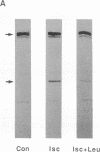
Abstract
Intense proteolysis of cytoskeletal proteins occurs in brain within minutes of transient ischemia, possibly because of the activation of calcium-sensitive proteases (calpains). This proteolytic event precedes overt signs of neuronal degeneration, is most pronounced in regions of selective neuronal vulnerability, and could have significant consequences for the integrity of cellular function. The present studies demonstrate that (i) the early phase of enhanced proteolysis is a direct response to hypoxia rather than other actions of ischemia, (ii) it is possible to pharmacologically inhibit the in vivo proteolytic response to ischemia, (iii) inhibition of proteolysis is associated with a marked reduction in the extent of neuronal death, and (iv) protected neurons exhibit normal-appearing electrophysiological responses and retain their capacity for expressing long-term potentiation, a form of physiological plasticity thought to be involved in memory function. These observations indicate that calcium-activated proteolysis is an important component of the post-ischemic neurodegenerative response and that targeting this response may be a viable therapeutic strategy for preserving both the structure and function of vulnerable neurons.
Full text
PDF
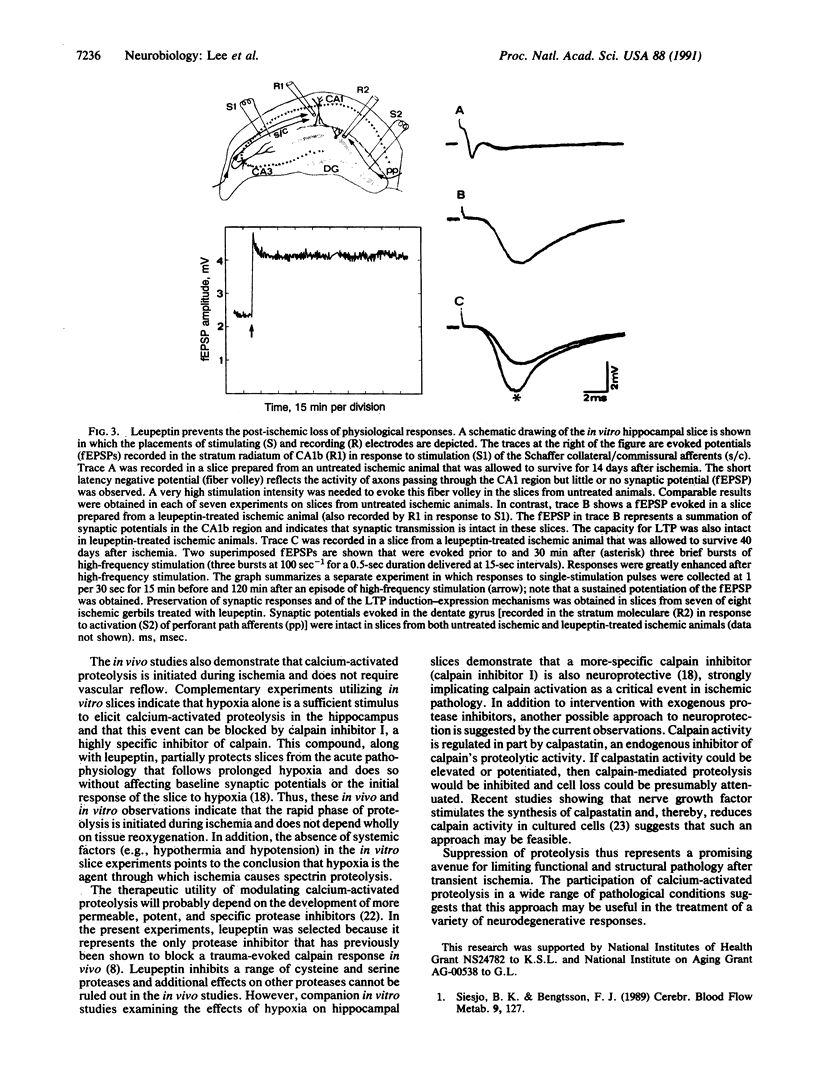

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai A., Kessler M., Lee K., Lynch G. Calpain inhibitors improve the recovery of synaptic transmission from hypoxia in hippocampal slices. Brain Res. 1990 Nov 5;532(1-2):63–68. doi: 10.1016/0006-8993(90)91742-y. [DOI] [PubMed] [Google Scholar]
- Gilbert D. S., Newby B. J. Neurofilament disguise, destruction and discipline. Nature. 1975 Aug 14;256(5518):586–589. doi: 10.1038/256586a0. [DOI] [PubMed] [Google Scholar]
- Ivy G. O., Seubert P., Baudry M., Lynch G. Presence of brain spectrin in dendrites of mammalian brain: technical factors involved in immunocytochemical detection. Synapse. 1988;2(3):329–333. doi: 10.1002/syn.890020324. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982 May 6;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kuwaki T., Satoh H., Ono T., Shibayama F., Yamashita T., Nishimura T. Nilvadipine attenuates ischemic degradation of gerbil brain cytoskeletal proteins. Stroke. 1989 Jan;20(1):78–83. doi: 10.1161/01.str.20.1.78. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Tetzlaff W., Kreutzberg G. W. Rapid down regulation of hippocampal adenosine receptors following brief anoxia. Brain Res. 1986 Aug 13;380(1):155–158. doi: 10.1016/0006-8993(86)91440-x. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Oshima M., Koizumi S., Fujita K., Guroff G. Nerve growth factor-induced decrease in the calpain activity of PC12 cells. J Biol Chem. 1989 Dec 5;264(34):20811–20816. [PubMed] [Google Scholar]
- Pulsinelli W. A., Brierley J. B., Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982 May;11(5):491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Calcium-induced inactivation of microtubule formation in brain extracts. Presence of a calcium-dependent protease acting on polymerization-stimulating microtubule-associated proteins. Eur J Biochem. 1978 Dec;92(2):463–470. doi: 10.1111/j.1432-1033.1978.tb12768.x. [DOI] [PubMed] [Google Scholar]
- Seubert P., Ivy G., Larson J., Lee J., Shahi K., Baudry M., Lynch G. Lesions of entorhinal cortex produce a calpain-mediated degradation of brain spectrin in dentate gyrus. I. Biochemical studies. Brain Res. 1988 Sep 6;459(2):226–232. doi: 10.1016/0006-8993(88)90638-5. [DOI] [PubMed] [Google Scholar]
- Seubert P., Larson J., Oliver M., Jung M. W., Baudry M., Lynch G. Stimulation of NMDA receptors induces proteolysis of spectrin in hippocampus. Brain Res. 1988 Sep 13;460(1):189–194. doi: 10.1016/0006-8993(88)91222-x. [DOI] [PubMed] [Google Scholar]
- Seubert P., Lee K., Lynch G. Ischemia triggers NMDA receptor-linked cytoskeletal proteolysis in hippocampus. Brain Res. 1989 Jul 17;492(1-2):366–370. doi: 10.1016/0006-8993(89)90921-9. [DOI] [PubMed] [Google Scholar]
- Seubert P., Nakagawa Y., Ivy G., Vanderklish P., Baudry M., Lynch G. Intrahippocampal colchicine injection results in spectrin proteolysis. Neuroscience. 1989;31(1):195–202. doi: 10.1016/0306-4522(89)90041-9. [DOI] [PubMed] [Google Scholar]
- Siesjö B. K., Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression: a unifying hypothesis. J Cereb Blood Flow Metab. 1989 Apr;9(2):127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Staubli U., Larson J., Thibault O., Baudry M., Lynch G. Chronic administration of a thiol-proteinase inhibitor blocks long-term potentiation of synaptic responses. Brain Res. 1988 Mar 15;444(1):153–158. doi: 10.1016/0006-8993(88)90922-5. [DOI] [PubMed] [Google Scholar]
- Urban L., Neill K. H., Crain B. J., Nadler J. V., Somjen G. G. Postischemic synaptic physiology in area CA1 of the gerbil hippocampus studied in vitro. J Neurosci. 1989 Nov;9(11):3966–3975. doi: 10.1523/JNEUROSCI.09-11-03966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986 Oct;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]