0% | 47 | 20.7% | |
| Other | 29 | 9.7% | 17 | 5.9% | 10 | 6.1% | 11 | 4.8% | |
| Clinical Variables | Mean | SD | Mean | SD | Mean | SD | |||
| PANSS Total | 65.19 | 17.0 | 67.7 | 15.3 | 53.4 | 14.1 | F=50.51§ e | ||
| PANSS Positive | 16.6 | 5.6 | 17.7 | 4.8 | 12.8 | 4.5 | F=52.26§ e | ||
| PANSS Negative | 16.6 | 5.9 | 15.7 | 4.9 | 12.1 | 4.0 | F=53.32§ e | ||
| YMRS | 5.3 | 5.8 | 7.0 | 6.6 | 5.9 | 6.6 | F=3.52* f | ||
| MADRS | 8.6 | 7.7 | 14.9 | 10.2 | 10.4 | 9.2 | F=25.81§ g | ||
p < .05,
p ≤ .001
Controls > Schizophrenia, Schizoaffective, and Bipolar; Schizoaffective>Schizophrenia and Bipolar
Disproportionate number of females in Bipolar group
Disproportionate number of males in Schizophrenia group
Disproportionate number of African-Americans and Caucasians in both Schizophrenia and Bipolar groups
Bipolar < Schizophrenia and Schizoaffective
Schizoaffective > Schizophrenia
Schizoaffective > Bipolar and Schizophrenia
Table 2.
Demographic data, history of psychosis, and psychosis spectrum personality traits for first-degree relatives
| Healthy Controls | Relatives of Schizophrenia Probands |
Relatives of Schizoaffective Probands |
Relatives of Bipolar w/ Psychosis Probands |
Findings | |||||
|---|---|---|---|---|---|---|---|---|---|
| n=289 | n=315 | n=193 | n=259 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 37.78 | (12.67) | 43.25 | (14.97) | 40.45 | (16.46) | 40.49 | (15.94) | F= 6.74§ a |
| Education (years) | 15.1 | (2.58) | 13.98 | (2.41) | 13.97 | (2.91) | 14.61 | (2.73) | F= 11.014§ b,c |
| Wide Range Achievement | 103.01 | (13.77) | 97.38 | (15.08) | 99.79 | (15.61) | 103.21 | (13.93) | F= 10.61§ c |
| Test–IV: Reading test (SS) | |||||||||
| Sex | n | % | n | % | n | % | n | % | |
| Male | 123 | 42.6% | 95 | 30.2% | 59 | 30.6% | 85 | 32.8% | χ2=12.6* d |
| Female | 166 | 57.4% | 220 | 69.8% | 134 | 69.4% | 174 | 67.2% | |
| Race | |||||||||
| Caucasian | 181 | 62.8% | 170 | 54% | 120 | 62.2% | 208 | 80.3% | χ2=54.3§ e |
| Afr.-American | 79 | 27.4% | 127 | 40.3% | 63 | 32.6% | 43 | 16.6% | |
| Other | 29 | 9.7% | 18 | 5.7% | 10 | 5.2% | 8 | 3.1% | |
| Positive psychosis history | 39 | 12.4% | 27 | 14.0% | 23 | 8.9% | |||
| Relatives with no psychosis history |
|||||||||
| Elevated Cluster A traits | 44 | 14.0% | 31 | 16.1% | 31 | 12.0% | |||
| Elevated Cluster B traits | 18 | 5.7% | 11 | 5.7% | 18 | 6.9% | |||
| Not Elevated | 214 | 67.9% | 122 | 63.2% | 184 | 71.0% | |||
p < .05
p ≤ .001
Controls < Relatives of Schizophrenia
Controls > Relatives of Schizophrenia and Schizoaffective
Controls, Relatives of Schizoaffective>Relatives of Schizophrenia
Disproportionate number of males in Schizophrenia and Control groups
Disproportionate number of African Americans and Caucasians in Relatives of Schizophrenia and Relatives of Bipolar groups
While BACS composite scores are widely accepted as an index of global cognitive impairment in schizophrenia, some subtests assess aspects of working memory. To address this issue, an alternate BACS composite score that did not include Digit Sequencing subtest was computed. Findings were comparable regardless of which BACS score was used and the relevant analyses below included the full BACS composite.
Familiality
A heritability analysis to estimate familiality of cognitive impairments was performed using Sequential Oligogenic Linkage Analysis Routine software (SOLAR) (Almasy and Blangero, 1998). In this design, estimates of familiality (h2) represent the portion of phenotypic variance accounted for by family membership. To test for the significance of familiality, a maximum likelihood ratio test compared phenotypic variation explained by family membership to a model assuming that no variation is explained by familial factors. A correction was applied to account for ascertainment bias as families were recruited through the identification of a psychotic proband and not a representative community sample (Beaty and Liang, 1987).
Results
Psychotic Probands
HLM analysis of raw spatial span scores indicated significant main effects for diagnostic group [F(3,966)=32.26, p<.001] and span type [F(3,966)=15.46, p<.001] as well as the diagnosis by span type interaction [F(3,966)=4.49, p<.01]. When BACS composite scores were included as a covariate the interaction remained significant [F(3,966)=2.59, p=.05] and separate analyses for forward and backward span were conducted to clarify this interaction.
Forward span
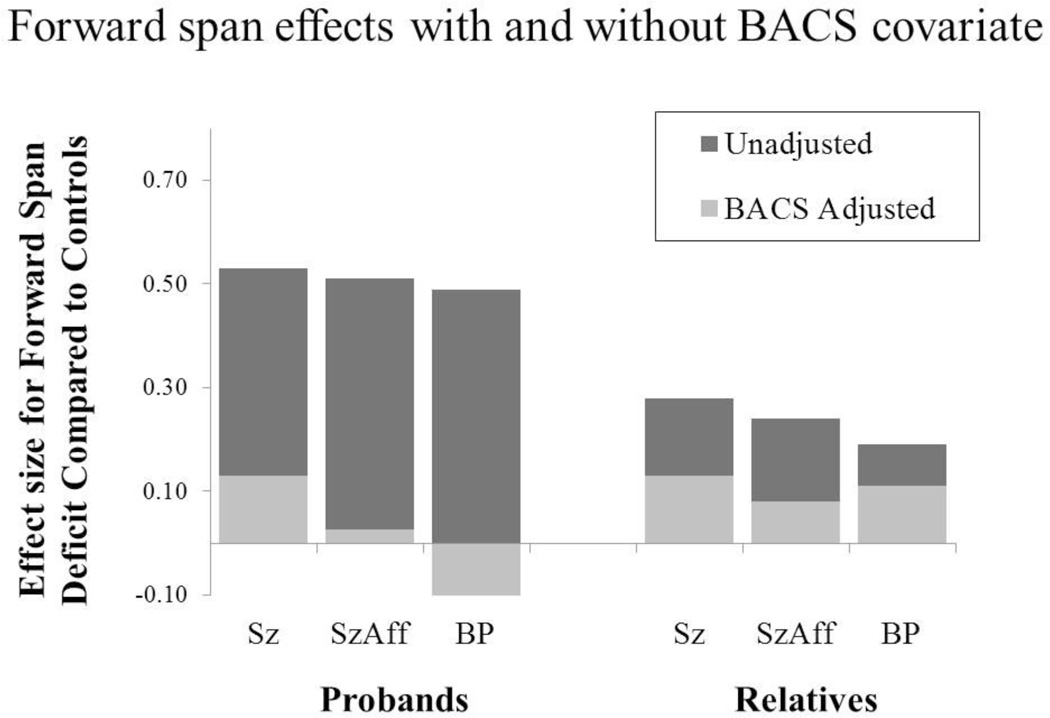
HLM analysis of raw forward span scores indicated significant group differences [F(3,966)=17.44, p<.001]. Post-hoc comparisons indicated impairments in all proband groups, compared to controls. Furthermore, deficits in the three proband groups were comparable (see Figure 1). When the BACS composite score was added as a covariate the effect sizes for deficits were reduced 75–94% and none of the proband groups remained significantly different than controls [Sz: t=1.64, p=0.10; SzAff: t=0.28, p=0.78; BP: t=1.25, p=0.22]. Thus, forward span deficits overlap considerably with the generalized deficit in probands.
Figure 1.
Robust group impairments compared to controls were seen initially for Spatial Span forward scores. However, after covarying BACS composite scores these effects were no longer significant as no proband or relative group differed from controls.
Backward span
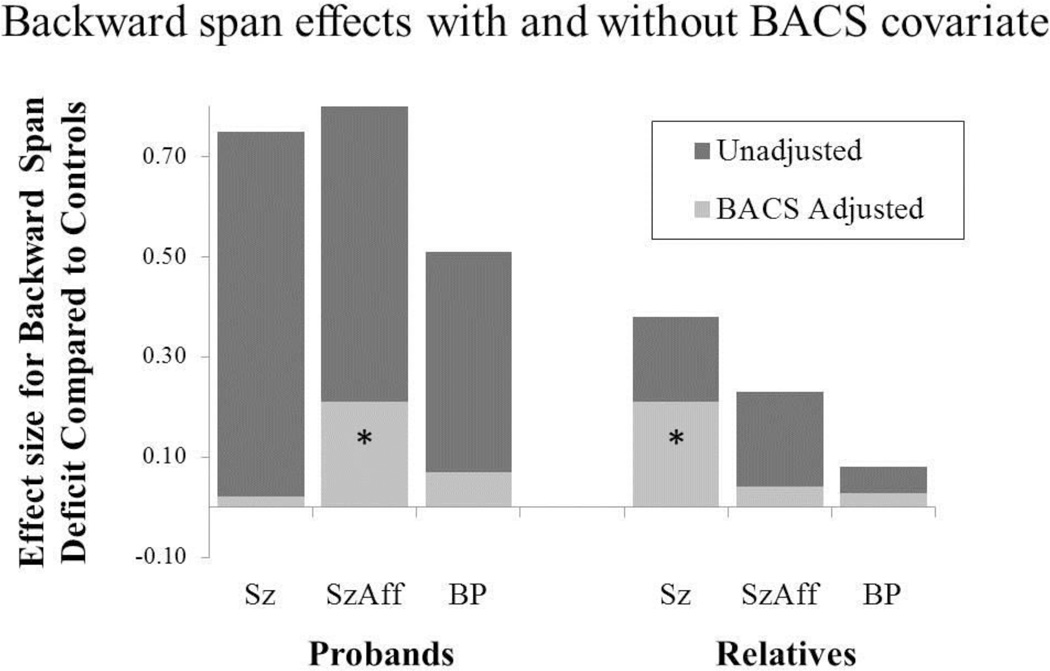
HLM analysis of backward span scores revealed significant group differences [F(3,966)=33.25, p<.001] and post-hoc contrasts indicated that all proband groups were impaired compared to controls. After controlling for the BACS composite score, only schizoaffective probands remained impaired compared to healthy controls (t=2.19, p<0.05) and unadjusted effect sizes were reduced 74–97% (see Figure 2). Thus, backward span deficits in schizophrenia and bipolar disorder overlapped considerably with impairments detected using the BACS index of generalized cognitive deficit.
Figure 2.
Backward span deficits were also robust initially and remained significant after taking into account generalized cognitive impairments (BACS composite scores). Specifically, both schizoaffective probands and relatives of schizophrenia patients showed working memory deficits that extended beyond those accounted for by general cognitive deficits.
Effects in First-degree Relatives
Forward span
HLM analysis indicated significant group differences [F(3,1050)=4.74, p<.01] (see Figure 2) in which all relative groups performed worse than healthy controls [SzRel: t=3.57, p<0.001; SzAffRel: t=2.67, p<0.01; BPRel: t=2.32, p<0.05]. When the BACS composite score was used as a covariate to control for general neuropsychological deficit, the omnibus test was no longer significant [F(3,1050)=0.99, p=.40]. Thus, forward span was not sensitive to a specific familial deficit beyond any general neuropsychological deficit in first-degree relatives.
Backward span
When assessing raw backward span total scores in first-degree relatives, there were significant group differences [F(3,1050)=8.31, p<.001]. Planned comparisons indicated impairments for relatives of schizophrenia (t=4.67, p<0.001) and schizoaffective (t=2.51, p=0.01) probands compared to controls. After covarying BACS composite scores effect size estimates were reduced 45–83% (Figure 2) with significant residual differences [F(3,1050)=3.52, p=.01] remaining for relatives of schizophrenia patients, compared to controls (t=2.64, p<0.01). Backward span deficits were also seen in unaffected relatives of schizophrenia probands, compared to controls [F(4,559)=8.23, p<.01]. This pattern was consistent among relatives of schizophrenia patients regardless of their personal history of psychosis or the presence of Cluster A personality traits as none of the subgroups of schizophrenia relatives differed significantly [F(3,1046)=0.83, p=.48]. Thus, the residual backward span deficits in relatives of schizophrenia patients extended beyond general cognitive deficits regardless of their history of psychosis or Cluster A personality traits.
Familiality
Familiality of forward and backward scores from the Spatial-span test were computed for schizophrenia and psychotic bipolar pedigrees (the two larger proband groups). Age, sex, and race were included as covariates in all analyses and separate estimates were computed with and without covarying for BACS scores. Familiality estimates remained significant after covarying for BACS scores, although estimates were somewhat lower, particularly for forward span scores. Estimates of familiality were also somewhat higher for bipolar compared to schizophrenia pedigrees (see Table 3).
Table 3.
Familiality estimates for forward and backward span scores with and without covarying BACS composite scores.
| Schizophrenia Pedigrees |
Bipolar Pedigrees |
|||
|---|---|---|---|---|
| Forward spam |
Backward span |
Forward span |
Backward span |
|
| BACS excluded from model | 0.35§ | 0.36§ | 0.50§ | 0.51§ |
| h2 (SD) | (.10) | (.11) | (.12) | (.12) |
| BACS included as covariate | 0.24† | 0.29† | 0.39§ | 0.43§ |
| h2 (SD) | (.11) | (.11) | (.13) | (.12) |
p ≤ .01
p ≤ .001
Comment
This is the first large scale study to assess the degree to which forward and backward spatial span measures are informative as a specific cognitive deficit both across a range of psychotic proband groups and in their first-degree relatives. Findings indicated robust deficits for forward span in probands that were primarily a manifestation of a generalized cognitive deficit (Figure 1). Likewise, robust backward span deficits were attenuated after covarying for BACS scores, with proband group deficits no longer significant in schizophrenia and bipolar probands. Backward span deficits remained significant in relatives of schizophrenia patients after BACS correction, regardless of their personal psychosis history or Cluster A personality traits. Familiality was significant in all pedigrees for both measures and remained significant, although somewhat reduced, after correcting for BACS scores. Thus, backward span deficits appear to reflect a familial and selective indicator of working memory deficits in relatives of schizophrenia probands.
Forward span performance overlaps with generalized cognitive ability
Working memory impairments in schizophrenia and psychotic bipolar disorder across a broad spectrum of tasks has been established (Lee and Park, 2005) (Tan et al., 2006) (Pirkola et al., 2005) (MacDonald, III et al., 2005) (Cannon et al., 2005) (Kim et al., 2010). However, differential deficits across disorders on maintenance and manipulation type tasks reflected in forward and backward span scores respectively, and in relation to generalized cognitive deficits has remained unclear. The present findings indicated deficits for maintaining and reproducing a sequence of spatial location targets in the same order in all proband groups and some relative groups. However, this effect was no longer significant after accounting for general cognitive impairments, regardless of diagnosis, suggesting that forward span provides little unique or specific information beyond an assessment of the generalized cognitive ability.
Backward span as a specific cognitive indicator
Unlike the case with forward span, backward span impairments were somewhat independent of generalized cognitive deficits in select groups as the deficits remained significant after controlling for BACS scores in schizoaffective probands and relatives of schizophrenia patients. For schizoaffective disorder, this might result from the combined presence of schizophrenia impact of psychotic and affective features on test performance. Because deficits were familial and significant after BACS correction, backward span deficits in relatives of schizophrenia patients may be useful in detecting a specific deficit associated with familial risk to illness. This observation parallels our prior report indicating behavioral flexibility dysfunction in this sample of first-degree relatives of schizophrenia remains significant after BACS correction (Hill et al., 2014). Thus, this combination of residual deficits in behavioral flexibility and working memory may reflect a familial pattern of specific deficits in executive processes in relatives of schizophrenia patients that might be usefully employed as a pattern of endophenotypes for family genetic research.
Familiality
Estimates of familiality for forward and backward span were on par with reports of familiality estimates in BACS subtests and somewhat lower than the BACS composite (Hill et al., 2013). Furthermore, familiality estimates were significant for both measures before and after a BACS correction in schizophrenia and bipolar pedigrees. Despite significant familiality of backward span in bipolar families, as is often the case with cognitive parameters linked to general intellectual ability, behavioral deficits were not significant after controlling for generalized deficits in either psychotic bipolar patients or their relatives. In contrast, residual backward span behavioral deficits were seen in schizophrenia relatives and this difference suggests that backward span may be a more informative intermediate cognitive phenotype for family genetic research in schizophrenia than bipolar disorder.
Limitations
One limitation is the potential for psychotropic medication effects on cognition. The majority of patients were treated with psychotropic medications, most commonly antipsychotics (Hill et al., 2013). The association between antipsychotic dose and drug class with either forward or backward span was low, yet there remains the possibility that medication effects could manifest as a threshold rather than a dose-related effect. Secondly, the present findings may not generalize to a broader sample of patients with psychotic disorders or their relatives. The study requirements that qualifying probands be clinically stable and have at least one first degree relative willing and able to participate may bias findings to a subgroup of higher functioning patients. Third, working memory deficits in psychotic disorders have been reported using a broad range of measures. The Spatial Span test is but one indicator and the degree to which other measures requiring manipulation of mental information in working memory would yield similar findings remains to be determined. Fourth, the issue of discriminating power (Chapman and Chapman, 1978) needs to be considered here. The backward span task may be somewhat more difficult and discriminating than the forward span task on psychometric grounds, which could account for the differences in findings rather than fundamental differences in maintenance and manipulation tests. Last, the issue of distinguishing generalized and specific deficits remains a challenge for the field. Additional studies are needed to clarify the degree to which individual provide incremental information beyond generalized cognitive dysfunction, which is an important requirement when planning neuropsychological test batteries for family and treatment outcome research.
Acknowledgements
This study was supported in part by NIMH grants MH078113, MH077945, MH077852, MH077851, MH077862, MH072767, and MH083888.
Role of the Funding Source
The funding agencies had no role in the design and conduct of the study collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts & Disclosures
Dr. Tamminga has received support from Intracellular Therapies (ITI, Inc.), PureTech Ventrues, Eli Lilly Pharmaceuticals, Sunovion, Astellas, Merck (ad hoc consulting), International Congress on Schizophrenia Research (unpaid volunteer), NAMI (unpaid volunteer), American Psychiatric Association (Deputy Editor), and Finnegan Henderson Farabow Garrett & Dunner, LLP. Dr. Hill has received support from Riley Nicholas. Dr. Keefe has received investigator initiated support from the Department of Veteran’s Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. Dr. Keefe has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biomarin, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, EnVivo, Helicon, Lundbeck, Merck, Mitsubishi, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Dr. Keefe is a shareholder in Sengenix and NeuroCog Trials, Inc. and receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding). Dr. Bishop has received research support from Ortho-McNeil Janssen. Dr. Keshavan has received support from Sunovion and GlaxoSmithKline. Dr. Sweeney has received support from Takeda, BMS, Roche, and Eli Lilly and research funding from Janssen. The other authors have nothing to disclose.
Contributors
Dr. Hill is the lead author and was involved in all aspects of the report. Dr. Sweeney is the senior author, was a site PI, and was involved in all aspects of the report. Ms. Buchholz was involved in data collection, data analysis, literature review, and writing early drafts of the report. Ms. Amsbaugh was involved in data analysis, literature review, compilation of the main tables, and writing early drafts of the report. Dr. Reilly has been involved in all aspects of the project including data collection, clinical characterization, data processing and quality control, conducted the heritability analysis, and advised on further data analysis and interpretation. Dr. Rubin conducted the hierarchical linear modeling and other statistical analyses. Dr. Gold was the driving force behind inclusion of the Spatial Span measure in the B-SNIP protocol. He also provided oversight for cognitive data collection and processing at the MPRC site. Dr. Keefe provided the BACS battery, training for the BACS, quality control of the BACS throughout the study, and consultation regarding the statistical analytic approach. Drs. Godfrey, Keshavan, and Tamminga were site PIs and PIs for the respective linked RO1 grants (see Acknowledgements). All authors have approved the final version.
Reference List
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty TH, Liang KY. Robust inference for variance components models in families ascertained through probands: I. Conditioning on proband’s phenotype. Genet Epidemiol. 1987;4:203–210. doi: 10.1002/gepi.1370040305. [DOI] [PubMed] [Google Scholar]
- Bora E, Vahip S, Akdeniz F, Ilerisoy H, Aldemir E, Alkan M. Executive and verbal working memory dysfunction in first-degree relatives of patients with bipolar disorder. Psychiatry Res. 2008;161:318–324. doi: 10.1016/j.psychres.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive impairment in affective psychoses: a meta-analysis. Schizophr Bull. 2010;36:112–125. doi: 10.1093/schbul/sbp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CL, Eichele T, Melle I, Sundet K, Server A, Agartz I, Hugdahl K, Jensen J, Andreassen OA. Working memory networks and activation patterns in schizophrenia and bipolar disorder: comparison with healthy controls. Br J Psychiatry. 2014;204:290–298. doi: 10.1192/bjp.bp.113.129254. Epub;%2014 Jan 16., 290-298. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, van Erp TG, Karlsgodt K, Cohen MS, Nuechterlein KH, Bava S, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62:1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of differential deficit. J Psychiatr Res. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- De HA, Wampers M, Vancampfort D, De HM, Vanhees L, Demunter H, Van BL, Brunner E, Probst M. Neurocognition in clinical high risk young adults who did or did not convert to a first schizophrenic psychosis: a meta-analysis. Schizophr Res. 2013;149:48–55. doi: 10.1016/j.schres.2013.06.017. [DOI] [PubMed] [Google Scholar]
- First MD, Gibbon GE, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders-patient edition Biometrics Research Department. New York: NYSPI; 1995. [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap ME, Maples N, Velligan DI, Soares JC. Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disord. 2006;8:117–123. doi: 10.1111/j.1399-5618.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological Impairments in Schizophrenia and Psychotic Bipolar Disorder: Findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. Am J Psychiatry. 2013:10. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Ragozzino ME, Rubin LH, Bishop JR, Gur RC, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Keefe RS, Sweeney JA. Regressing to Prior Response Preference After Set Switching Implicates Striatal Dysfunction Across Psychotic Disorders: Findings From the B-SNIP Study. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu130. sbu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of signicance. Biometrika. 1988;75:800–802. [Google Scholar]
- Kelleher I, Clarke MC, Rawdon C, Murphy J, Cannon M. Neurocognition in the extended psychosis phenotype: performance of a community sample of adolescents with psychotic symptoms on the MATRICS neurocognitive battery. Schizophr Bull. 2013a;39:1018–1026. doi: 10.1093/schbul/sbs086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher I, Clarke MC, Rawdon C, Murphy J, Cannon M. Neurocognition in the extended psychosis phenotype: performance of a community sample of adolescents with psychotic symptoms on the MATRICS neurocognitive battery. Schizophr Bull. 2013b;39:1018–1026. doi: 10.1093/schbul/sbs086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Glahn DC, Nuechterlein KH, Cannon TD. Maintenance and manipulation of information in schizophrenia: further evidence for impairment in the central executive component of working memory. Schizophr Res. 2004;68:173–187. doi: 10.1016/S0920-9964(03)00150-6. [DOI] [PubMed] [Google Scholar]
- Kim J, Matthews NL, Park S. An event-related FMRI study of phonological verbal working memory in schizophrenia. PLoS One. 2010;5:e12068. doi: 10.1371/journal.pone.0012068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koychev I, El-Deredy W, Mukherjee T, Haenschel C, Deakin JF. Core dysfunction in schizophrenia: electrophysiology trait biomarkers. Acta Psychiatr Scand. 2012;126:59–71. doi: 10.1111/j.1600-0447.2012.01849.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Lencz T, Bilder RM, Turkel E, Goldman RS, Robinson D, Kane JM, Lieberman JA. Impairments in perceptual competency and maintenance on a visual delayed match-to-sample test in first-episode schizophrenia. Arch Gen Psychiatry. 2003;60:238–243. doi: 10.1001/archpsyc.60.3.238. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Goghari VM, Hicks BM, Flory JD, Carter CS, Manuck SB. A convergent-divergent approach to context processing, general intellectual functioning, and the genetic liability to schizophrenia. Neuropsychology. 2005;19:814–821. doi: 10.1037/0894-4105.19.6.814. [DOI] [PubMed] [Google Scholar]
- Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. Am J Med Genet. 2002;114:609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Association of working memory deficit and eye tracking dysfunction in schizophrenia. Schizophr Res. 1993;11:55–61. doi: 10.1016/0920-9964(93)90038-k. [DOI] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, Lonnqvist J, Cannon TD. Spatial working memory function in twins with schizophrenia and bipolar disorder. Biol Psychiatry. 2005;58:930–936. doi: 10.1016/j.biopsych.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull. 2007;133:833–858. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiat. 2006;63:1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Antipsychotic drugs exacerbate impairment on a working memory task in first-episode schizophrenia. Biol Psychiatry. 2007;62:818–821. doi: 10.1016/j.biopsych.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Saperstein AM, Fuller RL, Avila MT, Adami H, McMahon RP, Thaker GK, Gold JM. Spatial working memory as a cognitive endophenotype of schizophrenia: assessing risk for pathophysiological dysfunction. Schizophr Bull. 2006;32:498–506. doi: 10.1093/schbul/sbj072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KK, Walshe M, Stahl D, Hall MH, Kravariti E, Morris R, Marshall N, McDonald C, Murray RM, Bramon E. Executive functioning in familial bipolar I disorder patients and their unaffected relatives. Bipolar Disord. 2011;13:208–216. doi: 10.1111/j.1399-5618.2011.00901.x. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical Phenotypes of Psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Am J Psychiatry. 2013:10. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Tan HY, Chen Q, Goldberg TE, Mattay VS, Meyer-Lindenberg A, Weinberger DR, Callicott JH. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Weinberger DR, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163:1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]