Abstract
Routine diagnostics and treatment monitoring of brain tumors is usually based on contrast-enhanced MRI. However, the capacity of conventional MRI to differentiate tumor tissue from posttherapeutic effects following neurosurgical resection, chemoradiation, alkylating chemotherapy, radiosurgery, and/or immunotherapy may be limited. Metabolic imaging using PET can provide relevant additional information on tumor metabolism, which allows for more accurate diagnostics especially in clinically equivocal situations. This review article focuses predominantly on the amino acid PET tracers 11C-methyl-l-methionine (MET), O-(2-[18F]fluoroethyl)-l-tyrosine (FET) and 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA) and summarizes investigations regarding monitoring of brain tumor therapy.
Keywords: PET, FET, MET, FDOPA, Glioma, Temozolomide, Bevacizumab, Immunotherapy, Checkpoint inhibitors, Pseudoprogression, Pseudoresponse
Highlights
-
•
Currently, the RANO group has strongly recommended the widespread diagnostic use of amino acid PET for brain tumor patients.
-
•
In this group of patients, the available literature provides strong evidence that amino acid PET can be a helpful adjunct.
-
•
The resulting improvement in diagnostic accuracy is expected to have patient-relevant benefits.
-
•
The ability of amino acid PET to quantify biological responses opens the door for its application in treatment monitoring.
1. Introduction
In general Oncology as well as in Neuro-Oncology, the evaluation of treatment response or monitoring of tumor therapy is of paramount importance. In particular, the early identification of non-response allows the termination of an ineffective therapy to avoid possible side effects, e.g., bone marrow depression, fatigue, nausea, and vomiting, and therefore to maintain or even improve life-quality. Furthermore, the early identification of non-response allows an earlier treatment change. For example, in the event of chemotherapy resistance, a switch to another chemotherapeutic agent is possible before bone marrow reserves are exhausted. Moreover, identification of treatment failure may help reduce costs. This is highly relevant because the expense of newer systemic treatment options (e.g., bevacizumab) is considerably higher than conventional alkylating chemotherapy (e.g., lomustine).
Additionally, following radiotherapy and temozolomide chemotherapy for newly diagnosed glioblastoma patients and antiangiogenic drugs such as bevacizumab in pretreated patients, “pseudoprogression” and “pseudoresponse” have been described (Brandes et al., 2008, Brandsma and van den Bent, 2009). These phenomena may complicate the assessment of treatment effect. Similarly, pseudoprogression or delayed responses may occur during immunotherapy of brain metastasis by blocking immune checkpoints such as CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) using ipilimumab and PD-1 (programmed cell death 1 receptor) using pembrolizumab or nivolumab (Okada et al., 2015, Preusser et al., 2015, Wolchok et al., 2009). Importantly, following immunotherapy, long-term survival and tumor regression can still occur after initial disease progression or even after the appearance of new lesions (Okada et al., 2015).
For decades, in patients with brain tumors, changes of contrast enhancement extent on MRI are traditionally used as an indicator of therapy response or tumor relapse (Macdonald et al., 1990, Wen et al., 2010). However, contrast enhancement resulting from increased blood-brain barrier (BBB) permeability is nonspecific and may not always be an accurate surrogate of neoplastic tissue, tumor extent or treatment effect (Ahluwalia and Wen, 2011, Dhermain et al., 2010, Kumar et al., 2000).
In order to overcome the limitations of the assessment of tumor response to antiangiogenic treatment by evaluation of contrast-enhancement changes only (according to the Macdonald criteria), the Response Assessment in Neuro-Oncology (RANO) group suggested in 2010 new recommendations for evaluating response (Wen et al., 2010). In particular, following anti-angiogenic drug treatment, FLAIR or T2 signal hyperintensity was recommended as a surrogate marker for nonenhancing tumor to help determine tumor progression, and thus nonenhancing FLAIR or T2 signal alterations were included as criteria for determining tumor response or progression (“non-enhancing tumor progression”) (Wen et al., 2010).
However, these criteria do not provide quantitative values of FLAIR or T2 signal change for the diagnosis of tumor progression. Various differential diagnoses such as tumor-related edema, radiation injury, demyelination, ischemia, and infection can result in hyperintense FLAIR or T2 signal hyperintensity, which is difficult to distinguish from nonenhancing tumor (Ahluwalia and Wen, 2011). Consequently, alternative diagnostic methods are necessary to improve the identification of treatment response.
Positron-Emission-Tomography (PET) is one of the most promising techniques for the imaging of specific molecular processes in vivo. This method uses biologically active molecules labeled with short-lived positron-emitting isotopes at micromolar or nanomolar concentrations. Molecular imaging using PET may provide relevant additional information on tumor metabolism, and may be helpful in clinical decision-making, especially in the case of ambiguous MRI findings following neurooncological treatment (Fig. 1). Further strengths of PET imaging is that the image acquisition can be standardized, the insensibility to susceptibility artifacts and the relatively uncomplicated post-processing for static images.
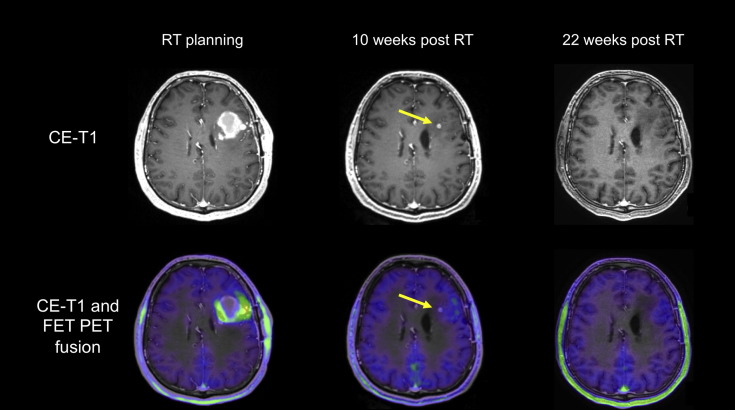
Fig. 1.
Patient with a newly diagnosed glioblastoma. After resection and chemoradiation with temozolomide, MR and FET PET images show residual tumor in the left frontal lobe (baseline imaging for radiotherapy planning) with complete metabolic response 10 weeks after radiotherapy. The residual contrast-enhancing lesion is metabolically inactive (arrows) indicating a post-treatment effect. This is confirmed 22 months later with complete resolution of this lesion.
This review article focuses predominantly on the amino acid PET tracers 11C-methyl-l-methionine (MET), O-(2-[18F]fluoroethyl)-l-tyrosine (FET) and 3,4-dihydroxy-6-[18F]-fluoro-l-phenylalanine (FDOPA) and summarizes investigations regarding monitoring of brain tumor therapy.
2. Most relevant pet tracers for (neuro-) oncological imaging
The classic and most common PET tracer for oncologic imaging has traditionally been 18F-2-fluoro-2-deoxy-d-glucose (FDG). FDG is accumulated in the majority of tumors due to increased energy demand and consequently elevated glucose metabolism. FDG uptake has been well characterized for extracranial tumors, and also has been applied to brain tumor imaging for many years. The relationship of FDG uptake to tumor glioma grade and prognosis has been reported in several studies (Herholz et al., 2012). As newer PET tracers have become available, the use of FDG for imaging in Neuro-Oncology has declined, in part due to several important limitations. These include the high rate of glucose metabolism in normal brain parenchyma resulting in diminished signal-to-noise ratio for brain tumors. Another problem with FDG is the high tracer uptake in inflammatory cells, which can occur in a variety of disease processes and can be independent of tumor growth or response.
Radiolabeled amino acids have been used in neurooncological practice since 1983 (Bergström et al., 1983). The most experience for this class of PET tracers for brain tumor imaging has been gained with MET. This tracer is an essential amino acid labeled with the positron-emitting isotope carbon-11, which has a half-life of 20 min (Galldiks et al., 2015b, Herholz et al., 2012). This relatively short half-life limits the use of MET to PET centers with an on-site cyclotron unit. More recently, amino acid tracers labeled with positron emitters that have longer half-lives have been synthesized. This has resulted in improved distribution, efficiency and cost-effectiveness (Huang and McConathy, 2013). For example, FET was developed in the late 1990s and is an 18F-labeled amino acid tracer (half-life, 110 min) resulting in logistic advantages for clinical practice compared to MET (Langen et al., 2006, Wester et al., 1999). The use of FET has grown rapidly in recent years, especially in Western Europe. Clinical results in brain tumors with PET using MET and FET appear similar (Grosu et al., 2011, Langen et al., 2003, Weber et al., 2000). Switzerland was the first country to approve FET PET as a medical drug in 2014 (Swissmedic, 2014). Another 18F-labeled amino acid analogue FDOPA, which was primarily developed to measure dopamine synthesis in the basal ganglia, has also increasingly been used as a tracer for brain tumor imaging (Becherer et al., 2003). FDOPA is currently approved for characterization of presynaptic dopaminergic activity in patients with Parkinsonian syndromes in the United States and Western Europe. The increased uptake of MET, FET and FDOPA in gliomas and brain metastases appears to be caused predominantly by increased transport via the amino acid transport system L for large neutral amino acids namely the subtypes LAT1 and LAT2 (Okubo et al., 2010, Papin-Michault et al., 2016, Wiriyasermkul et al., 2012, Youland et al., 2013). A feature that distinguishes FET from MET and FDOPA is the high metabolic stability of FET. After the transport via L-type amino acid transporters into tumor tissue, it has been demonstrated that MET and FDOPA show some metabolic degradation and incorporation into protein or participation in other metabolic pathways (Singhal et al., 2008), whereas FET is not metabolized (Langen et al., 2003). Furthermore, it has been shown that overexpression of LAT1 is closely correlated with a malignant phenotype and proliferation of gliomas (Haining et al., 2012).
In addition to static images, dynamic FET PET data can be acquired dynamically, allowing for the ability to characterize the temporal pattern of FET uptake by deriving a time-activity curve (TAC). It has been demonstrated that the configuration of TAC may contain additional biological information, which may be helpful for glioma grading (Albert et al., 2016b, Calcagni et al., 2011), the differentiation of both glioma and brain metastasis recurrence from radiation-induced changes (Ceccon et al., 2016, Galldiks et al., 2015a, Galldiks et al., 2015c) or the prognostication of untreated gliomas (Jansen et al., 2014, Jansen et al., 2015). For example, TACs of FET uptake in high-grade gliomas are characterized by an early peak of tracer uptake followed by a constant decent, whereas low-grade gliomas usually show a steadily increasing TAC (Pöpperl et al., 2007). Since this phenomenon has not observed for other amino acid tracers such as MET or FDOPA (Kratochwil et al., 2014, Moulin-Romsée et al., 2007), and it remains to be elucidated whether dynamic MET and FDOPA can contribute significantly to the characterization of brain tumors.
The value of other amino acid PET tracers such as α-[11C]-methyl-l-tryptophan (AMT) and [18F]Fluciclovine (FACBC) as well as glutamine-based amino acid PET tracers has been evaluated in glioma patients in terms of tumor delineation (Kamson et al., 2013, Kondo et al., 2016, Venneti et al., 2015), prognostication (Kamson et al., 2014) and the differentiation of tumor recurrence from radiation injury (Alkonyi et al., 2012). The observed findings are promising and suggest that these tracers have the potential to monitor treatment effects. However, the number of examined subjects is currently low and should be increased by subsequent studies.
Besides FDG and radiolabeled amino acids, several other radiopharmaceuticals have been used to image brain tumors, and can help identify malignant processes in tumors. For instance, the thymidine nucleoside analogue 3′-deoxy-3′-18F-fluorothymidine (FLT), the substrate for thymidine kinase-1, reflects cell proliferation. Previous studies suggest that FLT is a promising tool for glioma detection and grading (Chen et al., 2005, Jacobs et al., 2005) and is able to predict improved survival after bevacizumab therapy (Chen et al., 2007, Wardak et al., 2014). Unfortunately, FLT uptake is dependent on disruption of the blood-brain barrier, hampering its clinical value in Neuro-Oncology (Dhermain et al., 2010).
Imaging of hypoxia in brain tumors has been performed with the tracer 18F-Fluoromisonidazole (FMISO) (Lee and Scott, 2007). FMISO enters tumor cells by passive diffusion and becomes trapped in cells with reduced tissue oxygen partial pressure by nitroreductase enzymes. Clinically this tracer is of interest for the identification of hypoxic tumor areas, which are thought to be more resistant to irradiation (Spence et al., 2008), as well as a trigger for neoangiogenesis. A prospective study suggested that abnormal tumor vasculature and hypoxia, as measured with MRI and FMISO PET, have a negative impact on survival in patients with newly diagnosed glioblastoma (Gerstner et al., 2016). However, to date FMISO has predominantly been used in a preclinical setting (Suchorska et al., 2014). Furthermore, FMISO is relatively lipophilic and has a slow clearance from white matter, resulting in a low target-to-background ratio.
Another interesting PET target is the translocator protein (TSPO), a mitochondrial membrane protein that has been used as biomarker for neuroinflammation. TSPO is highly expressed in activated microglia, macrophages and neoplastic cells. Imaging with the TSPO ligand 11C-(R)PK11195 demonstrates increased binding in high-grade gliomas compared to low-grade gliomas and normal brain parenchyma (Su et al., 2013, Su et al., 2015). However, 11C-(R)PK11195, like MET, is limited to PET centers with a cyclotron due to its short half-life. More recently, the TSPO ligand 18F-DPA-714 labeled with 18F (half-life, 110 min) has been synthesized (Winkeler et al., 2012) and evaluated in glioma animal models (Awde et al., 2013). Results in human glioma patients are pending.
3. Local treatment options
3.1. Neurosurgical resection
To date, the spatial distribution of contrast enhancement on MRI is frequently used to define the tumor extent. Furthermore, the area of contrast enhancement is usually the target for stereotactic biopsy or for local treatment options such as neurosurgical resection. Tumor resection is a key treatment option in Neuro-Oncology because it has been demonstrated that a complete tumor resection is associated with a favorable outcome (Lacroix et al., 2001, Stummer et al., 2006). Furthermore, the importance of resection is dependent on tumor type - for some brain tumors, including multiple pediatric ones, complete removal is the only hope for cure. For the assessment of residual tumor after surgery, postoperative MRI within 24–72 h is commonly used. A complete tumor resection is defined by the lack on nodular contrast enhancement following surgery (Albert et al., 1994).
3.1.1. PET-guided neurosurgical planning and resection
The addition of MET PET data for resection-guidance of anaplastic gliomas and glioblastomas provides a target contour substantially different from that obtained by contrast enhancement MRI alone in about 80% of cases (Pirotte et al., 2009). In line with this, a recent study with 79 glioblastoma patients revealed that the metabolically active tumor volume on FET PET images prior to histological diagnosis is considerably larger than the volume of contrast enhancement (median volumes, 23.8 ml vs. 13.5 ml) (Suchorska et al., 2015). Similar findings could be observed in low-grade gliomas (Floeth et al., 2011). That biopsy-controlled study assessed the presurgical value of FET uptake, 5-aminolevulinic acid (5-ALA) fluorescence and contrast enhancement. 5-ALA is a non-fluorescent prodrug that leads to intracellular accumulation of fluorescent porphyrins in gliomas and is frequently used for intraoperative fluorescence-guided glioma resection. This can improve patient survival (Stummer et al., 2006), but, in contrast to FET PET, 5-ALA has a limited sensitivity for tumor tissue detection, especially in low-grade gliomas (Floeth et al., 2011).
Regarding PET-guided neurosurgical resection, Pirotte and co-workers showed that a complete resection of the tumor area with increased MET uptake resulted in significantly longer survival of patients with high-grade gliomas (Pirotte et al., 2009).
3.1.2. Postoperative evaluation of the extent of resection
A recent study suggests that findings on early postoperative MRI (24–72 h) can be falsely positive (i.e., due to reactive changes, ischemia, infarctions) or even falsely-negative, particularly when a 3T MR scanner is used (Lescher et al., 2014). In line with Lescher et al., a case series with high-grade glioma patients (n = 25) compared the early postoperative MRI performed within the first 72 h after surgery with an early FET PET scan within the same time frame. FET PET was more sensitive than MRI in 24% of cases in which MRI was falsely negative (wrongly indicating complete response) as proven by histopathology or short-term follow-up (Kläsner et al., 2015).
Moreover, the amount of residual tracer uptake in FET PET after surgery/prior to chemoradiation of glioblastomas (FET PET performed within 7–20 days after surgery) has a strong prognostic influence, even after adjustment by multivariate survival analyses for the effects of treatment, MGMT promoter methylation and other patient and tumor-related factors (Piroth et al., 2011a, Poulsen et al., 2016, Suchorska et al., 2015). These data indicate that resection of malignant gliomas guided by amino acid PET may increase the amount of neoplastic tissue removed, potentially improving patient outcome.
3.2. Radiotherapy
Similar to neurosurgical resection, radiotherapy is one of the most important local treatment options and is the main treatment component for both newly diagnosed and relapsed brain tumors. The radiation dose can be fractionated, directly placed inside the tumor (i.e., brachytherapy) or can be applied as a single high dose fraction (i.e., radiosurgery).
3.2.1. Pseudoprogression after chemoradiation
Since the introduction of chemoradiation with temozolomide as the current standard of care for patients with glioblastoma, there has been an increasing awareness of progressive enhancing lesions on MRI, which are not related to tumor progression, but which are due to treatment effect, i.e., pseudoprogression. Pseudoprogression is typically regarded as a phenomenon of the first 12 weeks after radiotherapy (Brandsma et al., 2008, Brandsma and van den Bent, 2009, Wen et al., 2010) and this time-dependent definition has been incorporated into the RANO criteria (Wen et al., 2010). Although pseudoprogression most often occurs within the first 12 weeks after radiochemotherapy completion, some cases occurring later have been observed, particularly after radiochemotherapy using temozolomide in combination with lomustine (Kruser et al., 2013, Stuplich et al., 2012). Compared to conventional MRI, more recent studies with a larger glioblastoma patient cohort reported a diagnostic accuracy of FET PET of at least 85% for differentiating both typical (within 12 weeks) and late (> 12 weeks) pseudoprogression after radiochemotherapy completion from true tumor progression (Galldiks et al., 2015a, Kebir et al., 2016a).
3.2.2. Fractionated external beam radiation therapy
A prospective study assessed the prognostic value of early changes of FET uptake 6–8 weeks after postoperative radiochemotherapy in glioblastoma patients (Galldiks et al., 2012b, Piroth et al., 2011b). PET responders with a decrease in the tumor/brain ratio of > 10% had a significantly longer disease-free and overall survival than patients with stable or increasing tracer uptake after radiochemotherapy. However, the kinetic analysis of FET uptake was not helpful in the evaluation of treatment effects to radiochemotherapy (Piroth et al., 2013).
3.2.3. Brachytherapy
Amino acid PET has also been investigated as a way to assess response or failure of iodine-125 seed brachytherapy. For instance, 12 months after brachytherapy in patients with low-grade glioma, MET uptake was significantly reduced, whereas glucose metabolism as assessed by FDG PET was unchanged (Voges et al., 1997, Würker et al., 1996). In a more recent FET PET study, tumor/brain ratios, uptake kinetics and PET tumor volumes were evaluated for their value in monitoring stereotactic brachytherapy using iodine-125 seeds (Jansen et al., 2013). In that study, FET PET correctly differentiated with a high diagnostic accuracy late posttherapeutic effects after 6 months from local tumor progression in patients with recurrent high-grade glioma.
3.2.4. Radiosurgery
In view of the demographic changes with an increasing elderly population as well as of a wider spectrum of diagnostic measurements and therapy options for extracranial tumors (e.g., biomarker-guided patient stratification, whole-genome sequencing, targeted therapy, immunotherapy etc.) resulting in an improvement of prognosis, an increasing number of patients diagnosed with brain metastasis is expected. Besides neurosurgical resection, various types of radiation therapy such as radiosurgery, brachytherapy, and whole-brain radiation therapy are commonly used to treat secondary brain neoplasms.
However, following radiotherapy, and in particular after radiosurgery, conventional MRI cannot reliably differentiate brain metastasis recurrence or progression from radiation-induced changes (e.g., radiation necrosis). In gliomas, radiation necrosis usually manifests within 6 months after standard radiotherapy, occurring in approximately 5–25% of these patients (Kumar et al., 2000, Shah et al., 2013). For patients with brain metastasis treated by radiosurgery, a similar fraction of radiation necrosis (24% of 310 cerebral metastases) has been reported (Minniti et al., 2011), which may increase to as high as 47%, depending on the irradiated volume receiving a specific radiation dose (Minniti et al., 2011).
Amino acid PET has been investigated as a problem-solving tool to address this common problem in clinical practice. For instance, MET PET may be effective in differentiating recurrent metastatic brain tumor from radiation-induced changes. A simple semiquantitative regions-of-interest analysis for the calculation of tumor/brain ratios demonstrated a sensitivity and specificity of 70–80% (Terakawa et al., 2008, Tsuyuguchi et al., 2003). FDOPA PET has also been shown to differentiate recurrent or progressive brain metastasis from radiation-induced changes with high sensitivity (81%) and specificity (84%) (Lizarraga et al., 2014). FDOPA PET has been compared to perfusion-weighted MRI in a study of patients with brain metastases after stereotactic radiosurgery. The accuracy of FDOPA PET was 91% and superior to PWI MRI, which yielded an accuracy of 76% (Cicone et al., 2015) in identifying metastases. A similar diagnostic accuracy has also been reported for FET PET: the combination of tumor/brain ratios with dynamic FET parameters differentiated local brain metastasis recurrence from radiation-induced changes with a sensitivity of 95% and specificity of 91% (Galldiks et al., 2012c). And a similar diagnostic performance was confirmed in a subsequent study with a greater number of patients (n = 62) (Ceccon et al., 2016).
4. Systemic treatment options
In Neuro-Oncology, frequently used systemic treatment options are cytotoxic chemotherapy and antiangiogenic therapy. Immunotherapy is effective in patients with extracranial tumors and brain metastasis and seems to be a promising systemic approach for malignant gliomas (Berghoff and Preusser, 2016, Nduom et al., 2016), that is currently under intensive investigation.
4.1. Alkylating chemotherapy
For patients treated with alkylating chemotherapy, MET and FET PET may improve response assessment (Fig. 2). For instance, MET PET has been evaluated as a means to assess the effects of alkylating chemotherapy. Reliable monitoring of temozolomide and nitrosourea-based chemotherapy (PCV scheme including procarbazine, CCNU and vincristine or CCNU monotherapy) has been demonstrated in patients with recurrent high-grade glioma (Galldiks et al., 2006, Galldiks et al., 2010, Galldiks and Langen, 2016, Herholz et al., 2003). Similarly, FET PET has been used to assess effects of temozolomide chemotherapy according to the EORTC protocol 22,033–26,033 (application of 75 mg/m2 temozolomide per day over 21 days in a 28-day cycle) (Wyss et al., 2009). In that prospective study, changes of FET uptake were compared with FLAIR signal alterations on MRI for evaluation of response to temozolomide regime 21/28 in 11 patients with progressive nonenhancing low-grade glioma (WHO Grade II). In responding patients, a reduction of the metabolically active tumor volume after initiation of treatment could be observed substantially earlier than volume reductions on FLAIR sequences, suggesting that FET PET may be an earlier marker of successful treatment than standard MRI for this patient group. These findings described by Wyss and colleagues were confirmed by a subsequent multicenter PET study in a greater patient number (Roelcke et al., 2016).
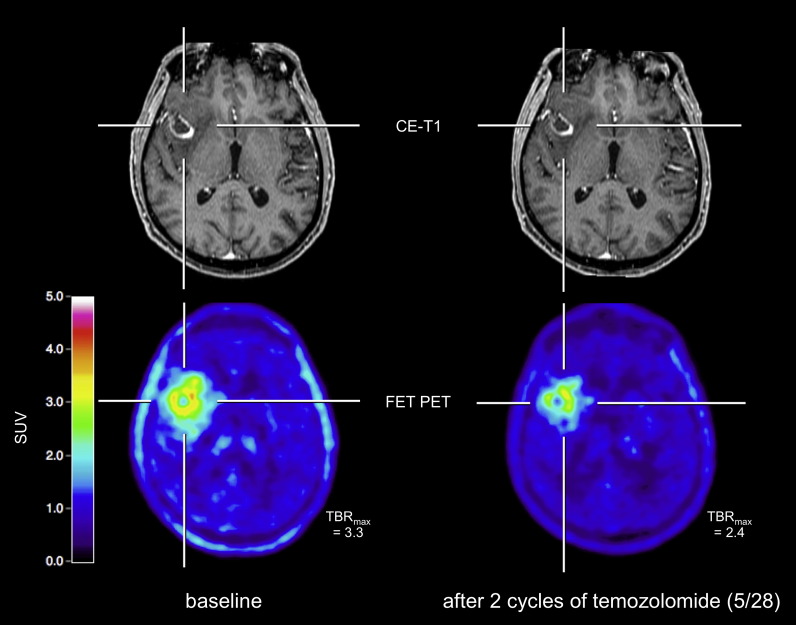
Fig. 2.
A 67-year old glioblastoma patient prior to adjuvant chemotherapy (images on the left). After two cycles of temozolomide chemotherapy (images on the right), a clear decrease of both the metabolically active tumor volume and tumor/brain ratios can be observed whereas conventional MRI shows no change of contrast enhancement (“stable disease” according to RANO criteria).
4.2. Antiangiogenic therapy with bevacizumab
In 2010, the RANO group addressed the problem of pseudoresponse related to antiangiogenic drugs and recommended new criteria for response assessment by including FLAIR or T2 signal alterations as criteria for determining tumor response or progression (“non-enhancing tumor progression”) (Wen et al., 2010).
However, the problem of accurately identifying non-enhancing tumor remains, and thus amino acid PET has been investigated as an alternative imaging method to assess treatment response to antiangiogenic therapy (Reithmeier et al., 2013) (Fig. 3). Recent studies and case reports indicate that FET and FDOPA PET are useful in detection of pseudoresponse (Galldiks et al., 2012a, Galldiks et al., 2013a, Galldiks et al., 2013b, Hutterer et al., 2011, Morana et al., 2013). FET and FDOPA PET have also been used to predict a favorable outcome in responders to bevacizumab (Galldiks et al., 2013b, Hutterer et al., 2011, Schwarzenberg et al., 2014).
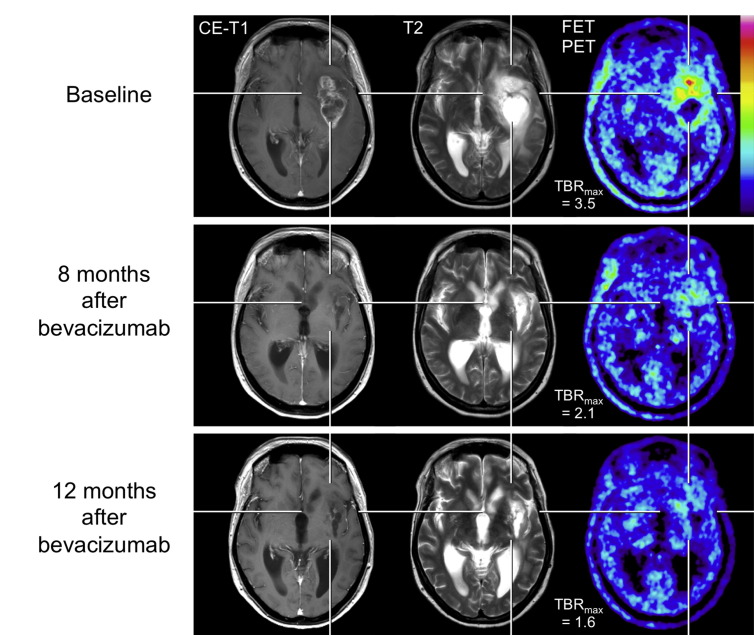
Fig. 3.
A 52-year-old patient with a progressive anaplastic oligoastrocytoma according to the WHO classification 2007 (top row). During follow-up after 8 and 12 months (middle and bottom row) of biweekly bevacizumab therapy, MRI shows a markedly reduction of contrast enhancement and T2 hyperintensity. Correspondingly, FET PET shows a decrease of metabolic activity by means of maximum tumor/brain ratio reduction (TBRmax).
Additionally, the cost effectiveness of FET PET for therapy monitoring of antiangiogenic therapy has been analyzed (Heinzel et al., 2013). The data suggest that the additional use of FET PET in the management of these patients have the potential to avoid overtreatment and corresponding costs, as well as unnecessary patient side effects.
4.3. Immunotherapy with checkpoint Inhibitors
Identifying patients with pseudoprogression is crucial because a successful treatment might be erroneously and prematurely discontinued with a potentially negative influence on survival. Following treatment of melanoma patients with checkpoint inhibitors such as ipilimumab, nivolumab, or pembrolizumab, in particular, detecting pseudoprogression has become a major challenge, with approximately 10% incidence in clinical practice (Hodi et al., 2016). In order to overcome this issue, a working group recommended in 2015 criteria for immunotherapy response assessment in Neuro-Oncology (iRANO) based on clinical parameter and standard MRI findings (Okada et al., 2015). The iRANO group noted that to date there is no non-invasive method that can confidently identify pseudoprogression in these patients. Thus, there remains an urgent need for the acquisition of additional information potentially derived from advanced imaging techniques. Recently a small retrospective pilot study addressed this issue and showed for the first time the potential of FET PET to detect pseudoprogression in patients with malignant melanoma brain metastasis treated with ipilimumab or nivolumab (Kebir et al., 2016b).
5. Other experimental therapy approaches
Other experimental treatment options for gliomas such as convection-enhanced delivery of paclitaxel, intracavitary radioimmunotherapy, stereotaxy-guided laser-induced interstitial thermotherapy, as well as adjuvant maintenance therapy with imatinib in combination with hydroxyurea may also benefit from PET imaging. For instance several studies (Galldiks et al., 2009, Galldiks et al., 2012d, Pöpperl et al., 2005, Pöpperl et al., 2006) have shown that such treatment effects could be successfully monitored by PET using MET and FET.
6. Outlook
The present literature in neuroimaging using PET highlights the ability of amino acid PET to quantify biological responses to treatment and allows its application to monitor patients to identify early disease relapse and response to treatment. The more widespread use of amino acid PET for the management of patients with brain tumors has been strongly recommended by the RANO group (Albert et al., 2016a, Langen and Watts, 2016). However, therapy-monitoring PET data remain limited, necessitating more comprehensive (i.e., biopsy-controlled), prospective studies in larger clinical cohorts. In particular, continued progress is impeded by the lack of stereotactically guided biopsy-controlled studies. Lastly, the diagnostic impact of amino acid PET needs to be compared with a variety of promising advanced MR imaging techniques to develop the most accurate and useful multi-modal biomarkers possible.
References
- Ahluwalia M.S., Wen P.Y. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert. Rev. Anticancer. Ther. 2011;11:653–656. doi: 10.1586/era.11.35. [DOI] [PubMed] [Google Scholar]
- Albert F.K., Forsting M., Sartor K., Adams H.P., Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–60. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Albert N.L., Weller M., Suchorska B., Galldiks N., Soffietti R., Kim M.M., la Fougere C., Pope W., Law I., Arbizu J., Chamberlain M.C., Vogelbaum M., Ellingson B.M., Tonn J.C. Response assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology. 2016;18:1199–1208. doi: 10.1093/neuonc/now058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert N.L., Winkelmann I., Suchorska B., Wenter V., Schmid-Tannwald C., Mille E., Todica A., Brendel M., Tonn J.C., Bartenstein P., la Fougere C. Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20–40 min scans. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1105–1114. doi: 10.1007/s00259-015-3276-2. [DOI] [PubMed] [Google Scholar]
- Alkonyi B., Barger G.R., Mittal S., Muzik O., Chugani D.C., Bahl G., Robinette N.L., Kupsky W.J., Chakraborty P.K., Juhasz C. Accurate differentiation of recurrent gliomas from radiation injury by kinetic analysis of alpha-11C-methyl-l-tryptophan PET. J. Nucl. Med. 2012;53:1058–1064. doi: 10.2967/jnumed.111.097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awde A.R., Boisgard R., Theze B., Dubois A., Zheng J., Dolle F., Jacobs A.H., Tavitian B., Winkeler A. The translocator protein radioligand 18F-DPA-714 monitors antitumor effect of erufosine in a rat 9L intracranial glioma model. J. Nucl. Med. 2013;54:2125–2131. doi: 10.2967/jnumed.112.118794. [DOI] [PubMed] [Google Scholar]
- Becherer A., Karanikas G., Szabo M., Zettinig G., Asenbaum S., Marosi C., Henk C., Wunderbaldinger P., Czech T., Wadsak W., Kletter K. Brain tumour imaging with PET: a comparison between [18F]fluorodopa and [11C]methionine. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:1561–1567. doi: 10.1007/s00259-003-1259-1. [DOI] [PubMed] [Google Scholar]
- Berghoff A.S., Preusser M. In search of a target: PD-1 and PD-L1 profiling across glioma types. Neuro-Oncology. 2016;18(10):1331–1332. doi: 10.1093/neuonc/now162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström M., Collins V.P., Ehrin E., Ericson K., Eriksson L., Greitz T., Halldin C., von Holst H., Langstrom B., Lilja A. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. J. Comput. Assist. Tomogr. 1983;7:1062–1066. doi: 10.1097/00004728-198312000-00022. [DOI] [PubMed] [Google Scholar]
- Brandes A.A., Franceschi E., Tosoni A., Blatt V., Pession A., Tallini G., Bertorelle R., Bartolini S., Calbucci F., Andreoli A., Frezza G., Leonardi M., Spagnolli F., Ermani M. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J. Clin. Oncol. 2008;26:2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- Brandsma D., Stalpers L., Taal W., Sminia P., van den Bent M.J. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- Brandsma D., van den Bent M.J. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr. Opin. Neurol. 2009;22:633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]
- Calcagni M.L., Galli G., Giordano A., Taralli S., Anile C., Niesen A., Baum R.P. Dynamic O-(2-[18F]fluoroethyl)-l-tyrosine (F-18 FET) PET for glioma grading: assessment of individual probability of malignancy. Clin. Nucl. Med. 2011;36:841–847. doi: 10.1097/RLU.0b013e3182291b40. [DOI] [PubMed] [Google Scholar]
- Ceccon G., Lohmann P., Stoffels G., Judov N., Filss C.P., Rapp M., Bauer E., Hamisch C., Ruge M.I., Kocher M., Kuchelmeister K., Sellhaus B., Sabel M., Fink G.R., Shah N.J., Langen K.J., Galldiks N. Dynamic O-(2-18F-fluoroethyl)-l-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncology. 2016 doi: 10.1093/neuonc/now149. (Jul 28 Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Cloughesy T., Kamdar N., Satyamurthy N., Bergsneider M., Liau L., Mischel P., Czernin J., Phelps M.E., Silverman D.H. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J. Nucl. Med. 2005;46:945–952. [PubMed] [Google Scholar]
- Chen W., Delaloye S., Silverman D.H., Geist C., Czernin J., Sayre J., Satyamurthy N., Pope W., Lai A., Phelps M.E., Cloughesy T. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J. Clin. Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- Cicone F., Minniti G., Romano A., Papa A., Scaringi C., Tavanti F., Bozzao A., Maurizi Enrici R., Scopinaro F. Accuracy of F-DOPA PET and perfusion-MRI for differentiating radionecrotic from progressive brain metastases after radiosurgery. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:103–111. doi: 10.1007/s00259-014-2886-4. [DOI] [PubMed] [Google Scholar]
- Dhermain F.G., Hau P., Lanfermann H., Jacobs A.H., van den Bent M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9:906–920. doi: 10.1016/S1474-4422(10)70181-2. [DOI] [PubMed] [Google Scholar]
- Floeth F.W., Sabel M., Ewelt C., Stummer W., Felsberg J., Reifenberger G., Steiger H.J., Stoffels G., Coenen H.H., Langen K.J. Comparison of (18)F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:731–741. doi: 10.1007/s00259-010-1690-z. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Dunkl V., Stoffels G., Hutterer M., Rapp M., Sabel M., Reifenberger G., Kebir S., Dorn F., Blau T., Herrlinger U., Hau P., Ruge M.I., Kocher M., Goldbrunner R., Fink G.R., Drzezga A., Schmidt M., Langen K.J. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[(18)F]fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging. 2015;42:685–695. doi: 10.1007/s00259-014-2959-4. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Filss C.P., Goldbrunner R., Langen K.J. Discrepant MR and [(18)F]fluoroethyl-l-tyrosine PET imaging findings in a patient with bevacizumab failure. Case Rep. Oncol. 2012;5:490–494. doi: 10.1159/000342480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galldiks N., Kracht L.W., Burghaus L., Thomas A., Jacobs A.H., Heiss W.D., Herholz K. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:516–524. doi: 10.1007/s00259-005-0002-5. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Kracht L.W., Burghaus L., Ullrich R.T., Backes H., Brunn A., Heiss W.D., Jacobs A.H. Patient-tailored, imaging-guided, long-term temozolomide chemotherapy in patients with glioblastoma. Mol. Imaging. 2010;9:40–46. [PubMed] [Google Scholar]
- Galldiks N., Langen K., Holy R., Pinkawa M., Stoffels G., Nolte K., Kaiser H., Filss C., Fink G., Coenen H., Eble M., Piroth M. Assessment of treatment response in patients with glioblastoma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. J. Nucl. Med. 2012;53:1048–1057. doi: 10.2967/jnumed.111.098590. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Langen K.J. Amino acid PET - an imaging option to identify treatment response, posttherapeutic effects, and tumor recurrence? Front. Neurol. 2016;7:120. doi: 10.3389/fneur.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galldiks N., Langen K.J., Pope W.B. From the clinician's point of view - what is the status quo of positron emission tomography in patients with brain tumors? Neuro-Oncology. 2015;17:1434–1444. doi: 10.1093/neuonc/nov118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galldiks N., Rapp M., Stoffels G., Dunkl V., Sabel M., Langen K.J. Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-l-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mol. Imaging. 2013;12:273–276. [PubMed] [Google Scholar]
- Galldiks N., Rapp M., Stoffels G., Fink G.R., Shah N.J., Coenen H.H., Sabel M., Langen K.J. Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-l-tyrosine PET in comparison to MRI. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:22–33. doi: 10.1007/s00259-012-2251-4. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Stoffels G., Filss C., Rapp M., Blau T., Tscherpel C., Ceccon G., Dunkl V., Weinzierl M., Stoffel M., Sabel M., Fink G.R., Shah N.J., Langen K.J. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro-Oncology. 2015;17:1293–1300. doi: 10.1093/neuonc/nov088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galldiks N., Stoffels G., Filss C.P., Piroth M.D., Sabel M., Ruge M.I., Herzog H., Shah N.J., Fink G.R., Coenen H.H., Langen K.J. Role of O-(2-18F-fluoroethyl)-l-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J. Nucl. Med. 2012;53:1367–1374. doi: 10.2967/jnumed.112.103325. [DOI] [PubMed] [Google Scholar]
- Galldiks N., Ullrich R., Schroeter M., Fink G.R., Kracht L.W. Imaging biological activity of a glioblastoma treated with an individual patient-tailored, experimental therapy regimen. J. Neuro-Oncol. 2009;93:425–430. doi: 10.1007/s11060-008-9790-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galldiks N., von Tempelhoff W., Kahraman D., Kracht L.W., Vollmar S., Fink G.R., Schroeter M., Goldbrunner R., Schmidt M., Maarouf M. 11C-methionine positron emission tomographic imaging of biologic activity of a recurrent glioblastoma treated with stereotaxy-guided laser-induced interstitial thermotherapy. Mol. Imaging. 2012;11:265–271. [PubMed] [Google Scholar]
- Gerstner E.R., Zhang Z., Fink J.R., Muzi M., Hanna L., Greco E., Prah M., Schmainda K.M., Mintz A., Kostakoglu L., Eikman E.A., Ellingson B.M., Ratai E.M., Sorensen A.G., Barboriak D.P., Mankoff D.A., Group, A.T ACRIN 6684: assessment of tumor hypoxia in newly diagnosed glioblastoma using 18F-FMISO PET and MRI. Clin. Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosu A.L., Astner S.T., Riedel E., Nieder C., Wiedenmann N., Heinemann F., Schwaiger M., Molls M., Wester H.J., Weber W.A. An interindividual comparison of O-(2-[(18)F]fluoroethyl)-l-tyrosine (FET)- and l-[methyl-(11)C]methionine (MET)-PET in patients with brain gliomas and metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011;81:1049–1058. doi: 10.1016/j.ijrobp.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Haining Z., Kawai N., Miyake K., Okada M., Okubo S., Zhang X., Fei Z., Tamiya T. Relation of LAT1/4F2hc expression with pathological grade, proliferation and angiogenesis in human gliomas. BMC Clin. Pathol. 2012;12:4. doi: 10.1186/1472-6890-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel A., Müller D., Langen K.J., Blaum M., Verburg F.A., Mottaghy F.M., Galldiks N. The use of O-(2-18F-fluoroethyl)-l-tyrosine PET for treatment management of bevacizumab and irinotecan in patients with recurrent high-grade glioma: a cost-effectiveness analysis. J. Nucl. Med. 2013;54:1217–1222. doi: 10.2967/jnumed.113.120089. [DOI] [PubMed] [Google Scholar]
- Herholz K., Kracht L.W., Heiss W.D. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J. Neuroimaging. 2003;13:269–271. [PubMed] [Google Scholar]
- Herholz K., Langen K.J., Schiepers C., Mountz J.M. Brain tumors. Semin. Nucl. Med. 2012;42:356–370. doi: 10.1053/j.semnuclmed.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi F.S., Hwu W.J., Kefford R., Weber J.S., Daud A., Hamid O., Patnaik A., Ribas A., Robert C., Gangadhar T.C., Joshua A.M., Hersey P., Dronca R., Joseph R., Hille D., Xue D., Li X.N., Kang S.P., Ebbinghaus S., Perrone A., Wolchok J.D. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 2016;34:1510–1517. doi: 10.1200/JCO.2015.64.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., McConathy J. Radiolabeled amino acids for oncologic imaging. J. Nucl. Med. 2013;54:1007–1010. doi: 10.2967/jnumed.112.113100. [DOI] [PubMed] [Google Scholar]
- Hutterer M., Nowosielski M., Putzer D., Waitz D., Tinkhauser G., Kostron H., Muigg A., Virgolini I.J., Staffen W., Trinka E., Gotwald T., Jacobs A.H., Stockhammer G. O-(2-18F-fluoroethyl)-l-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J. Nucl. Med. 2011;52:856–864. doi: 10.2967/jnumed.110.086645. [DOI] [PubMed] [Google Scholar]
- Jacobs A.H., Thomas A., Kracht L.W., Li H., Dittmar C., Garlip G., Galldiks N., Klein J.C., Sobesky J., Hilker R., Vollmar S., Herholz K., Wienhard K., Heiss W.D. 18F-fluoro-l-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J. Nucl. Med. 2005;46:1948–1958. [PubMed] [Google Scholar]
- Jansen N.L., Suchorska B., Schwarz S.B., Eigenbrod S., Lutz J., Graute V., Bartenstein P., Belka C., Kreth F.W., la Fougere C. [18F]fluoroethyltyrosine-positron emission tomography-based therapy monitoring after stereotactic iodine-125 brachytherapy in patients with recurrent high-grade glioma. Mol. Imaging. 2013;12:137–147. [PubMed] [Google Scholar]
- Jansen N.L., Suchorska B., Wenter V., Eigenbrod S., Schmid-Tannwald C., Zwergal A., Niyazi M., Drexler M., Bartenstein P., Schnell O., Tonn J.C., Thon N., Kreth F.W., la Fougere C. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J. Nucl. Med. 2014;55:198–203. doi: 10.2967/jnumed.113.122333. [DOI] [PubMed] [Google Scholar]
- Jansen N.L., Suchorska B., Wenter V., Schmid-Tannwald C., Todica A., Eigenbrod S., Niyazi M., Tonn J.C., Bartenstein P., Kreth F.W., la Fougere C. Prognostic significance of dynamic 18F-FET PET in newly diagnosed astrocytic high-grade glioma. J. Nucl. Med. 2015;56:9–15. doi: 10.2967/jnumed.114.144675. [DOI] [PubMed] [Google Scholar]
- Kamson D.O., Juhasz C., Buth A., Kupsky W.J., Barger G.R., Chakraborty P.K., Muzik O., Mittal S. Tryptophan PET in pretreatment delineation of newly-diagnosed gliomas: MRI and histopathologic correlates. J. Neuro-Oncol. 2013;112:121–132. doi: 10.1007/s11060-013-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamson D.O., Mittal S., Robinette N.L., Muzik O., Kupsky W.J., Barger G.R., Juhasz C. Increased tryptophan uptake on PET has strong independent prognostic value in patients with a previously treated high-grade glioma. Neuro-Oncology. 2014;16:1373–1383. doi: 10.1093/neuonc/nou042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir S., Fimmers R., Galldiks N., Schafer N., Mack F., Schaub C., Stuplich M., Niessen M., Tzaridis T., Simon M., Stoffels G., Langen K.J., Scheffler B., Glas M., Herrlinger U. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET. Clin. Cancer Res. 2016;22:2190–2196. doi: 10.1158/1078-0432.CCR-15-1334. [DOI] [PubMed] [Google Scholar]
- Kebir S., Rauschenbach L., Galldiks N., Schlaak M., Hattingen E., Landsberg J., Bundschuh R.A., Langen K.J., Scheffler B., Herrlinger U., Glas M. Dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET imaging for the detection of checkpoint inhibitor-related pseudoprogression in melanoma brain metastases. Neuro-Oncology. 2016;18(10):1462–1464. doi: 10.1093/neuonc/now154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kläsner B., Buchmann N., Gempt J., Ringel F., Lapa C., Krause B.J. Early [18F]FET-PET in gliomas after surgical resection: comparison with MRI and histopathology. PLoS One. 2015;10 doi: 10.1371/journal.pone.0141153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo A., Ishii H., Aoki S., Suzuki M., Nagasawa H., Kubota K., Minamimoto R., Arakawa A., Tominaga M., Arai H. Phase IIa clinical study of [18F]fluciclovine: efficacy and safety of a new PET tracer for brain tumors. Ann. Nucl. Med. 2016;30:608–618. doi: 10.1007/s12149-016-1102-y. [DOI] [PubMed] [Google Scholar]
- Kratochwil C., Combs S.E., Leotta K., Afshar-Oromieh A., Rieken S., Debus J., Haberkorn U., Giesel F.L. Intra-individual comparison of (18)F-FET and (18)F-DOPA in PET imaging of recurrent brain tumors. Neuro-Oncology. 2014;16:434–440. doi: 10.1093/neuonc/not199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruser T.J., Mehta M.P., Robins H.I. Pseudoprogression after glioma therapy: a comprehensive review. Expert. Rev. Neurother. 2013;13:389–403. doi: 10.1586/ern.13.7. [DOI] [PubMed] [Google Scholar]
- Kumar A.J., Leeds N.E., Fuller G.N., Van Tassel P., Maor M.H., Sawaya R.E., Levin V.A. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., Lang F.F., McCutcheon I.E., Hassenbusch S.J., Holland E., Hess K., Michael C., Miller D., Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- Langen K.J., Hamacher K., Weckesser M., Floeth F., Stoffels G., Bauer D., Coenen H.H., Pauleit D. O-(2-[18F]fluoroethyl)-l-tyrosine: uptake mechanisms and clinical applications. Nucl. Med. Biol. 2006;33:287–294. doi: 10.1016/j.nucmedbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Langen K.J., Jarosch M., Mühlensiepen H., Hamacher K., Broer S., Jansen P., Zilles K., Coenen H.H. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl. Med. Biol. 2003;30:501–508. doi: 10.1016/s0969-8051(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Langen K.J., Watts C. Neuro-oncology: amino acid PET for brain tumours - ready for the clinic? Nat. Rev. Neurol. 2016;12:375–376. doi: 10.1038/nrneurol.2016.80. [DOI] [PubMed] [Google Scholar]
- Lee S.T., Scott A.M. Hypoxia positron emission tomography imaging with 18f-fluoromisonidazole. Semin. Nucl. Med. 2007;37:451–461. doi: 10.1053/j.semnuclmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Lescher S., Schniewindt S., Jurcoane A., Senft C., Hattingen E. Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg. Focus. 2014;37 doi: 10.3171/2014.9.FOCUS14479. [DOI] [PubMed] [Google Scholar]
- Lizarraga K.J., Allen-Auerbach M., Czernin J., DeSalles A.A., Yong W.H., Phelps M.E., Chen W. (18)F-FDOPA PET for differentiating recurrent or progressive brain metastatic tumors from late or delayed radiation injury after radiation treatment. J. Nucl. Med. 2014;55:30–36. doi: 10.2967/jnumed.113.121418. [DOI] [PubMed] [Google Scholar]
- Macdonald D.R., Cascino T.L., Schold S.C., Jr., Cairncross J.G. Response criteria for phase II studies of supratentorial malignant glioma. J. Clin. Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- Minniti G., Clarke E., Lanzetta G., Osti M.F., Trasimeni G., Bozzao A., Romano A., Enrici R.M. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011:48. doi: 10.1186/1748-717X-6-48. (May 15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morana G., Piccardo A., Garre M.L., Nozza P., Consales A., Rossi A. Multimodal magnetic resonance imaging and 18F-l-dihydroxyphenylalanine positron emission tomography in early characterization of pseudoresponse and nonenhancing tumor progression in a pediatric patient with malignant transformation of ganglioglioma treated with bevacizumab. J. Clin. Oncol. 2013;31:e1–e5. doi: 10.1200/JCO.2012.43.6113. [DOI] [PubMed] [Google Scholar]
- Moulin-Romsée G., D'Hondt E., de Groot T., Goffin J., Sciot R., Mortelmans L., Menten J., Bormans G., Van Laere K. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur. J. Nucl. Med. Mol. Imaging. 2007;34:2082–2087. doi: 10.1007/s00259-007-0557-4. [DOI] [PubMed] [Google Scholar]
- Nduom E.K., Wei J., Yaghi N.K., Huang N., Kong L.Y., Gabrusiewicz K., Ling X., Zhou S., Ivan C., Chen J.Q., Burks J.K., Fuller G.N., Calin G.A., Conrad C.A., Creasy C., Ritthipichai K., Radvanyi L., Heimberger A.B. PD-L1 expression and prognostic impact in glioblastoma. Neuro-Oncology. 2016;18:195–205. doi: 10.1093/neuonc/nov172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H., Weller M., Huang R., Finocchiaro G., Gilbert M.R., Wick W., Ellingson B.M., Hashimoto N., Pollack I.F., Brandes A.A., Franceschi E., Herold-Mende C., Nayak L., Panigrahy A., Pope W.B., Prins R., Sampson J.H., Wen P.Y., Reardon D.A. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015;16:e534–e542. doi: 10.1016/S1470-2045(15)00088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Zhen H.N., Kawai N., Nishiyama Y., Haba R., Tamiya T. Correlation of l-methyl-11C-methionine (MET) uptake with l-type amino acid transporter 1 in human gliomas. J. Neuro-Oncol. 2010;99:217–225. doi: 10.1007/s11060-010-0117-9. [DOI] [PubMed] [Google Scholar]
- Papin-Michault C., Bonnetaud C., Dufour M., Almairac F., Coutts M., Patouraux S., Virolle T., Darcourt J., Burel-Vandenbos F. Study of LAT1 expression in brain metastases: towards a better understanding of the results of positron emission tomography using amino acid tracers. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piroth M.D., Holy R., Pinkawa M., Stoffels G., Kaiser H.J., Galldiks N., Herzog H., Coenen H.H., Eble M.J., Langen K.J. Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother. Oncol. 2011;99:218–224. doi: 10.1016/j.radonc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Piroth M.D., Liebenstund S., Galldiks N., Stoffels G., Shah N.J., Eble M.J., Coenen H.H., Langen K.J. Monitoring of radiochemotherapy in patients with glioblastoma using O-(2-(18)fluoroethyl)-l-tyrosine positron emission tomography: is dynamic imaging helpful? Mol. Imaging. 2013;12:388–395. [PubMed] [Google Scholar]
- Piroth M.D., Pinkawa M., Holy R., Klotz J., Nussen S., Stoffels G., Coenen H.H., Kaiser H.J., Langen K.J., Eble M.J. Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int. J. Radiat. Oncol. Biol. Phys. 2011;80:176–184. doi: 10.1016/j.ijrobp.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Pirotte B.J., Levivier M., Goldman S., Massager N., Wikler D., Dewitte O., Bruneau M., Rorive S., David P., Brotchi J. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery. 2009;64:471–481. doi: 10.1227/01.NEU.0000338949.94496.85. [DOI] [PubMed] [Google Scholar]
- Pöpperl G., Goldbrunner R., Gildehaus F.J., Kreth F.W., Tanner P., Holtmannspotter M., Tonn J.C., Tatsch K. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:1018–1025. doi: 10.1007/s00259-005-1819-7. [DOI] [PubMed] [Google Scholar]
- Pöpperl G., Götz C., Rachinger W., Schnell O., Gildehaus F.J., Tonn J.C., Tatsch K. Serial O-(2-[(18)F]fluoroethyl)-l:-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur. J. Nucl. Med. Mol. Imaging. 2006;33:792–800. doi: 10.1007/s00259-005-0053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöpperl G., Kreth F.W., Mehrkens J.H., Herms J., Seelos K., Koch W., Gildehaus F.J., Kretzschmar H.A., Tonn J.C., Tatsch K. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:1933–1942. doi: 10.1007/s00259-007-0534-y. [DOI] [PubMed] [Google Scholar]
- Poulsen S.H., Urup T., Grunnet K., Christensen I.J., Larsen V.A., Jensen M.L., Af Rosenschold P.M., Poulsen H.S., Law I. The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur. J. Nucl. Med. Mol. Imaging. 2016 doi: 10.1007/s00259-016-3494-2. (Epub ahead of print 23 Aug) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusser M., Lim M., Hafler D.A., Reardon D.A., Sampson J.H. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat. Rev. Neurol. 2015;11:504–514. doi: 10.1038/nrneurol.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier T., Lopez W.O., Spehl T.S., Nguyen T., Mader I., Nikkhah G., Pinsker M.O. Bevacizumab as salvage therapy for progressive brain stem gliomas. Clin. Neurol. Neurosurg. 2013;115:165–169. doi: 10.1016/j.clineuro.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Roelcke U., Wyss M.T., Nowosielski M., Ruda R., Roth P., Hofer S., Galldiks N., Crippa F., Weller M., Soffietti R. Amino acid positron emission tomography to monitor chemotherapy response and predict seizure control and progression-free survival in WHO grade II gliomas. Neuro-Oncology. 2016;18:744–751. doi: 10.1093/neuonc/nov282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzenberg J., Czernin J., Cloughesy T.F., Ellingson B.M., Pope W.B., Grogan T., Elashoff D., Geist C., Silverman D.H., Phelps M.E., Chen W. Treatment response evaluation using 18F-FDOPA PET in patients with recurrent malignant glioma on bevacizumab therapy. Clin. Cancer Res. 2014;20:3550–3559. doi: 10.1158/1078-0432.CCR-13-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.H., Snelling B., Bregy A., Patel P.R., Tememe D., Bhatia R., Sklar E., Komotar R.J. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J. Neuro-Oncol. 2013;112:141–152. doi: 10.1007/s11060-013-1059-9. [DOI] [PubMed] [Google Scholar]
- Singhal T., Narayanan T.K., Jain V., Mukherjee J., Mantil J. 11C-l-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol. Imaging Biol. 2008;10:1–18. doi: 10.1007/s11307-007-0115-2. [DOI] [PubMed] [Google Scholar]
- Spence A.M., Muzi M., Swanson K.R., O'Sullivan F., Rockhill J.K., Rajendran J.G., Adamsen T.C., Link J.M., Swanson P.E., Yagle K.J., Rostomily R.C., Silbergeld D.L., Krohn K.A. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin. Cancer Res. 2008;14:2623–2630. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- Stuplich M., Hadizadeh D.R., Kuchelmeister K., Scorzin J., Filss C., Langen K.J., Schafer N., Mack F., Schuller H., Simon M., Glas M., Pietsch T., Urbach H., Herrlinger U. Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J. Clin. Oncol. 2012;30:e180–e183. doi: 10.1200/JCO.2011.40.9565. [DOI] [PubMed] [Google Scholar]
- Su Z., Herholz K., Gerhard A., Roncaroli F., Du Plessis D., Jackson A., Turkheimer F., Hinz R. [(11)C]-(R)PK11195 tracer kinetics in the brain of glioma patients and a comparison of two referencing approaches. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:1406–1419. doi: 10.1007/s00259-013-2447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z., Roncaroli F., Durrenberger P.F., Coope D.J., Karabatsou K., Hinz R., Thompson G., Turkheimer F.E., Janczar K., Du Plessis D., Brodbelt A., Jackson A., Gerhard A., Herholz K. The 18-kDa mitochondrial translocator protein in human gliomas: a 11C-(R)PK11195 PET imaging and neuropathology study. J. Nucl. Med. 2015;56:512–517. doi: 10.2967/jnumed.114.151621. [DOI] [PubMed] [Google Scholar]
- Suchorska B., Jansen N.L., Linn J., Kretzschmar H., Janssen H., Eigenbrod S., Simon M., Popperl G., Kreth F.W., la Fougere C., Weller M., Tonn J.C., German Glioma N. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology. 2015;84:710–719. doi: 10.1212/WNL.0000000000001262. [DOI] [PubMed] [Google Scholar]
- Suchorska B., Tonn J.C., Jansen N.L. PET imaging for brain tumor diagnostics. Curr. Opin. Neurol. 2014;27:683–688. doi: 10.1097/WCO.0000000000000143. [DOI] [PubMed] [Google Scholar]
- Swissmedic Swiss agency for therapeutic products. J. Swissmedic. 2014;13:651. [Google Scholar]
- Terakawa Y., Tsuyuguchi N., Iwai Y., Yamanaka K., Higashiyama S., Takami T., Ohata K. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J. Nucl. Med. 2008;49:694–699. doi: 10.2967/jnumed.107.048082. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi N., Sunada I., Iwai Y., Yamanaka K., Tanaka K., Takami T., Otsuka Y., Sakamoto S., Ohata K., Goto T., Hara M. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J. Neurosurg. 2003;98:1056–1064. doi: 10.3171/jns.2003.98.5.1056. [DOI] [PubMed] [Google Scholar]
- Venneti S., Dunphy M.P., Zhang H., Pitter K.L., Zanzonico P., Campos C., Carlin S.D., La Rocca G., Lyashchenko S., Ploessl K., Rohle D., Omuro A.M., Cross J.R., Brennan C.W., Weber W.A., Holland E.C., Mellinghoff I.K., Kung H.F., Lewis J.S., Thompson C.B. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aaa1009. (274ra217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voges J., Herholz K., Holzer T., Würker M., Bauer B., Pietrzyk U., Treuer H., Schroder R., Sturm V., Heiss W.D. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact. Funct. Neurosurg. 1997;69:129–135. doi: 10.1159/000099864. [DOI] [PubMed] [Google Scholar]
- Wardak M., Schiepers C., Cloughesy T.F., Dahlbom M., Phelps M.E., Huang S.C. 18F-FLT and 18F-FDOPA PET kinetics in recurrent brain tumors. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1199–1209. doi: 10.1007/s00259-013-2678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W.A., Wester H.J., Grosu A.L., Herz M., Dzewas B., Feldmann H.J., Molls M., Stocklin G., Schwaiger M. O-(2-[18F]fluoroethyl)-l-tyrosine and l-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur. J. Nucl. Med. 2000;27:542–549. doi: 10.1007/s002590050541. [DOI] [PubMed] [Google Scholar]
- Wen P.Y., Macdonald D.R., Reardon D.A., Cloughesy T.F., Sorensen A.G., Galanis E., Degroot J., Wick W., Gilbert M.R., Lassman A.B., Tsien C., Mikkelsen T., Wong E.T., Chamberlain M.C., Stupp R., Lamborn K.R., Vogelbaum M.A., van den Bent M.J., Chang S.M. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- Wester H.J., Herz M., Weber W., Heiss P., Senekowitsch-Schmidtke R., Schwaiger M., Stocklin G. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-l-tyrosine for tumor imaging. J. Nucl. Med. 1999;40:205–212. [PubMed] [Google Scholar]
- Winkeler A., Boisgard R., Awde A.R., Dubois A., Theze B., Zheng J., Ciobanu L., Dolle F., Viel T., Jacobs A.H., Tavitian B. The translocator protein ligand [18F]DPA-714 images glioma and activated microglia in vivo. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:811–823. doi: 10.1007/s00259-011-2041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiriyasermkul P., Nagamori S., Tominaga H., Oriuchi N., Kaira K., Nakao H., Kitashoji T., Ohgaki R., Tanaka H., Endou H., Endo K., Sakurai H., Kanai Y. Transport of 3-fluoro-l-alpha-methyl-tyrosine by tumor-upregulated l-type amino acid transporter 1: a cause of the tumor uptake in PET. J. Nucl. Med. 2012;53:1253–1261. doi: 10.2967/jnumed.112.103069. [DOI] [PubMed] [Google Scholar]
- Wolchok J.D., Hoos A., O'Day S., Weber J.S., Hamid O., Lebbe C., Maio M., Binder M., Bohnsack O., Nichol G., Humphrey R., Hodi F.S. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- Würker M., Herholz K., Voges J., Pietrzyk U., Treuer H., Bauer B., Sturm V., Heiss W.D. Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur. J. Nucl. Med. 1996;23:583–586. doi: 10.1007/BF00833397. [DOI] [PubMed] [Google Scholar]
- Wyss M., Hofer S., Bruehlmeier M., Hefti M., Uhlmann C., Bartschi E., Buettner U.W., Roelcke U. Early metabolic responses in temozolomide treated low-grade glioma patients. J. Neuro-Oncol. 2009;95:87–93. doi: 10.1007/s11060-009-9896-2. [DOI] [PubMed] [Google Scholar]
- Youland R.S., Kitange G.J., Peterson T.E., Pafundi D.H., Ramiscal J.A., Pokorny J.L., Giannini C., Laack N.N., Parney I.F., Lowe V.J., Brinkmann D.H., Sarkaria J.N. The role of LAT1 in (18)F-DOPA uptake in malignant gliomas. J. Neuro-Oncol. 2013;111:11–18. doi: 10.1007/s11060-012-0986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]