Abstract
It is often stated that short-term memory is consolidated in a protein-synthesis-dependent manner into long-term memory. Alternatively, memories might consist of distinct molecular traces that last for different periods of time. These traces can be graded by their ‘volatility’; traces encoded by activation of protein kinases are more volatile than traces encoded by morphological changes at preexisting synapses. The least volatile (‘static’) traces are due to the generation and stabilization of new synapses. Importantly, whereas at the cellular level these traces are generated independently of each other, they might be linked at the network level where volatile memory traces are required to set up a cellular network that is in turn required to induce the static memory trace.
Thinking about memory in terms of the molecular trace
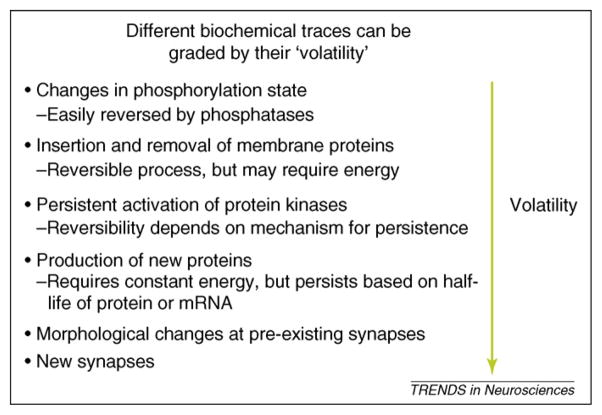
Memories are defined by behavioral experiments, where the distinction between short-term and long-term memory is time based and the conversion of short-term to long-term memory is called ‘consolidation.’ I propose that it is preferable to use a biochemical-based differentiation of memory traces that does not depend on absolute time but on the mechanism of the molecular trace. In computer science, the terms ‘volatile’ and ‘static’ differentiate types of memory. For example, memory that is stored in the state of a transistor requires constant input and is termed volatile, whereas memory stored on a magnetic disc does not require constant input and is termed static. Memories in the brain can also be differentiated based on the physical trace underlying the memory; however, unlike computer memory that either requires constant input or does not, physical memory traces are graded based on how long they last and how easily they are maintained. I will use the terms from computer science, volatile and static, to define this characteristic of memory traces, although this is not meant to imply an isomorphic relationship between computer and neuronal memory storage. Memory traces that depend on modification of preexisting proteins (such as phosphorylation) will be more volatile than those that depend on changes in protein levels, and these in turn will be more volatile than those due to morphological changes (Figure 1).
Figure 1.
Illustration of biochemical mechanisms underlying memory traces in order of their relative volatility.
Serial versus parallel pathways for memory formation: the three little pigs
Are volatile memory traces made into static memory traces through consolidation, or are distinct volatile and static memory traces initiated after experience? An instructive analogy is to envision that the static memory trace is a brick house. If volatile traces are stabilized into static memories, the structure of the house would be built as a volatile memory, stabilized by adding plaster and then consolidated by adding bricks. This model is implicit in the term ‘consolidation’ or when we talk about the ‘conversion’ of short- to long-term memory. By contrast, memory traces might resemble the houses built by the three little pigs, with the straw house representing the most volatile trace, a stick house representing a less volatile trace and a brick house representing the static trace. All three traces could be initiated in parallel after an experience occurs. If production of the straw house is blocked, building and consolidation of the brick house continues. The strong prediction of this model is that long-term memories can be formed in the absence of short-term memories. The most obvious instantiation of this at the cellular level is where the static memory trace is stored at new synapses, whereas more volatile memory traces are stored in changes at preexisting synapses. In this case, the molecular steps in formation and stabilization of new synapses are distinct from the molecular steps in strengthening preexisting synapses, and could easily occur in parallel. Below I will outline the evidence from invertebrate and vertebrate models that support this model and highlight important implications of this model for our understanding of memory.
An important limitation of this model is that it only addresses memory traces at the cellular level. Formation of a long-term memory might require the generation of a multicellular network that through repeated activation initiates and stabilizes a static memory trace. If the formation of this network requires volatile traces, then there will appear to be a serial relationship between the volatile and static memory traces. However, at the molecular and cellular level, these traces would still be made in parallel.
Cellular memory in Aplysia
The best evidence for parallel biochemical traces underlying memory comes from studying the changes at the sensory-motor neuron synapse in Aplysia californica that underlie behavioral sensitization [1]. When Aplysia are given a noxious stimulus, interneurons that release serotonin (5-HT) are activated [2]. The release of 5-HT causes a complex series of biochemical events that lead to changes in the circuit from receiving the sensory touch to moving the muscle such that defensive reflexes are increased. The most prominent cellular changes associated with behavioral memory are changes in output from the sensory neurons, including an increase in the strength of the direct sensory-motor neuron synapses. This increase in synaptic strength is termed ‘facilitation.’
A long-term cellular memory in Aplysia that does not depend on short-term memory
The first evidence that one could generate long-term facilitation (LTF) (lasting 24 h) without short-term facilitation (STF) in this system was by Emptage and Carew, who found that 5-HT applied selectively to the cell body led to LTF in the absence of STF [3]; also see [4]. Further work in cell culture showed that 5-HT application to the cell body gave a comparable amount of facilitation as 5-HT applied both to the cell body and synapse, but without STF [5,6], and this form of LTF required gene expression [6]. The gene expression required for Aplysia LTF is downstream of the transcription factor CREB [7]. Indeed, injection of phosphorylated CREB is sufficient to generate LTF in the absence of STF [6,7]. However, this type of cellular memory is relatively volatile; it disappears by 72 h [6] and new synapses are not formed [5,6]. Thus, this form of LTF is more volatile because it only involves changes at pre-existing synapses, whereas LTF that is encoded at new synapses is more static and lasts longer.
Independent biochemical traces coexist at the sensory-motor neuron synapse
Multiple parallel traces are created at the sensory-motor neuron synapse during facilitation (Figure 2). The most volatile trace, underlying STF, is a result of cAMP-mediated PKA activation and phosphorylation of proteins leading to an increase in transmitter release [8]. Multiple spaced sensitization training trials or multiple spaced applications of 5-HT lead to a persistent activation of PKA [9,10] that maintains the memory trace up to ~12 h, but not at 24 h [11,12]. Both the facilitation and the persistent activation of PKA initially require protein synthesis, but not gene expression, but then does require gene expression at later times [10,13,14]. Thus, there are multiple overlapping memory traces induced by the same stimulus (five spaced applications of 5-HT) over the first 24 h, when facilitation is dependent on changes at preexisting synapses (Figure 2).
Figure 2.
The maintenance of the increase in synaptic strength seen after five pulses of 5-HT consist with five possibly parallel mechanisms listed in order of volatility (bottom to top). The first three depend on activation of PKA (bottom) through different mechanisms. Another parallel memory trace depends on morphological change at preexisting synapses. These occur first in a protein-synthesis-independent manner, but then are consolidated in a protein-synthesis- and gene-expression-dependent manner [59]. The synaptic tag is important for this phase of memory and for stabilizing new synapses, but does not itself increase synaptic strength. Finally, the most static memory is stored in new synapses.
Which of these changes are serial or parallel? The best way to determine this is to find biochemical pathways involved specifically in one type of memory and determine whether blocking this pathway blocks later phases of memory formation. For example, in the initial studies by Emptage and Carew, cyproheptadine blocked STF without blocking LTF, demonstrating that LTF was induced in parallel with STF [3]. Another way to probe whether the memory traces are induced in a serial or parallel process is to determine whether one can induce later traces in the absence of induction of earlier traces. For example, the molecular trace underlying translation-dependent, transcription-independent changes in synaptic strength (often called intermediate-term facilitation; ITF) requires spaced applications of 5-HT at the synapse [15]. However, when a single pulse of 5-HT at the synapse is coupled with a separate stimulus that activates gene expression [6], the later traces are formed in the absence of ITF [16]. In addition, the trace lasting 12 h that depends on gene-expression-dependent persistent activation of PKA is not required for the later PKA-independent biochemical trace because after injection of phospho-CREB, the facilitation seen at 12 h is not observed but facilitation is still seen at 24 h [6]. Thus, there are at least three traces induced by five applications of 5-HT to sensory-motor neuron synapses: (i) translationally dependent persistent PKA activation; (ii) gene-expression-dependent persistent PKA activation; and (iii) gene expression changes independent of PKA. The longest-lasting trace can be induced in the absence of the two previous traces, suggesting that these traces are made in parallel (Figure 2).
The synaptic tag is a latent trace
When 5-HT is applied to only one of the two branches of a bifurcating sensory neuron, only one of the branches undergoes synaptic facilitation [17]. Thus, addition of 5-HT to the synapse stimulates the formation of a process (often called a tag or a mark) to allow this synapse to capture more of the products produced by gene expression, preventing their capture by the nonactivated synapse. Induction of the tag does not induce ITF or require protein synthesis [17]. Thus, formation of the synaptic tag is another parallel process (Figure 2); however, this process by itself is not a memory trace, because it does not increase synaptic strength on its own. To define biochemical processes that are required for later memory traces but do not in themselves increase synaptic strength, I use the term ‘latent’ trace.
New synapses are a static trace requiring multiple consolidation steps
Whereas new synapses are not required for 24 h LTF, blocking the maintenance of these synapses blocks 72 h LTF [6]. What is the biochemical basis for the induction and stabilization of the new synapses? A 5 min pulse of 5-HT is sufficient to induce the growth at the synapse [6]. This nascent growth is another example of a latent trace, because it is required for the formation of new synapses, but in the absence of additional events does not cause an increase in synaptic strength. Initial synaptic growth is independent of protein synthesis, whereas maintenance and elaboration of that growth requires local protein synthesis [18,19]. Moreover, maintenance and maturation of the synapse at 24 h requires gene expression [18]. Even after 24 h, the new synapses are unstable and require further events to become consolidated into more permanent structures [6]. Thus, the formation of new synapses is a physical trace that represents a serial process that fits well into the consolidation model. After all, making the brick house requires multiple steps. However, while this static trace is being built, more volatile traces represent the increase in synaptic strength that underlies the memory. Moreover, these volatile traces are not required for the formation of the static trace.
Behavioral sensitization studies support distinct volatile and static traces underlying memory
New sensory-to-motor neuron synapses are observed after 4 days of sensitization training, and although morphological changes at preexisting synapses are seen early after training, the most persistent change is in the number of synapses [20]. Interestingly, only 1 day of training leads to behavioral sensitization at 24 h in the absence of new synapses [21], consistent with the evidence discussed above from cultured sensory-motor neurons that changes in the strength of preexisting synapses are more important for memory at this time. However, unlike in cultured neurons, training sessions on multiple days are required for observation of new synapses in the animal [21]. mRNAs that are required to be locally translated to elaborate or maintain synaptic growth might not be present at the synapse at rest. The initial training session could induce these products and stimulate their transport to the synapse, where they can be used the next day during training to induce morphological changes. This might explain certain priming effects observed during sensitization in Aplysia [22,23]. In cultures, these messages might already be present at the synapse because of the synaptic growth induced by culturing the neurons. This priming gene expression is another example of a latent biochemical trace.
Lessons from Aplysia
There are many parallel memory traces that underlie increases in synaptic strength. These traces last various periods of time. However, the most static trace involves the formation and stabilization of new synapses. Formation of the static trace requires several latent traces, such as the synaptic tag, transport of mRNAs to synapses and the formation of nascent morphological changes that do not themselves cause changes in synaptic strength.
Parallel volatile and static memory traces in mammals
Is LTP in CA3-CA1 neurons a serial or a parallel process? Elegant studies have demonstrated that insertion of GluR1-containing AMPA receptors into synapses is the mechanism underlying the early phase of LTP (E-LTP) in the CA1 region of the hippocampus [24]. Similar to Aplysia LTF, there is also a late phase of LTP (L-LTP) that requires protein synthesis and gene expression [20]. Does L-LTP require E-LTP? This question is still open. Similar to Aplysia, L-LTP has an initial translation-dependent transcription-independent phase [25], followed by a later gene-expression-dependent phase. Also similar to Aplysia, the gene-expression-dependent phase requires a synaptic tag [26]. It is unclear whether, similar to Aplysia, the combination of activating gene expression and setting the synaptic tag would be sufficient for synaptic increases in the absence of E-LTP. One indication that it could is that activation of cAMP can lead to a slowly rising increase in synaptic strength, although cAMP is not sufficient to induce E-LTP [27]. To explicitly test this hypothesis, one could determine whether one could generate L-LTP in slices from adult GluR1 KO mice that lack E-LTP owing to the lack of AMPA receptor insertion [28].
The maintenance of L-LTP depends on translation of the persistently active kinase PKMζ, but E-LTP does not require PKMζ [29,30]. Because expression of PKMζ is sufficient for increases in synaptic strength [31], it suggests that the maintenance phase could occur in parallel with and independently of the earlier insertion of AMPA receptors. Strikingly, unlike in Aplysia, where persistent activation of protein kinases plays a time-limited role in maintaining synaptic strength, inhibition of PKMζ can reverse both LTP at 24 h and memories after weeks [32]. Thus, PKMζ is an example of a memory trace that can last for a significant period of time. However, this long-lasting memory can be reversed using a single application of a pharmacological agent that inhibits the kinase. Thus, this memory is still volatile in the sense that it can be easily reversed.
The late phase of LTP in CA1 neurons can also involve presynaptic changes that are downstream of BDNF [33–35]. Again, it is possible that this trace occurs in parallel to the traces mentioned above. Thus, although parallel processes have not been proven to occur during LTP, I propose the possibility of three independent traces: insertion of AMPA receptors through activation of calcium-activated kinases; translation of PKMζ; and gene-expression-dependent production of BDNF that induces presynaptic changes (Figure 3). These might represent parallel processes induced by the same stimulus, or distinct traces that are activated using different stimulus paradigms. Nevertheless, it suggests that increases in synaptic strength rely on multiple types of molecular traces.
Figure 3.

The maintenance of synaptic change after experience in vertebrates might also consist of parallel biochemical traces. Five possible parallel traces are suggested.
Long-term memory without short-term memory in mammals
Although there might be distinct molecular traces underlying LTP, can they be linked to different stages of memory? Whereas answering this issue definitively will require a much better understanding of the relationship between LTP and memory, there are some intriguing results suggesting that distinct molecular traces are linked to different stages of memory. The lack of LTP and LTD in the hippocampus of adult GluR1 KO mice has provided an important test for the role of E-LTP in memory. Indeed, mice lacking GluR1 have a severe deficit in short-term spatial memory when the animal must remember a location that it has recently visited [36]. This deficit can be seen even 45 min after training, distinguishing it from ‘working memory’ [37]. Surprisingly, long-term spatial memory or ‘reference’ memory is not perturbed, even in the same task [36]. Thus, the traces required for short-term memory might not be required for the formation of the less volatile traces needed for reference memory.
Another interesting genetically modified animal is the CAMKII (T-A) mouse. This mutation blunts the ability of CAMKII to respond more strongly to strong stimulation, and in these animals LTP and memory are both abrogated in many tasks [38]. However, when multiple training tasks are given, these impairments disappear and memory is normal [39]. This might be a result of recruitment of the same pathway (i.e. insertion of AMPA receptors) owing to alternative molecular mechanisms, or recruitment of alternative circuits that are not sensitive to this molecular pathway [40]. However, as discussed above, this might be evidence for recruitment of a long-term trace in the absence of short-term traces.
Izquierdo and colleagues have argued strongly for the parallel nature of STM and LTM in inhibitory avoidance learning [41] because, with some pharmacological inhibitors, for example a 5-HT1A antagonist, the expression of memory is blocked at 1.5 h but not at 24 h, demonstrating in these cases the presence of LTM in the absence of STM [41].
Morphological changes during memory formation in vertebrates
LTP in CA1 neurons causes increases in the size of spines at preexisting synapses that have undergone LTP [42]. Interestingly, although this requires GluR1 insertion, similar to E-LTP, E-LTP itself is not required because when pore-dead forms of GluR1 that would not express E-LTP are inserted, there is still an increase in spine size [43]. Thus, although E-LTP and spine changes are linked, at some level they are parallel memory traces.
There is considerable controversy over whether long-term memories are stored in new synapses in mammals [44,45]. Intriguing long-term experiments have been done using in vivo imaging of mice expressing GFP in a subset of neurons. These studies show that most spines and synaptic boutons are stable over time, suggesting that they could form the basis for a static memory [46–48]. When new spines are seen after imaging, they usually have a short half-life [47,48], suggesting additional steps are required to stabilize new synapses, similar to Aplysia. Another important result from these studies is that different neurons have different abilities to undergo morphological changes [46,49]. Thus, choosing the right place to look might be critical for finding the static memories formed in vertebrates.
One interesting finding is that overexpression of a form of CREB was sufficient to increase the number of spines in hippocampal neurons; these spines had presynaptic partners, but apparently lacked AMPA receptors, matching the description of silent synapses [50]. This might suggest that previous activation of a neuron is sufficient to generate the template for the next phase of plasticity; these silent synapses might be another example of a latent trace.
Are volatile traces required to set up circuits that induce static traces?
Formation of memories might require ongoing network activity following the initial changes induced by experience to consolidate the memory. At the cellular level, consolidation is used to imply that short-term molecular traces are converted into long-term traces through protein synthesis and gene expression. I am suggesting that at the cellular level, the use of this word might be misleading if indeed these lasting molecular traces are formed in parallel with the short-term trace. However, this does not mean that ongoing network activity is not needed for generation or stabilization of LTM. Indeed, it might be that if the organization of the cellular network required for this persistent network activity requires volatile molecular traces, and this network activity is required to form or stabilize the static memory trace, then experiments will reveal an apparent serial relationship between volatile and static traces even if at the cellular and molecular level, these two traces represent parallel processes.
Experiments with inhibitory avoidance have examined this question in detail. Pharmacological inhibitors act in different brain regions at different times, suggesting initially short-term changes set up a network in one brain region and then activation of that circuit causes changes in another brain region, required for later phases of memory [51]. In a recent example, inhibitors of protein synthesis or BDNF applied 6 h after learning blocked memory at 1 week, but not at 2 days. This suggests that short-term changes in the hippocampus results in a network that then induces additional static memory traces that are only required after the volatile traces have decayed [52].
Evidence for activation of a circuit that then can act to facilitate long-term changes is most evident from studies of replay. When a rodent goes around a circuit, hippo-campal neurons that act as place cells fire in the order that the animal moves through the circuit. One can then observe a replay of this circuit, both immediately after behavior and during stages of sleep following behavior [53]. I suggest that setting up a circuit to allow replay might involve volatile changes, and activation of this circuit is then required to encode later static memory formation.
LTP in lateral amygdyla neurons is believed to be part of the mechanism underlying memory for auditory fear conditioning [54]. In this paradigm, inhibiting AMPA receptor insertion using viral delivery of a peptide blocked both short- and long-term learning, suggesting the requirement of E-LTP for long-term memory [55]. However, this block occurred when insertion is blocked in only 20% of the lateral amygdyla neurons, consistent with the model that a network needs to be created by these volatile changes that is required for later more permanent changes. Indeed, the transcription factor CREB is also required for auditory fear conditioning [56]. Replacing CREB in a CREB KO mouse using a similar viral strategy revealed that CREB is only required in 20% of amygdyla neurons for learning to be rescued [57]. At face value, these results appear to be conflicting; how can only 20% of the neurons be required when blocking only 20% of the neurons is sufficient to interrupt the memory? However, the two results can be accommodated in a model where the initial learning sets up a circuit that needs to be activated to generate the long-term trace. Blocking the early changes in only 20% of the neurons is enough to block the formation of the circuit; however, fewer neurons are required for the static memory trace.
Conclusions
The concept that short-term memory is converted into long-term memory or that short-term memories are consolidated might not semantically reflect the underlying process where multiple parallel biochemical processes are occurring at the same time. One can grade biochemical traces underlying memory by their volatility. Volatile traces might last a long time, such as traces encoded by persistent activation of PKMζ [32], but I predict that these types of memory are less stable, perhaps more susceptible to events such as reconsolidation [58]. It is also important to realize that there are many latent memory traces, such as the synaptic tag, new synaptic growth, transport of mRNA to processes, and silent synapses that might be required for memory formation but do not in themselves change synaptic strength. Finally, even if distinct traces are induced using parallel pathways, they might be linked at the systems level if volatile traces are required to set up circuits that are then required to induce a static trace. A critical step in establishing this model will be to identify whether or not new synapses represent the static traces that encode long-term memory in vertebrate systems and to then determine the requirements for formation of these synapses.
Acknowledgments
This work was supported by Canadian Institutes of Health Research (CIHR) grants MOP 12046 and 15121 (W.S.S.). W.S.S. is a William Dawson Scholar and a Fonds de la Recherche en Santé du Québec (FRSQ) Chercheur National. I thank Dr Karim Nader and Ed Ruthazer for comments on an earlier form of this manuscript.
References
- 1.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 2.Marinesco S, et al. Serotonergic modulation in Aplysia I Distributed serotonergic network persistently activated by sensitizing stimuli. J Neurophysiol. 2004;92:2468–2486. doi: 10.1152/jn.00209.2004. [DOI] [PubMed] [Google Scholar]
- 3.Emptage NJ, Carew TJ. Long-term synaptic facilitation in the absence of short-term facilitation in Aplysia neurons. Science. 1993;262:253–256. doi: 10.1126/science.8211146. [DOI] [PubMed] [Google Scholar]
- 4.Clark GA, Kandel ER. Induction of long-term facilitation in Aplysia sensory neurons by local application of serotonin to remote synapses. Proc Natl Acad Sci U S A. 1993;90:11411–11415. doi: 10.1073/pnas.90.23.11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun ZY, Schacher S. Binding of serotonin to receptors at multiple sites is required for structural plasticity accompanying long-term facilitation of Aplysia sensorimotor synapses. J Neurosci. 1998;18:3991–4000. doi: 10.1523/JNEUROSCI.18-11-03991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadio A, et al. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch D, et al. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins RD, et al. Molecular mechanisms of memory storage in Aplysia. Biol Bull. 2006;210:174–191. doi: 10.2307/4134556. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg SM, et al. A molecular mechanism for long-term sensitization in Aplysia. Nature. 1987;329:62–65. doi: 10.1038/329062a0. [DOI] [PubMed] [Google Scholar]
- 10.Muller U, Carew TJ. Serotonin induces temporally and mechanistically distinct phases of persistent PKA activity in Aplysia sensory neurons. Neuron. 1998;21:1423–1434. doi: 10.1016/s0896-6273(00)80660-1. [DOI] [PubMed] [Google Scholar]
- 11.Chain DG, et al. Mechanisms for generating the autonomous cAMP-dependent protein kinase required for long-term facilitation in Aplysia. Neuron. 1999;22:147–156. doi: 10.1016/s0896-6273(00)80686-8. [DOI] [PubMed] [Google Scholar]
- 12.Hegde AN, et al. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 13.Ghirardi M, et al. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 14.Sutton MA, et al. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 15.Sherff CM, Carew TJ. Parallel somatic and synaptic processing in the induction of intermediate-term and long-term synaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 2004;101:7463–7468. doi: 10.1073/pnas.0402163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauelshagen J, et al. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin KC, et al. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 18.Grabham PW, et al. Initiating morphological changes associated with long-term facilitation in Aplysia is independent of transcription or translation in the cell body. J Neurobiol. 2005;64:202–212. doi: 10.1002/neu.20133. [DOI] [PubMed] [Google Scholar]
- 19.Udo H, et al. Serotonin-induced regulation of the actin network for learning-related synaptic growth requires Cdc42, N-WASP, and PAK in Aplysia sensory neurons. Neuron. 2005;45:887–901. doi: 10.1016/j.neuron.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Barco A, et al. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 21.Wainwright ML, et al. Localized neuronal outgrowth induced by long-term sensitization training in Aplysia. J Neurosci. 2002;22:4132–4141. doi: 10.1523/JNEUROSCI.22-10-04132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antzoulatos EG, et al. Long-term sensitization training primes Aplysia for further learning. Learn Mem. 2006;13:422–425. doi: 10.1101/lm.230306. [DOI] [PubMed] [Google Scholar]
- 23.Philips GT, et al. Latent memory for sensitization in Aplysia. Learn Mem. 2006;13:224–229. doi: 10.1101/lm.111506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher RJ, III, et al. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 27.Navakkode S, et al. Synergistic requirements for the induction of dopaminergic D1/D5-receptor-mediated LTP in hippocampal slices of rat CA1 in vitro. Neuropharmacology. 2007;52:1547–1554. doi: 10.1016/j.neuropharm.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Zamanillo D, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 29.Osten P, et al. Protein synthesis-dependent formation of protein kinase Mζ in long-term potentiation. J Neurosci. 1996;16:2444–2451. doi: 10.1523/JNEUROSCI.16-08-02444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serrano P, et al. Persistent phosphorylation by protein kinase Mζ maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ling DS, et al. Protein kinase Mζ is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- 32.Pastalkova E, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 33.Huang YY, et al. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci U S A. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakharenko SS, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 35.Barco A, et al. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt WB, et al. A within-subjects, within-task demonstration of intact spatial reference memory and impaired spatial working memory in glutamate receptor-A-deficient mice. J Neurosci. 2003;23:3953–3959. doi: 10.1523/JNEUROSCI.23-09-03953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanderson DJ, et al. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav Neurosci. 2007;121:559–569. doi: 10.1037/0735-7044.121.3.559. [DOI] [PubMed] [Google Scholar]
- 38.Giese KP, et al. Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 39.Irvine EE, et al. αCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat Neurosci. 2005;8:411–412. doi: 10.1038/nn1431. [DOI] [PubMed] [Google Scholar]
- 40.Cooke SF, et al. Autophosphorylation of αCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J Physiol. 2006;574:805–818. doi: 10.1113/jphysiol.2006.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izquierdo LA, et al. Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol. 2002;22:269–287. doi: 10.1023/A:1020715800956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopec CD, et al. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopec CD, et al. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segal M. Dendritic spines and long-term plasticity. Nat Rev Neurosci. 2005;6:277–284. doi: 10.1038/nrn1649. [DOI] [PubMed] [Google Scholar]
- 45.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 46.De Paola V, et al. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 48.Zuo Y, et al. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46:181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Holtmaat A, et al. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441:979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 50.Marie H, et al. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 51.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki WA. Encoding new episodes and making them stick. Neuron. 2006;50:19–21. doi: 10.1016/j.neuron.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 54.Rodrigues SM, et al. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Rumpel S, et al. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 56.Bourtchuladze R, et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 57.Han JH, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 58.Dudai Y, Eisenberg M. Rites of passage of the engram: reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Kim JH, et al. Presynaptic activation of silent synapses and growth of new synapses contribute to intermediate and long-term facilitation in Aplysia. Neuron. 2003;40:151–165. doi: 10.1016/s0896-6273(03)00595-6. [DOI] [PubMed] [Google Scholar]