Abstract
Wear debris seems to be the most likely reason for osteolysis in THA. The aim was to show the influence of head and acetabular liner revision surgery on osteolytic zones around the femoral component.
Can head and inlay revision surgery reduce the size of the osteolytic zones around the femoral component? Which patients could benefit?
47 patients (51 hips), who had undergone THA head and inlay revision surgery between 1999 and 2011, were reviewed. The mean lifetime for the prosthesis was 15.1 years (8–26, standard deviation 4.5) and the mean follow-up for head and inlay was 39.2 months (12–113, standard deviation 30). The heads used in revision surgery were made of metal (26) and ceramics (25). In 36 cases a ultra-high-molecular-weight polyethylene inlay was taken, in 15 cases a regular PE-inlay. We compared the pre-surgical and follow-up anterior–posterior X-rays.
The mean size of radiolucent areas before revision surgery was 147 sq.mm (5–389 sq.mm, standard deviation 115).
Thirty-nine months (12–113) after surgery, their mean size was 145 sq.mm (7–604 sq.mm, standard deviation 124). Radiolucent zones exceeding 100 sq.mm could be reduced by an average of 28% in 18 out of 29 cases.
The results showed an improvement in 29 out of 51 cases and a stop of progress in one case. According to the findings there may be a benefit for patients with big radiolucent areas.
Keywords: Total hip arthroplasty, Revision surgery, Head, Liner, Inlay, Osteolysis, Radiolucent lines
1. Introduction
Although it is commonly accepted, that wear debris in total joint replacement may cause osteolysis and loosening, a radiological study of the effects on osteolytic processes after changing head and inlay in total hip arthroplasty is missing. Most data available deals with the clinical outcome after revision surgery, so it was tried to assess the radiological changes of the bone-ingrowth of the implant. The aim was to show that head and inlay revision surgery can reduce the growth of osteolytic zones around the stem in uncemented total hip replacement. The questions we wanted to answer were: Can head and inlay revision surgery in patients with osteolytic areas around the femoral component reduce the size of the osteolytic zones? For which patients can we find a benefit?
2. Materials and methods
By using the history of performed surgeries, 178 patients who had to undergo revision of head and inlay between 1999 and 2011, were selected. For the retrospect study it was necessary to exclude patients who had received either a new femoral or acetabular (63) component and those who had suffered from traumatic loosening (8) or infection (14). Further more reasons for exclusion were the second revision of head and inlay (3), removal of the prosthesis (2) and a cemented femoral shaft (1). A short time from primary to revision surgery (7) as well as death (5) led to exclusion from our study.
So 75 patients remained. It was possible to review 47 of them (62.6%, 18 male, 29 female, 27 right, 24 left hips). The mean lifetime for the prosthesis was 13.6 years (8–20, standard deviation 3.7) and the mean follow-up for head and inlay was 15.1 years (8–26, standard deviation 4.5). In the cohort the most common primary head was ceramics (35) followed by metal (11) and the Metasul (Zimmer, Freiburg, Baden-Württemberg, Germany) tribological pairing (5). The heads used in revision surgery were made of metal (DePuy, Warsaw, Indiana, USA, 26; Zimmer Tribosul 2; Zimmer Durasul 15) and ceramics (Zimmer Sulox/Biolox 8). The primary size was 28 mm in 27 cases and 32 mm in 24 cases. In revision surgery a 28 mm head was chosen 28 times and a 32 mm head 23 times. In 15 hips a ultra-high-molecular-weight inlay was taken, in 36 hips a Zimmer Durasul cross-linked ultra-high-molecular-weight one.
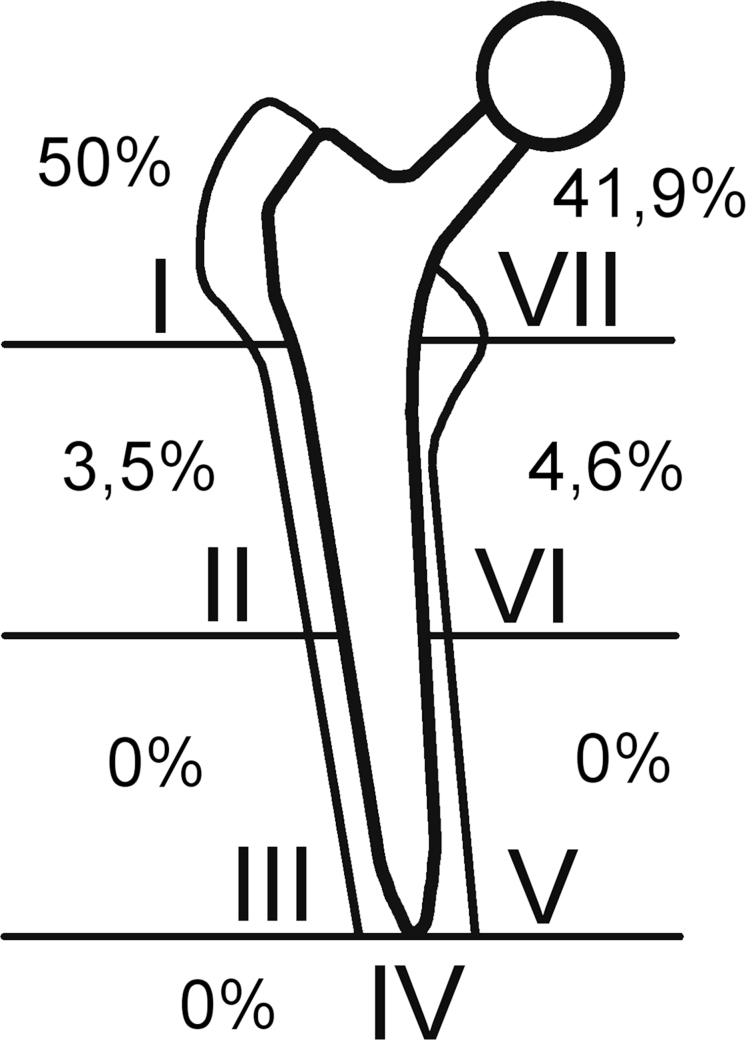
For analyzing the osteolytic zones on the anterior–posterior X-rays, an image editing software called GIMP, version 2.8, was used. Because the aim was to assess the quantity of radiolucent zones around the femoral shaft, it was necessary to find a way to measure their size as accurate as possible. By knowing the size of the ball head and coloring it, it was possible to calculate any other isochromatic stain in the X-ray. Histograms were used to analyze the amount of colored pixels which was needed to cover the ball head and the radiolucent zones. Then the size of the osteolysis could be easily calculated. The X-rays taken before and after revision surgery were compared and the change of size of the periprosthetic radiolucent zones was analyzed. The results were double-checked and it was noticed, that the measurement error did not exceed five percent in any case. For describing the localization of the osteolytic zones the classification of Gruen et al.1 was used (Fig. 1).
Fig. 1.
The majority of the radiolucent areas was, as expected, evident in the Gruen zones 1 and 7.
2.1. Statistical methods
The arithmetic average size was used to compare the preoperative size of radiolucent areas to the postoperative. The standard deviation for both numbers was calculated.
3. Results
Although we did not have the chance to get volumetric data of the osteolytic areas, we were able to find an interesting development in our cohort on follow-up.
The pre-revision anterior–posterior X-rays, on average taken nine years after implantation, showed osteolytic zones with a wide-spread size. Their mean size was 147 mm2 (5–389 mm2, standard deviation 115).
Fifty-four months (12–113) after surgery, their mean size was 145 mm2 (7–604 mm2, standard deviation 124). In both cases the enormous standard deviation proves the broad distribution.
The mean relative enlargement of the osteolytic zones was 10.5% (−86% to +305%). In 29 cases (57%) a reduction of radiolucent areas could be found, in 22 cases (44%) an increase was evident (Figs. 2 and 3).
Figs. 2 and 3.
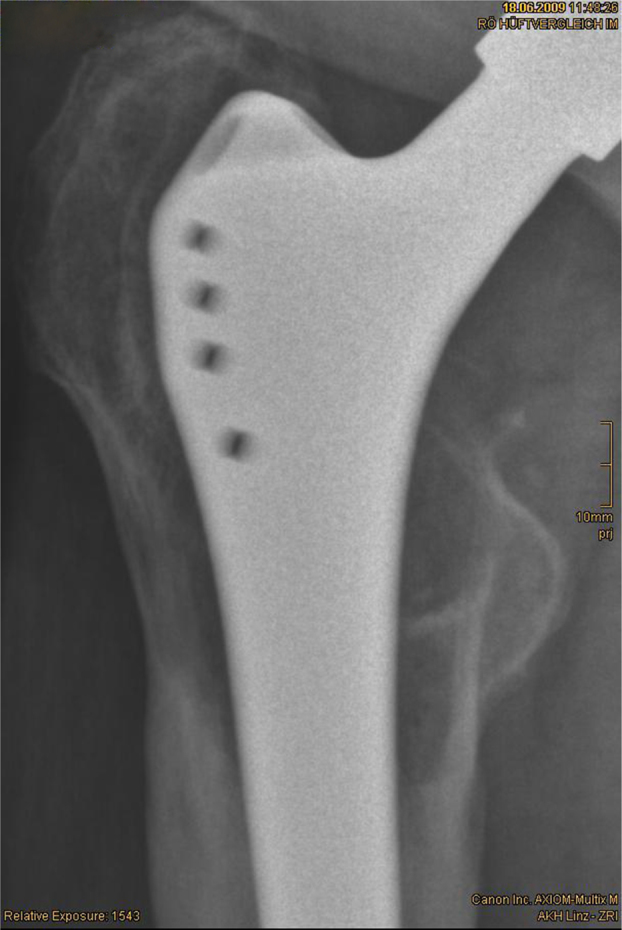
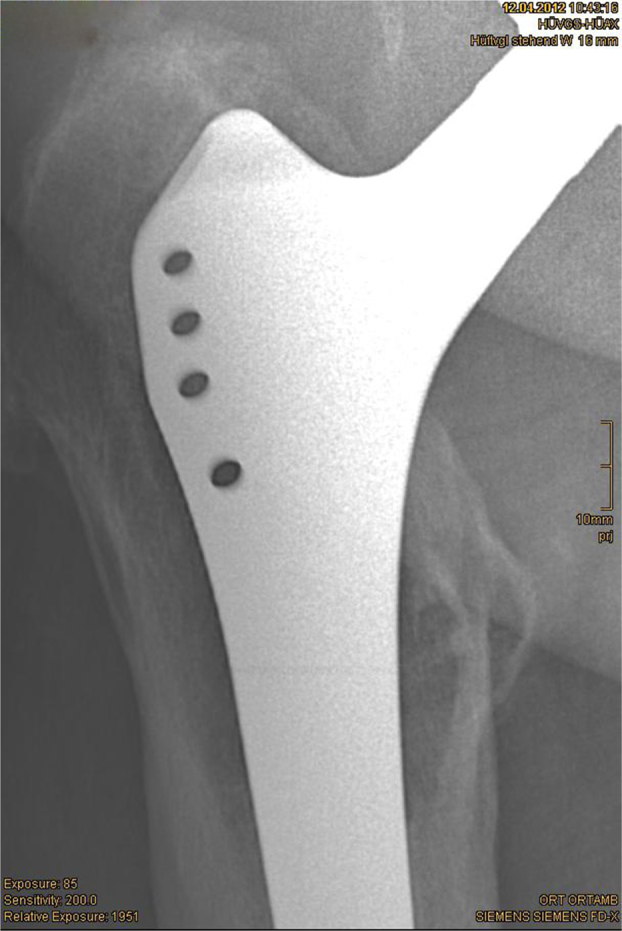
Reduction of osteolytic zones by 46% within 33 months.
Due to our small cohort, it was not possible to find any relation between material or size of the femoral head and the progression of osteolysis.
No relationship between the lateral opening of the acetabular component and the rate of osteolysis, as Schmalzried et al.2 described it, was found.
4. Discussion
Schmalzried et al. showed that there is a relationship between polyethylene debris particles, the macrophages and the presence of osteolytic zones.3 Apart from that, his study made clear, that wear particles may be found far away from the articular surface.
Margevicius et al.4 as well as Maloney et al.5 tried to characterize the quality, the quantity and the size of wear particles around total hip prostheses and showed that the amount of debris in failed arthroplasty is often underestimated.
According to Dattani,6 reasons for radiolucent zones around the femoral component in THA may be aging, adaptive bone remodeling, migration of the prosthesis, fluid pressure and wear debris. In combination with the other reasons mentioned above, debris, whether an ionic one from metal-on-metal tribological pairings, polyethylene, or cement, may lead to a release of proinflammatory mediators and the activation of osteoclasts.7
Oparaugo et al.8 showed a direct correlation between volumetric wear-rates, incidence of osteolysis and revision rates. Although we could not compare volumetric wear-rates to the size of osteolytic zones and their development after revision surgery, it is likely that the early interruption of tribological effects may avoid replacement of the whole prosthesis.
Since wear debris may be the most likely reason for osteolysis in prosthetic surgery, it was possible to show that changing the weight bearing and articulating parts of the prostheses can stop or even reverse the progress of bone loss in certain cases. 47 patients at an average of 39.2 months after they had had revision surgery could be reviewed, which showed an improvement of the situation in 29 out of 51 cases. Despite the little number of patients, the interval between surgery and follow-up gave us the chance to observe the developments very accurately and get reliable data.
Due to our findings in cases with large osteolytic areas, which showed an improvement in 29 out of 51 cases we believe that the revision surgery and change of the weight-bearing parts is a reasonable way to avoid a worse situation for the patient.
As expected, osteolytic areas were more often found in the proximal Gruen zones.
The main problem was to find a way to measure the osteolytic zones properly. By having the femoral head as a reliable reference it was possible to evaluate any area on a radiograph obtaining exact numbers. Using a freeware tool seemed to be the easiest way of calculation. One source for measurement errors could be the malalignment of the leg when taking the X-ray, which may lead to occultation of radiolucent areas. It is believed, that this is the main reason for the limitation of the study. There are only two ways to avoid that: It is either necessary to take the radiograph with a X-ray TV system, which makes it possible to check the position of the implant properly or to use a CAT scan. The CAT scan is definitely the most exact way to calculate osteolysis9 because it is possible to evaluate volumetric data and not only plane surfaces. Despite those possible sources for errors, the methods are on the one hand less burdensome for the patient and on the other hand easy to handle and exact enough to obtain reliable data.
There have not been any complications in the cohort after revision surgery. Even those patients, whose radiograph showed heavy bone loss, did not appear clinically affected.
There is definitely a need for further studies on this topic, especially to make it possible to assess the different materials for head and inlay in revision surgery.
Although the study is limited due to the described problems with measuring the osteolytic zones, the results showed an improvement of the situation in 29 out of 51 cases and a stop of progress in one case, which makes the authors assume that there may be a benefit for the survival rate of the implant, especially in patients with a high rate of osteolysis. It is necessary to keep in mind that wear is a function of use, not time10 with the consequence of a close follow-up in patients who are more active in order to avoid the need of full revision surgery.
Ethical approval
The conducted research (in retrospect performed radiological research) is not related to either human or animals use.
Conflicts of interest
The authors have none to declare.
Contributor Information
Lorenz Pisecky, Email: lorenz.pisecky@kepleruniklinikum.at.
Günter Hipmair, Email: guenter.hipmair@kepleruniklinikum.at.
Bernhard Schauer, Email: bernhard.schauer@kepleruniklinikum.at.
Nikolaus Böhler, Email: nikolaus.boehler@kepleruniklinikum.at.
References
- 1.Gruen T.A., McNeice G.M., Amstutz H.C. “Modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17–27. [PubMed] [Google Scholar]
- 2.Schmalzried T.P., Guttmann D., Grecula M., Amstutz H.C. The relationship between the design, position, and articular wear of acetabular components inserted without cement and the development of pelvic osteolysis. J Bone Joint Surg Am. 1994;76:5. doi: 10.2106/00004623-199405000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Schmalzried T.P., Jasty M., Harris W.H. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am. 1992;74(6):849–863. [PubMed] [Google Scholar]
- 4.Margevicius K.J., Bauer T.W., McMahon J.T., Brown S.A., Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76(11):1664–1675. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Maloney W.J., Smith R.L., Schmalzried T.P., Chiba J., Huene D., Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77(9):1301–1310. doi: 10.2106/00004623-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Dattani R. Femoral osteolysis following total hip replacement. Postgrad Med J. 2007;83(979):312–316. doi: 10.1136/pgmj.2006.053215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purdue P.E., Koulouvaris P., Nestor B.J., Sculco T.P. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2(2):102–113. doi: 10.1007/s11420-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oparaugo P.C., Clarke I.C., Malchau H., Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72(1):22–28. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- 9.Walde T.A., Weiland D.E., Leung S.B. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res. 2005;(437):138–144. doi: 10.1097/01.blo.0000164028.14504.46. [DOI] [PubMed] [Google Scholar]
- 10.Schmalzried T.P., Shepherd E.F., Dorey F.J. The John Charnley Award. Wear is a function of use, not time. Clin Orthop Relat Res. 2008;(381):36–46. doi: 10.1097/00003086-200012000-00005. [DOI] [PubMed] [Google Scholar]