Abstract
The role of natural killer (NK) cell function in HIV disease especially in the setting of long-term antiretroviral therapy (ART) and viral suppression is not fully understood. In the current study, we have investigated NK cell activation in healthy controls and aviremic ART-treated HIV+ subjects with different degrees of immune restoration. We performed a cross sectional study in 12 healthy controls and 24 aviremic ART-treated HIV-infected subjects including 13 HIV+ subjects with CD4+ T cells above 500 cells/μL defined as “immunologic responders” and 11 HIV+ subjects with CD4+ T cells below 350 cells/μL defined as “immunologic non-responders”. We analyzed NK cell number, subset, and activation by expression of CD107a and NKG2D and co-expression of CD38 and HLA-DR. NK cell-mediated cytotoxicity against uninfected CD4+ T cells was tested in vitro. We found that NK cell absolute number, percentage of NK cells, and percentage of NK cell subsets were similar in the three study groups. The increased NK cell activation was found predominantly in CD56dimCD16+ subset of immunologic non-responders but not immunologic responders compared to healthy controls. The activation of NK cells was inversely correlated with the peripheral CD4+ T cell count in HIV+ subjects, even after controlling for chronic T cell activation, sex, and age, potential contributors for CD4+ T cell counts in HIV disease. Interestingly, NK cells from immunologic non-responders mediated cytotoxicity against uninfected CD4+ T cells ex vivo. NK cells may play a role in blunted CD4+ T cell recovery in ART-treated HIV disease.
Introduction
Antiretroviral therapy (ART) dramatically suppresses HIV viral replication, improves immune function, restores CD4+ T cells, increases survival, and delays disease progression in HIV disease [1–3]. However, up to 25% patients fail to reconstitute their CD4+ T cells to the levels similar to healthy controls despite HIV suppression under ART treatment [2, 4, 5]. Inflammatory syndrome, heightened morbidity and mortality, are seen in persons who fail to increase their CD4+ T cell counts under ART treatment [3, 6–10]. Patients under viral-suppressive ART treatment with peripheral CD4+ T cell counts > 500 cells/μl are defined as “immunologic responders” and patients under viral-suppressive ART treatment with peripheral CD4+ T cell counts ≤ 350 cells/μl are defined as “immunologic non-responders” [11–13]. The mechanisms of incomplete CD4+ T cell restoration have been extensively studied, including thymic and lymphoid fibrosis, low nadir CD4+ T cell counts, heightened microbial translocation and inflammation, high T cell activation, and virus-mediated cytopathogenicity [14–23]; but are still not fully understood.
Chronic T cell activation is a predictive marker for peripheral CD4+ T cell count and disease progression in HIV disease [24–26]. Although ART treatment significantly decreases chronic T cell activation, residual activation persists even after many years of ART treatment [27, 28]. The magnitude of T cell activation in this setting is associated with the degrees of CD4+ T cell recovery [29].
Natural killer (NK) cells are a subset of granular lymphocytes that are critical in the innate immunity against infection [30]. They are capable to kill infected cells through cytolysis [31]. Moreover, NK cell mediated antibody-dependent cellular cytotoxicity (ADCC) has been associated with protection from infection and disease progression [32], and is an important mechanism to control HIV infection [33–35]. Human NK cells are commonly defined to two subsets, CD56dimCD16+ and CD56brightCD16− subpopulations [36]. CD56dimCD16+ NK cells predominate in the peripheral blood, and CD56brightCD16− NK cells constitute the majority of NK cells in secondary lymphoid tissues [36]. Chronic HIV infection is associated with reduced proportion and absolute number of CD3−CD56+ NK cells compared to healthy controls [37, 38], as well as increased proportion of CD56dimCD16+ NK cell subset [39, 40]. These alterations of NK cells are largely associated with HIV viral replication in ART-naïve patients [41].
Several markers are associated with NK cell activation and cytolytic function. For example, co-expression of CD38 and HLA-DR is an activation marker on NK cells [42]. CD107a is a marker of lysosomal granule exocytosis [43]. Upon antibody-mediated NK cell activation, CD107a expression increases and mediates cytolytic activity [43, 44]. NKG2D is evolutionary conserved and encoded in human chromosome 12 within the NK cell gene complex [45]. NKG2D has been shown to regulate NK cell cytotoxicity and cytokine production [46]. Notably, increased levels of NK cell activation and functional markers have been reported in HIV disease and are largely controlled by ART treatment [42, 47]. For example, the percentages of IFN-γ, TNF-α, and CD107a-expressing NK cells were significantly higher in ART-naïve patients but were similar in treated patients compared to healthy controls [43]. Moreover, NK cells express Fc receptor CD16, which can bind to IgG1 and IgG3 subclasses [30]. Fc of HIV specific antibodies binds to its receptors on HIV infected cells and triggers cell lysis through Fc receptor on NK cells [31]. However, NK cell cytolysis was impaired and was associated with HIV disease progression [48, 49]. Notably, chronic immune activation has a significant impact on NK cell dysfunction in HIV disease [50]. Nonetheless, the role of NK cells in ART-treated HIV-infected patients with poor CD4+ T cell recovery is not fully understood.
To better understand the role of NK cells in CD4+ T cell recovery after ART treatment, we examined and analyzed NK cell activation in healthy controls, HIV+ immunologic non-responders and HIV+ immunologic responders. We found that NK cells were activated predominantly in CD56dimCD16− subset in immunologic non-responders compared to responders and healthy controls. The activation of NK cells was inversely correlated with peripheral CD4+ T cell counts even after controlling for confounding factors.
Methods
Study subjects
This study was approved by the Institutional Review Board for Human Research (IRB) at the Medical University of South Carolina. All participants were adult ages above 20 and provided written consent. In the current study, 12 healthy controls and 24 HIV+ ART-treated aviremic HIV-infected subjects were evaluated in a cross sectional study. The clinical characteristics of healthy controls, 13 immunologic responders, and 11 immunologic non-responders are shown in Table 1 and S1 Table.
Table 1. Clinical characteristics.
| Healthy control | HIV+/IR | HIV+/INR | P value (IR vs INR) | |
|---|---|---|---|---|
| Total no. of subjects | 12 | 13 | 11 | |
| No. of male/female | 3 ⁄ 9 | 11 ⁄ 2 | 6 ⁄ 5 | 0.08 |
| Age (yr) | 44 (34–58) | 43 (31–49) | 47 (36–56) | 0.21 |
| Plasma HIV RNA | Not detectable | Not detectable | ||
| CD4+ T cell counts | 782 (513–944) | 733 (658–777) | 322 (225–349) | < 0.0001 |
| Years of ART | 15 (13.8–15.0) | 14 (11.0–16.0) | 0.46 | |
| %CD38+HLA-DR+CD4+ | 1.17 (1.0–2.2) | 1.96 (1.2–3.2) | 0.09 | |
| Nadir CD4+ T cell counts | 320 (215–507) | 170 (92–195) | 0.001 |
CD4+ T cell counts (cells/μl).
Plasma HIV RNA (copies/ml).
Data are medians (interquartile ranges).
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated over a Ficoll-Hypaque cushion (GE, Pittsburgh, PA) from EDTA-contained blood, aliquot, and stored at -80°C before use. Antibodies were incubated with PBMC at 4°C for 30 min for surface staining. After surface staining, the cells were washed and analyzed by flow cytometry. The following fluorochrome-labeled monoclonal antibodies were used (clone): anti-human CD3-percp (OKT3), anti-CD56-APC (B-159), anti-CD16-PEcy7 (3G8), anti-human CD4-BV421 (RPA-T4), anti-human CD107a-BV500 (H4A3), anti-human CD38-FITC (HIT2), anti-human HLA-DR-PE (G46-6), anti-human NKG2D-BV500 (1D11), and Ghost Red 780 (Tonbo Biosciences, San Diego, CA). Mouse IgG1-BV500, IgG1-APC and IgG2a-PE isotype antibodies were used to gate on CD107a/NKG2D-BV500, CD38 and HLA-DR respectively. Cells were collected by BD FACSVerse Flow Cytometer (BD Biosciences) and data were analyzed by FlowJo software (Version 10.0.8).
NK cell-mediated CD4+ T cell cytotoxicity
NK cell-mediated CD4+ T cell cytotoxicity was assessed by flow cytometry. Briefly, purified CD4+ T cells from the same healthy control donor were co-cultured with or without purified NK cells at a 1:3 ratio in 96-well V-bottom plates (Corning). Cells were incubated for 15 min at room temperature, spun at 300 g for 1 min, and incubated for 6 h at 37°C. After incubation, cells were surface stained with antibodies against CD3, and fixed with 2% paraformaldehyde solution containing a constant number of flow cytometry particles (5*104/ml) (AccuCount blank particles, 5.3 μm; Spherotech, Lake Forest, IL). A constant number of particles (2.5*103) were counted during cytometry acquisition in order to normalize the number of CD4+ T cells. The percentage of CD4+ T cell cytolysis was calculated using the formula: %cytolysis = [(number of CD3+ T cells in the absence of NK cells)–(number of CD3+ T cells in the presence of NK cells)]/(number of CD3+ T cells in the absence of NK cells) *100.
Statistical analysis
All data were analyzed and graphed using Prism software (GraphPad 6.0) and SPSS (Version 23). Statistical significance between two groups was determined by the Mann-Whitney test (non-parametric). In the pre-specified hypothesis, we were interested in the comparisons of HIV+ immunologic non-responders versus HIV+ immunologic responders or healthy controls; therefore, p-values from comparing HIV+ immunologic non-responders to each of control groups were not adjusted for multiple comparisons [51]. Associations between pairs of continuous variables were analyzed by Spearman Correlation test. Partial correlation (controlling for age, sex, and frequency of CD38+ on CD4+ T cells) between CD4+ T cell counts and NK cell activation was analyzed using SPSS (Version 23).
Results
NK cell number and subsets in healthy controls, HIV-infected immunologic responders, and HIV-infected immunologic non-responders
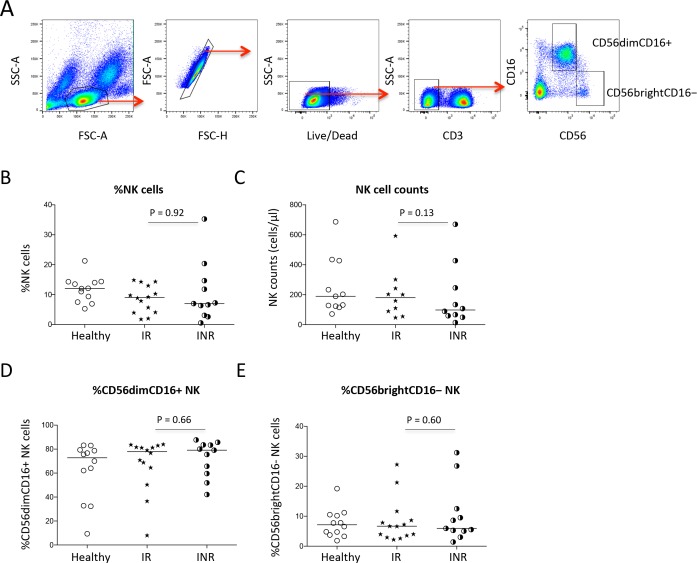
To study NK cells in the setting of HIV+ subjects with different degrees of immune restoration, the absolute cell count and percentages of NK cell and subset (CD56dimCD16+ and CD56brightCD16−) were measured in 12 healthy controls, 11 HIV+ immunologic responders and 13 HIV+ immunologic non-responders patients under viral-suppressive ART treatment. Consistent with previously studies [40], NK cell counts and the percentages of NK cell in PBMC tended to be lower in immunologic non-responders compare to immunologic responders and healthy controls, but the differences did not achieve statistical significance (Fig 1A–1C). The percentages of CD56dimCD16+ and CD56brightCD16− NK cell subpopulations were also similar in the three study groups (Fig 1D and 1E).
Fig 1. NK cells and subsets distribution.
Blood samples were stained with fluorescent-labeled antibodies and tested by flow cytometry. (A) Representative dot plots, showing the gating strategies used to assess NK cells, and CD56dimCD16+ or CD56brightCD16− NK cell subsets. The median frequency (B) and absolute count (C) of NK cells were shown in immunologic non-responders, immunologic responders, and healthy controls. The median frequencies of CD56dimCD16+ (D) or CD56brightCD16− subsets (E) in NK cells were shown in the three study groups.
NK cell activation, CD107a, and NKG2D expression in HIV-infected immunologic non-responders
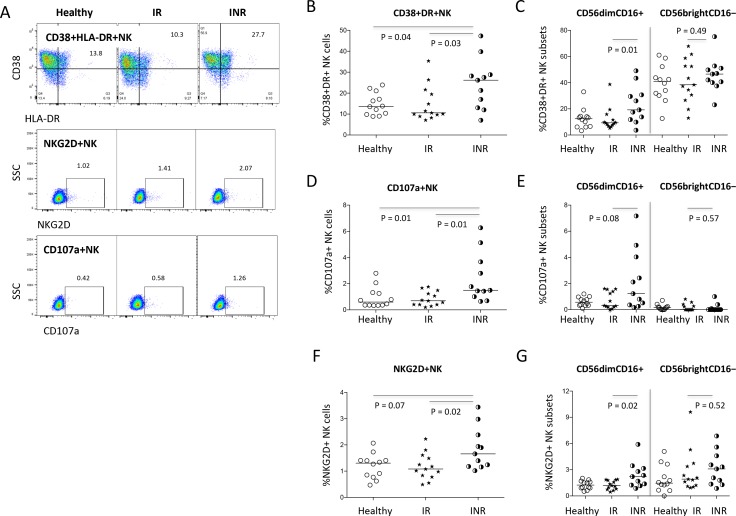
To further determine NK cell activation in HIV+ subjects with different degrees of immune restoration after long-term ART treatment and viral suppression, we analyzed the expression of CD38, HLA-DR, CD107a, and NKG2D on NK cells in the current study. In the current study, co-expression of CD38 and HLA-DR on NK cells in all ART-treated patients was similar compared to healthy controls (P = 0.68, Fig 2A and 2B). However, if HIV+ subjects were stratified into immunologic responders and non-responders, the non-responders had significant higher frequency of CD38 and HLA-DR co-expression compared to responders and healthy controls (Fig 2A and 2B). As expected, CD38+HLA-DR+ co-expression on CD56dimCD16+ NK subset was significantly increased in immunologic non-responders compared to the other two study groups (p < 0.05, Fig 2C). The other NK cell subset, CD56brightCD16−, without expression of the IgG receptor FcRγIIIA (CD16), had similar levels of CD38 and HLA-DR co-expression in the three study groups (P > 0.05, Fig 2C).
Fig 2. NK cell activation in treated HIV disease.
(A) Dot plots represent the gating strategies from one representative donor of each study group. The median frequencies of CD38+HLA-DR+ NK cells (B) and NK cell subsets (C) among healthy controls, immunologic responders, and immunologic non-responders. The median frequencies of CD107a+ on NK cells (D) and NK cell subsets (E) in healthy controls, immunologic responders, and immunologic non-responders. The median frequencies of NKG2D+ on NK cells (F) and NK cell subsets (G) in healthy controls, immunologic responders, and immunologic non-responders.
To analyze NK cell parameters related to cytotoxicity, we have assessed surface CD107a and NKG2D expression on NK cells. CD107a expression on NK cells was elevated in immunologic non-responders compared to immunologic responders and healthy controls (Fig 2D, p < 0.05), while CD107a expression in NK cells was similar in immunologic responders and healthy controls (Fig 2D). Notably, CD107a-expressing NK cells were predominantly in CD56dimCD16+ NK cells but not in CD56brightCD16− NK cells (Fig 2E), suggesting that cytolytic function is enriched in CD56dimCD16+ NK cell subset. Moreover, the frequency of surface NKG2D-expressing NK cells increased in NK cells, especially in the CD56dimCD16+ subset among immunologic non-responders compare to immunologic responders (Fig 2F and 2G); there was no difference of CD107a and NKG2D expression in CD56brightCD16- NK cells among the three study groups (Fig 2E and 2G). These results suggest that CD56dimCD16+ NK cell subset are activated and may have cytolytic function in vivo in aviremic ART-treated HIV+ subjects with incomplete CD4+ T cell recovery.
Correlations of CD38 and HLA-DR co-expression, CD107a, and NKG2D expression on NK cells
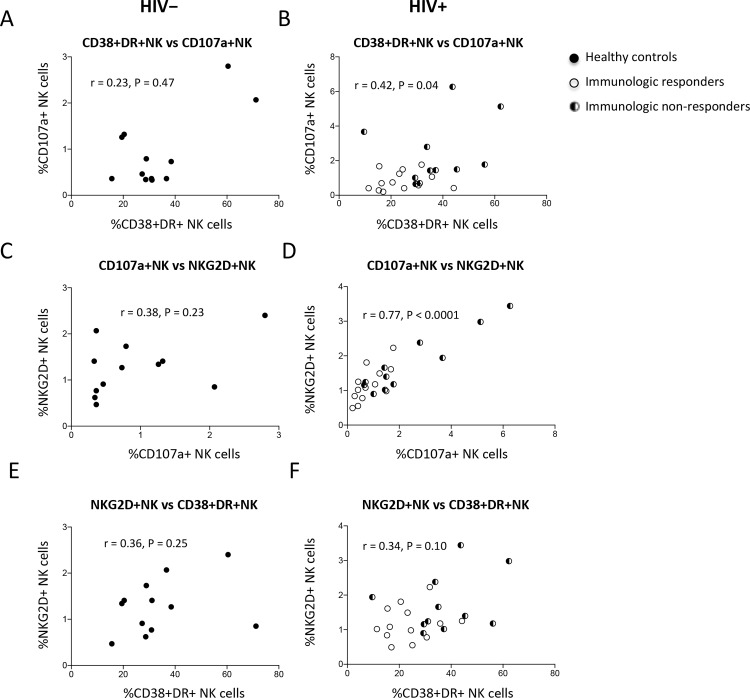
Next, we analyzed the correlations between activation and functional markers on NK cells in healthy controls and HIV+ subjects (Fig 3A–3F). Interestingly, no correlation was found in healthy controls (Fig 3A, 3C, and 3E), but there were direct correlations between the percentages of CD107a-expressing NK cells and co-expression of CD38 and HLA-DR on NK cells (Fig 3B), and between the percentages of CD107a-expressing NK cells and NKG2D-expressing NK cells (Fig 3D) in all HIV+ subjects. The correlation between the percentages of NKG2D-expressing NK cells and co-expression of CD38 and HLA-DR on NK cells in HIV+ subjects tended to correlate, however did not achieve significant difference (Fig 3F). These results suggest that NK cells may be activated to express these activation and functional markers by different mechanisms in healthy individuals but by a similar mechanism in treated HIV-infected patients.
Fig 3. Correlations of NK cell activation in healthy controls and ART-treated HIV disease.
Correlations between the percentages of CD107a-expressing NK cells and co-expression of CD38 and HLA-DR on NK cells in healthy controls (A) and HIV+ subjects (B), between the percentages of CD107a-expressing NK cells and NKG2D-expressing NK cells in healthy controls (C) and HIV+ subjects (D), and between the percentages of NKG2D-expressing NK cells and co-expression of CD38 and HLA-DR on NK cells in healthy controls (E) and HIV+ subjects (F).
Correlation between NK cell activation and CD4+ T cell counts
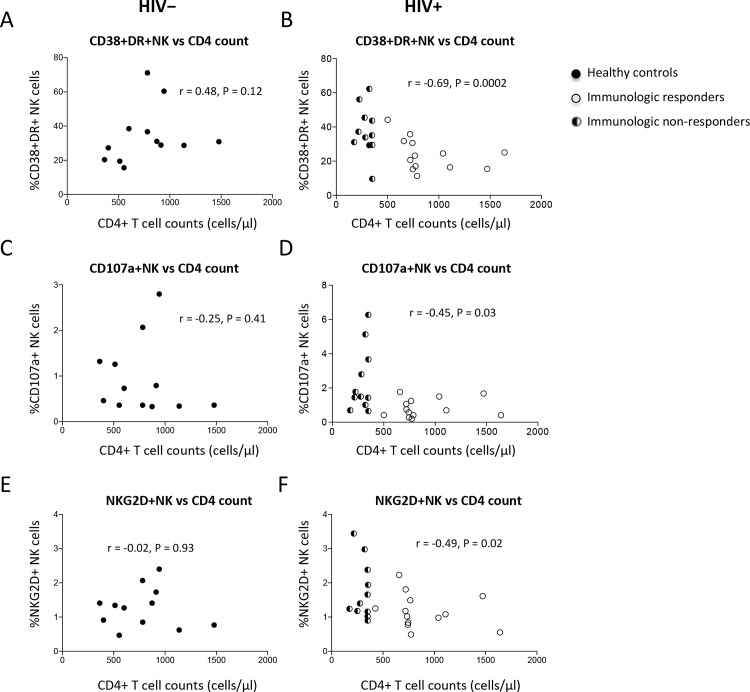
To investigate NK cells in HIV disease, we analyze the correlation between NK cell activation and CD4+ T cell reconstitution after long-term ART treatment and viral suppression in HIV+ subjects. Notably, NK cell activation and function reflected by co-expression of CD38 and HLA-DR, and expression of CD107a and NKG2D, were all inversely correlated with peripheral CD4+ T cell counts in HIV+ subjects, but not in healthy controls (Fig 4). It is well know that chronic T cell activation contributes to CD4+ T cell depletion in chronic HIV infection [52, 53], and sex and age are associated with CD4+ T cell counts [54, 55]; we therefore have analyzed the inverse correlation between NK activation and CD4+ T cell counts after controlling these potential contributors. Interestingly, the correlation between CD4+ T cell count and the percentage of CD38 and HLA-DR co-expression on NK cells (r = -0.48, P = 0.03) was still significant, but neither the correlation between CD4+ T cell count and the percentage of CD107a-expressing (r = -0.34, P = 0.20) nor the correlation between CD4+ T cell count and NKG2D-expressing (r = -0.40, P = 0.13) NK cells was significant in HIV+ subjects after controlling of age, sex, and CD4+ T cell activation. These results suggest that long-term ART treatment did not fully normalize NK cell activation, and NK cell activation is associated with CD4+ T cell reconstitution.
Fig 4. NK cell activation and peripheral CD4+ T cell counts.
Correlations between the percentages of co-expression of CD38 and HLA-DR on NK cells and CD4+ T cell counts in healthy controls (A) and HIV+ subjects (B), between the percentages of CD107a-expressing NK cells and CD4+ T cell counts in healthy controls (C) and HIV+ subjects (D), and between the percentages of NKG2D-expressing NK cells and CD4+ T cell counts in healthy controls (E) and HIV+ subjects (F).
Correlations between NK cell subset activation and CD4+ T cell counts in viral-suppressed and ART-treated HIV+ subjects
To determine which NK cell subpopulation is associated with CD4+ T cell recovery, we analyzed the correlations between the percentages of activation in NK cell subsets and CD4+ T cell counts (Table 2). Interestingly, we found that the frequency of NKG2D expression and CD38 and HLA-DR co-expression on CD56dimCD16+ NK cells was significantly correlated with CD4+ T cell counts (Table 2). Furthermore, after controlling of CD4+ T cell activation, sex and age, the correlation between CD4+ T cell counts and co-expression of CD38 and HLA-DR on CD56dimCD16+ NK cell subsets was still significant (r = -0.48, P = 0.03), but the correlation between CD4+ T cell counts and NKG2D (r = -0.31, P = 0.18) or CD107a (r = -0.23, P = 0.33) expression in CD56dimCD16+ NK cell subsets was not significant in HIV+ subjects. Together these data reveal that the activation and function of CD56dimCD16+ NK cell subset were associated with CD4+ T cell recovery in viral-suppressed and long-term ART-treated HIV disease.
Table 2. Correlations between NK cell subset activation and CD4+ T cell counts in HIV+ subjects.
| NK subset activation | %CD107a+ NK | %NKG2D+ NK | %CD38+DR+NK |
|---|---|---|---|
| CD4 count vs CD56dimCD16+ | r = -0.28 | r = -0.48 | r = -0.66 |
| P = 0.17 | P = 0.02 | P < 0.0001 | |
| CD4 count vs CD56brightCD16− | r = 0.08 | r = -0.23 | r = -0.33 |
| P = 0.63 | P = 0.18 | P = 0.05 |
r: correlation coefficient.
P: P value.
Cytotoxicity of NK cells from immunologic non-responders
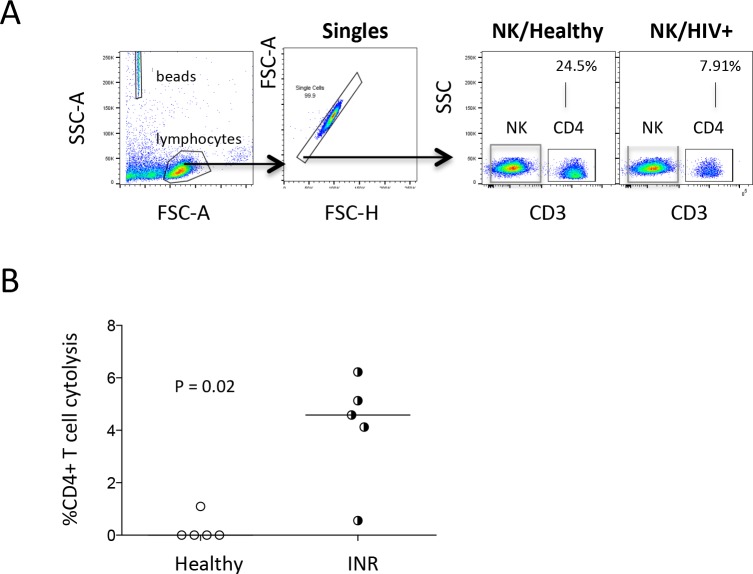
Cytotoxic or activated NK cells have been found to accumulate in lymph nodes after HIV/SIV infection [56, 57]. To determine whether NK cells from HIV+ immune non-responders were activated to gain the function of cytotoxicity against CD4+ T cells, we have isolated NK cells ex vivo and co-cultured with CD4+ T cells from the same healthy control donor. NK cell-mediated cytotoxicity was assessed by flow cytometry. Notably, activated NK cells have been shown to express CD4 [58], we thus used CD3 antibody to stain CD4+ T cells in the co-cultured system containing NK cells and CD4+ T cells to avoid miscalculation of CD4+ T cells. Interestingly, NK cells from immunologic non-responders but not from healthy controls had the ability to induce CD4+ T cell death (Fig 5), suggesting that NK cells from immunologic non-responders may be activated and cytotoxic to induce uninfected CD4+ T cell death in immunologic non-responders in vivo. These results suggest that NK cells may play a role in CD4+ T cell depletion in HIV disease.
Fig 5. NK cells from immunologic non-responders mediate CD4+ T cell death in vitro.
NK cells were isolated from 5 healthy controls and 5 ART-treated viral-suppressed immunologic non-responders. CD4+ T cells were isolated from the same healthy donor. CD4+ T cells were cultured with isolated NK cells at a ratio of 1:3. The percentage of CD4+ T cell cytolysis was analyzed. (A) Dot plots were shown from a representative healthy control donor and a representative HIV+ donor. (B) The median percentages of CD4+ T cell cytolysis were shown with co-cultured NK cells from healthy controls and immunologic non-responders.
Discussion
In this study, we found that long-term ART treatment and viral suppression controls NK cell activation in HIV-infected immunologic responders to the level similar to healthy controls. However, HIV-infected immunologic non-responders still exhibit elevated NK cell activation, and the magnitude of NK cell activation, CD107a, and NKG2D expression were inversely related to CD4+ T cell counts in HIV+ subjects.
The results on the role of NK cell function in HIV disease are conflicting [31, 33, 35, 42, 47, 59]. NK cells are shown to control HIV replication and kill HIV-infected cells through ADCC [60]. On the other hand, NK cells have been shown to associate with HIV disease pathogenesis. For example, in untreated HIV disease, NK cell activation was correlated with CD4+ T cell counts, plasma level of HIV RNA, T cell activation, plasma sCD14 and inflammation [41, 42]. However, in another study in ART-treated and untreated patients, neither plasma level of HIV RNA nor CD4+ T cell counts was correlated with NK cell activation [47]. Notably, NK cells from long-term non-progressors who naturally control viremia have greater NK cell function compared to progressors and healthy controls [61, 62]. Nonetheless, NK cell function is impaired during HIV infection [33]. The possible mechanisms include the followings: 1) HIV virus induces activation and dysfunction of NK cells; 2) decreased levels of perforin and granzyme A in NK cells may account for impaired NK cytotoxicity [63]; 3) NK cells are activated in vivo in HIV disease (Figs 2 and 5), therefore they are desensitized to be re-stimulated and function as ADCC in vitro; 4) DC and NK cell cross-talk is dysregulated through IL-12/IL-15 during HIV infection [64, 65]; and 5) changes in NK cell subsets in untreated HIV disease may play a role in their function. Most studies on NK cell dysfunction were among high viremia and untreated patients [38, 42]. In the current study, we focus on the role of NK cells in CD4+ T cell recovery among ART-treated and viral-suppressed HIV-infected patients.
CD56brightCD16− NK cell subset is the majority of NK cells in lymph nodes [36]; they do not have strong cytotoxic activity but produce cytokines such as IFN-γ and TNF-α upon activation [30]. They may be precursors of CD56dimCD16+ NK cells [36], previously research shows that NK cells in HIV-1-infected patients from Ugandans display elevated activation, and low CD4+ T cell counts were associated with increased levels of IFN-γ and degranulation in CD56bright NK cells [66]. CD56dimCD16+ NK cell subset is the predominant NK cell subset in the periphery; they express FcγR IIIa (CD16) and enable NK cell cytotoxicity [30]. In HIV/SIV infection, NK cells have impaired function of cytotoxicity and IFN-γ production [59, 61]. Notably, CD56dimCD16+ subset has been shown to play a role in HIV pathogenesis [61]. Consistently, we found that levels of NK cell activation and functional markers increased in CD56dimCD16+ NK cells from immunologic non-responders but not responders compared to healthy controls, and that NK cell activation and functional markers in CD56dimCD16+ NK cells was inversely correlated with CD4+ T cell counts in aviremic ART-treated HIV+ subjects.
NK cell activation has been correlated with plasma level of soluble CD14, a factor associated with microbial translocation in one study [42], but not in another study [47]. The drivers of NK cell activation in immunologic non-responders in viral-suppressed HIV disease are not known. The virus still actively replicates in lymph nodes [67–69] in patients (most likely immunologic non-responders) even after long-term plasma viral-suppressive ART treatment [70–72], thus the virus may play a role in NK cell activation in lymph nodes. Furthermore, antibodies may contribute to NK cell activation due to their ability to activate NK cells via Fc receptor and mediate cell death through NK cell cytotoxicity [30]. Altogether, the residual active HIV replication in lymph nodes, inflammation, or antibodies may play a role in NK cell activation among immunologic non-responders in treated HIV disease.
The mechanisms of blunted CD4+ T cell restoration after long-term viral-suppressive ART treatment is not fully understood. Thymic output is measured by the T cell receptor excision circle (TREC) assay, but results are inconclusive [73–77]. Increased T cell activation and expansion result in decreased TREC numbers, which has been linked to increased T cell activation and expansion rather than impaired thymic output in HIV [77, 78]. Other factors such as sustained increases in microbial translocation and inflammation, low nadir and pre-ART naïve CD4+ T cell counts, and high T cell activation, have been associated with CD4+ T cell recovery post-ART treatment [14, 52, 79–84]. In the current study, we found that markers of NK cell activation and function were inversely related to CD4+ T cell counts even after controlling of T cell activation, sex, and age. Isolated NK cells from immunologic non-responders induced CD4+ T cell death from the healthy donor in vitro. Our results reveal a novel mechanism that NK cell activation may play a role in blunted CD4+ T cell recovery in ART-treated HIV disease.
Supporting Information
(PDF)
Acknowledgments
This work was supported by NIH grants AI091526 and AI128864, the University of Alabama at Birmingham, Center for AIDS Research P30 AI027767, and the 12th Five Year Research Project of People's Liberation Army (CWS11J160).
Data Availability
All relevant data are within the paper and the Supporting Information file.
Funding Statement
This work was supported by NIH grants AI091526 (Wei Jiang), the University of Alabama at Birmingham, Center for AIDS Research P30 AI027767 (Sonya Heath), and the 12th Five Year Research Project of People's Liberation Army (CWS11J160) (Lei Huang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. The Lancet. 2010;375(9731):2092–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocroft A, Phillips AN, Gatell J, Ledergerber B, Fisher M, Clumeck N, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. The Lancet. 2007;370(9585):407–13. [DOI] [PubMed] [Google Scholar]
- 3.Battegay M, Nüesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. The Lancet infectious diseases. 2006;6(5):280–7. 10.1016/S1473-3099(06)70463-7 [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, Determinants, and Clinical Relevance of CD4 T Cell Recovery to< 500 Cells/μL in HIV Type 1—Infected Individuals Receiving Potent Antiretroviral Therapy. Clinical infectious diseases. 2005;41(3):361–72. 10.1086/431484 [DOI] [PubMed] [Google Scholar]
- 5.Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends Immunol. 2011;32(3):131–7. 10.1016/j.it.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS (London, England). 2008;22(7):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El-Sadr W, Lundgren JD, Neaton J, Gordin F, Abrams D, Arduino R, et al. CD4+ count-guided interruption of antiretroviral treatment. New Engl J Med. 2006;355(22):2283–96. 10.1056/NEJMoa062360 [DOI] [PubMed] [Google Scholar]
- 8.Silverberg MJ, Neuhaus J, Bower M, Gey D, Hatzakis A, Henry K, et al. Risk of cancers during interrupted antiretroviral therapy in the SMART study. Aids. 2007;21(14):1957–63. 10.1097/QAD.0b013e3282ed6338 [DOI] [PubMed] [Google Scholar]
- 9.Lapadula G, Cozzi-Lepri A, Marchetti G, Antinori A, Chiodera A, Nicastri E, et al. Risk of clinical progression among patients with immunological nonresponse despite virological suppression after combination antiretroviral treatment. Aids. 2013;27(5):769–79. 10.1097/QAD.0b013e32835cb747 [DOI] [PubMed] [Google Scholar]
- 10.Group DCoAEoA-HDS. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS (London, England). 2008;22(16):2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley CF, Kitchen CM, Hunt PW, Rodriguez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clinical Infectious Diseases. 2009;48(6):787–94. 10.1086/597093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clinical Infectious Diseases. 2007;44(3):441–6. 10.1086/510746 [DOI] [PubMed] [Google Scholar]
- 13.Bandera A, Mangioni D, Incontri A, Perseghin P, Gori A. Characterization of Immune Failure by Monocyte Activation Phenotypes in HIV-Infected Patients Receiving Antiretroviral Therapy. Journal of Infectious Diseases. 2015:jiv166. [DOI] [PubMed] [Google Scholar]
- 14.Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. The Journal of infectious diseases. 2011;204(8):1217–26. PubMed Central PMCID: PMC3218674. 10.1093/infdis/jir507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–33. PubMed Central PMCID: PMC3466592. 10.1086/524143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, et al. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143(5):789–801. PubMed Central PMCID: PMC3026834. 10.1016/j.cell.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12(3):289–95. 10.1038/nm1380 [DOI] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- 19.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505(7484):509–14. PubMed Central PMCID: PMC4047036. 10.1038/nature12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434(7037):1093–7. 10.1038/nature03501 [DOI] [PubMed] [Google Scholar]
- 21.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013;254(1):54–64. PubMed Central PMCID: PMC3729334. 10.1111/imr.12066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwata T, Nishimura Y, Whitted S, Ourmanov I, Brown CR, Dang Q, et al. Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS pathogens. 2009;5(4):e1000372 PubMed Central PMCID: PMC2662887. 10.1371/journal.ppat.1000372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature medicine. 2006;12(12):1365–71. 10.1038/nm1511 [DOI] [PubMed] [Google Scholar]
- 24.Fahey JL, Taylor JM, Manna B, Nishanian P, Aziz N, Giorgi JV, et al. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T‐cell measurements. Aids. 1998;12(13):1581–90. [DOI] [PubMed] [Google Scholar]
- 25.Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, et al. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. New England Journal of Medicine. 1990;322(3):166–72. 10.1056/NEJM199001183220305 [DOI] [PubMed] [Google Scholar]
- 26.Medina E, Borthwick NJ, Sabin CA, Timms A, Winter M, Baptista L, et al. Increased number of primed activated CD8+ CD38+ CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. Aids. 1996;10(8):827–34. [DOI] [PubMed] [Google Scholar]
- 27.Mohri H, Perelson AS, Tung K, Ribeiro RM, Ramratnam B, Markowitz M, et al. Increased turnover of T lymphocytes in HIV-1 infection and its reduction by antiretroviral therapy. The Journal of experimental medicine. 2001;194(9):1277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazenberg MD, Stuart JWC, Otto SA, Borleffs JC, Boucher CA, de Boer RJ, et al. T-cell division in human immunodeficiency virus (HIV)-1 infection is mainly due to immune activation: a longitudinal analysis in patients before and during highly active antiretroviral therapy (HAART). Blood. 2000;95(1):249–55. [PubMed] [Google Scholar]
- 29.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. Journal of Infectious Diseases. 2003;187(10):1534–43. 10.1086/374786 [DOI] [PubMed] [Google Scholar]
- 30.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9. PubMed Central PMCID: PMCPMC2481557. 10.1182/blood-2007-09-077438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844 PubMed Central PMCID: PMCPMC4782064. 10.1038/ncomms10844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, et al. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol. 2011;85(14):6906–12. PubMed Central PMCID: PMCPMC3126600. 10.1128/JVI.00326-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, et al. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature. 2011;476(7358):96–100. PubMed Central PMCID: PMC3194000. 10.1038/nature10237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gooneratne SL, Richard J, Lee WS, Finzi A, Kent SJ, Parsons MS. Slaying the Trojan horse: natural killer cells exhibit robust anti-HIV-1 antibody-dependent activation and cytolysis against allogeneic T cells. Journal of virology. 2015;89(1):97–109. Epub 2014/10/17. PubMed Central PMCID: PMC4301139. 10.1128/JVI.02461-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko SY, et al. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol. 2012;86(16):8672–80. PubMed Central PMCID: PMC3421757. 10.1128/JVI.00287-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126(4):458–65. PubMed Central PMCID: PMCPMC2673358. 10.1111/j.1365-2567.2008.03027.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azzoni L, Rutstein RM, Chehimi J, Farabaugh MA, Nowmos A, Montaner LJ. Dendritic and natural killer cell subsets associated with stable or declining CD4+ cell counts in treated HIV-1-infected children. J Infect Dis. 2005;191(9):1451–9. 10.1086/429300 [DOI] [PubMed] [Google Scholar]
- 38.Eger KA, Unutmaz D. Perturbation of natural killer cell function and receptors during HIV infection. Trends Microbiol. 2004;12(7):301–3. 10.1016/j.tim.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 39.Tarazona R, Casado JG, Delarosa O, Torre-Cisneros J, Villanueva JL, Sanchez B, et al. Selective depletion of CD56(dim) NK cell subsets and maintenance of CD56(bright) NK cells in treatment-naive HIV-1-seropositive individuals. J Clin Immunol. 2002;22(3):176–83. [DOI] [PubMed] [Google Scholar]
- 40.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79(19):12365–74. Epub 2005/09/15. 79/19/12365 [pii].PubMed Central PMCID: PMC1211534. 10.1128/JVI.79.19.12365-12374.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bächle SM, Malone DF, Buggert M, Karlsson AC, Isberg P-E, Biague AJ, et al. Elevated levels of invariant natural killer T-cell and natural killer cell activation correlate with disease progression in HIV-1 and HIV-2 infections. AIDS (London, England). 2016;30(11):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuri-Cervantes L, de Oca GS, Avila-Rios S, Hernandez-Juan R, Reyes-Teran G. Activation of NK cells is associated with HIV-1 disease progression. J Leukoc Biol. 2014;96(1):7–16. 10.1189/jlb.0913514 [DOI] [PubMed] [Google Scholar]
- 43.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22. 10.1016/j.jim.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 44.Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G. Relationship between CD107a expression and cytotoxic activity. Cellular immunology. 2009;254(2):149–54. 10.1016/j.cellimm.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 45.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285(5428):727–9. [DOI] [PubMed] [Google Scholar]
- 46.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nature immunology. 2003;4(6):557–64. 10.1038/ni929 [DOI] [PubMed] [Google Scholar]
- 47.Lichtfuss GF, Cheng WJ, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J Immunol. 2012;189(3):1491–9. 10.4049/jimmunol.1200458 [DOI] [PubMed] [Google Scholar]
- 48.Ahmad A, Menezes J. Antibody-dependent cellular cytotoxicity in HIV infections. The FASEB journal. 1996;10(2):258–66. [DOI] [PubMed] [Google Scholar]
- 49.Brenner BG, Gryllis C, Wainberg MA. Role of antibody-dependent cellular cytotoxicity and lymphokine-activated killer cells in AIDS and related diseases. Journal of leukocyte biology. 1991;50(6):628–40. [DOI] [PubMed] [Google Scholar]
- 50.Lichtfuss GF, Cheng W-J, Farsakoglu Y, Paukovics G, Rajasuriar R, Velayudham P, et al. Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. The Journal of Immunology. 2012;189(3):1491–9. 10.4049/jimmunol.1200458 [DOI] [PubMed] [Google Scholar]
- 51.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 52.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. The Journal of infectious diseases. 2003;187(10):1534–43. 10.1086/374786 [DOI] [PubMed] [Google Scholar]
- 53.Marchetti G, Bellistri GM, Borghi E, Tincati C, Ferramosca S, La Francesca M, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. Aids. 2008;22(15):2035–8. 10.1097/QAD.0b013e3283112d29 [DOI] [PubMed] [Google Scholar]
- 54.Asher I, Guri KM, Elbirt D, Bezalel SR, Maldarelli F, Mor O, et al. Characteristics and Outcome of Patients Diagnosed With HIV at Older Age. Medicine (Baltimore). 2016;95(1):e2327. PubMed Central PMCID: PMCPMC4706254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dou Z, Xu J, Jiao JH, Ma Y, Durako S, Yu L, et al. Gender difference in 2-year mortality and immunological response to ART in an HIV-infected Chinese population, 2006–2008. PloS one. 2011;6(8):e22707 PubMed Central PMCID: PMCPMC3156700. 10.1371/journal.pone.0022707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taborda NA, Gonzalez SM, Alvarez CM, Correa LA, Montoya CJ, Rugeles MT. Higher Frequency of NK and CD4+ T-Cells in Mucosa and Potent Cytotoxic Response in HIV Controllers. PloS one. 2015;10(8):e0136292 10.1371/journal.pone.0136292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafer JL, Li H, Evans TI, Estes JD, Reeves RK. Accumulation of Cytotoxic CD16+ NK Cells in Simian Immunodeficiency Virus-Infected Lymph Nodes Associated with In Situ Differentiation and Functional Anergy. Journal of virology. 2015;89(13):6887–94. PubMed Central PMCID: PMCPMC4468491. 10.1128/JVI.00660-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bernstein HB, Plasterer MC, Schiff SE, Kitchen CM, Kitchen S, Zack JA. CD4 expression on activated NK cells: ligation of CD4 induces cytokine expression and cell migration. J Immunol. 2006;177(6):3669–76. [DOI] [PubMed] [Google Scholar]
- 59.He X, Li D, Luo Z, Liang H, Peng H, Zhao Y, et al. Compromised NK cell-mediated antibody-dependent cellular cytotoxicity in chronic SIV/SHIV infection. PLoS One. 2013;8(2):e56309 PubMed Central PMCID: PMCPMC3570461. 10.1371/journal.pone.0056309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kottilil S, Chun TW, Moir S, Liu S, McLaughlin M, Hallahan CW, et al. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J Infect Dis. 2003;187(7):1038–45. 10.1086/368222 [DOI] [PubMed] [Google Scholar]
- 61.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106(10):3366–9. 10.1182/blood-2005-03-1100 [DOI] [PubMed] [Google Scholar]
- 62.Vieillard V, Fausther-Bovendo H, Samri A, Debre P, French Asymptomatiques a Long Terme A-COSG. Specific phenotypic and functional features of natural killer cells from HIV-infected long-term nonprogressors and HIV controllers. J Acquir Immune Defic Syndr. 2010;53(5):564–73. 10.1097/QAI.0b013e3181d0c5b4 [DOI] [PubMed] [Google Scholar]
- 63.Portales P, Reynes J, Pinet V, Rouzier-Panis R, Baillat V, Clot J, et al. Interferon-alpha restores HIV-induced alteration of natural killer cell perforin expression in vivo. AIDS. 2003;17(4):495–504. 10.1097/01.aids.0000050816.06065.b1 [DOI] [PubMed] [Google Scholar]
- 64.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11(3):176–86. PubMed Central PMCID: PMCPMC3278081. 10.1038/nri2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101(47):16606–11. PubMed Central PMCID: PMCPMC534504. 10.1073/pnas.0407522101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eller MA, Eller LA, Ouma BJ, Thelian D, Gonzalez VD, Guwatudde D, et al. Elevated natural killer cell activity despite altered functional and phenotypic profile in Ugandans with HIV-1 clade A or clade D infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;51(4):380–9. 10.1097/QAI.0b013e3181aa256e [DOI] [PubMed] [Google Scholar]
- 67.Folkvord JM, Armon C, Connick E. Lymphoid follicles are sites of heightened human immunodeficiency virus type 1 (HIV-1) replication and reduced antiretroviral effector mechanisms. AIDS research and human retroviruses. 2005;21(5):363–70. 10.1089/aid.2005.21.363 [DOI] [PubMed] [Google Scholar]
- 68.Estaquier J, Hurtrel B. [Mesenteric lymph nodes, a sanctuary for the persistance of HIV. Escape mechanisms]. Medecine sciences: M/S. 2008;24(12):1055–60. Epub 2009/01/01. 10.1051/medsci/200824121055 [DOI] [PubMed] [Google Scholar]
- 69.Heesters BA, Lindqvist M, Vagefi PA, Scully EP, Schildberg FA, Altfeld M, et al. Follicular Dendritic Cells Retain Infectious HIV in Cycling Endosomes. PLoS pathogens. 2015;11(12):e1005285 PubMed Central PMCID: PMCPMC4666623. 10.1371/journal.ppat.1005285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ramratnam B, Ribeiro R, He T, Chung C, Simon V, Vanderhoeven J, et al. Intensification of antiretroviral therapy accelerates the decay of the HIV-1 latent reservoir and decreases, but does not eliminate, ongoing virus replication. Journal of acquired immune deficiency syndromes. 2004;35(1):33–7. [DOI] [PubMed] [Google Scholar]
- 71.Politch JA, Mayer KH, Welles SL, O'Brien WX, Xu C, Bowman FP, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. Aids. 2012;26(12):1535–43. Epub 2012/03/24. PubMed Central PMCID: PMC3806452. 10.1097/QAD.0b013e328353b11b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(10):E1126–34. Epub 2015/02/26. PubMed Central PMCID: PMC4364237. 10.1073/pnas.1414926112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teixeira L, Valdez H, McCune JM, Koup RA, Badley AD, Hellerstein MK, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001;15(14):1749–56. [DOI] [PubMed] [Google Scholar]
- 74.He S, Zhang Z, Fu Y, Qin C, Li S, Han X, et al. Thymic Function Is Most Severely Impaired in Chronic HIV-1 Infection, but Individuals With Faster Disease Progression During Early HIV-1 Infection Expressed Lower Levels of RTEs. Journal of acquired immune deficiency syndromes. 2015;70(5):472–8. 10.1097/QAI.0000000000000801 [DOI] [PubMed] [Google Scholar]
- 75.Fernandez S, Nolan RC, Price P, Krueger R, Wood C, Cameron D, et al. Thymic function in severely immunodeficient HIV type 1-infected patients receiving stable and effective antiretroviral therapy. AIDS research and human retroviruses. 2006;22(2):163–70. 10.1089/aid.2006.22.163 [DOI] [PubMed] [Google Scholar]
- 76.De Rossi A, Walker AS, Klein N, De Forni D, King D, Gibb DM. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. The Journal of infectious diseases. 2002;186(3):312–20. 10.1086/341657 [DOI] [PubMed] [Google Scholar]
- 77.Hazenberg MD, Otto SA, Cohen Stuart JW, Verschuren MC, Borleffs JC, Boucher CA, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nature medicine. 2000;6(9):1036–42. 10.1038/79549 [DOI] [PubMed] [Google Scholar]
- 78.Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nature immunology. 2003;4(2):97–9. 10.1038/ni0203-97 [DOI] [PubMed] [Google Scholar]
- 79.Anthony KB, Yoder C, Metcalf JA, DerSimonian R, Orenstein JM, Stevens RA, et al. Incomplete CD4 T cell recovery in HIV-1 infection after 12 months of highly active antiretroviral therapy is associated with ongoing increased CD4 T cell activation and turnover. Journal of acquired immune deficiency syndromes. 2003;33(2):125–33. [DOI] [PubMed] [Google Scholar]
- 80.Gandhi RT, Spritzler J, Chan E, Asmuth DM, Rodriguez B, Merigan TC, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. Journal of acquired immune deficiency syndromes. 2006;42(4):426–34. 10.1097/01.qai.0000226789.51992.3f [DOI] [PubMed] [Google Scholar]
- 81.Negredo E, Massanella M, Puig J, Perez-Alvarez N, Gallego-Escuredo JM, Villarroya J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(9):1300–8. [DOI] [PubMed] [Google Scholar]
- 82.Mavigner M, Cazabat M, Dubois M, L'Faqihi FE, Requena M, Pasquier C, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. The Journal of clinical investigation. 2012;122(1):62–9. Epub 2011/12/14. PubMed Central PMCID: PMC3248296. 10.1172/JCI59011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schacker TW, Bosch RJ, Bennett K, Pollard R, Robbins GK, Collier AC, et al. Measurement of naive CD4 cells reliably predicts potential for immune reconstitution in HIV. Journal of acquired immune deficiency syndromes. 2010;54(1):59–62. Epub 2010/02/26. PubMed Central PMCID: PMC2955357. 10.1097/QAI.0b013e3181c96520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yukl SA, Shergill AK, Girling V, Li Q, Killian M, Epling L, et al. Site-specific differences in T cell frequencies and phenotypes in the blood and gut of HIV-uninfected and ART-treated HIV+ adults. PloS one. 2015;10(3):e0121290 PubMed Central PMCID: PMCPMC4374729. 10.1371/journal.pone.0121290 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and the Supporting Information file.