Abstract
PC12 cells are a well-established model to study how differences in signal transduction duration can elicit distinct cell behaviors. Epidermal growth factor (EGF) activates transient ERK signaling in PC12 cells that lasts 30–60 min, which in turn promotes proliferation; nerve growth factor (NGF) activates more sustained ERK signaling that lasts 4–6 h, which in turns induces neuronal differentiation. Data presented here extend a previous study by Mullenbrock et al. (2011) that demonstrated that sustained ERK signaling in response to NGF induces preferential expression of a 69-member gene set compared to transient ERK signaling in response to EGF and that the transcription factors AP-1 and CREB play a major role in the preferential expression of several genes within the set. Here, we examined whether the Egr family of transcription factors also contributes to the preferential expression of the gene set in response to NGF. Our data demonstrate that NGF causes transient induction of all Egr family member transcripts, but a corresponding induction of protein was detected for only Egr1 and 2. Chromatin immunoprecipitation experiments provided clearest evidence that, after induction, Egr1 binds 12 of the 69 genes that are preferentially expressed during sustained ERK signaling. In addition, Egr1 expression and binding upstream of its target genes were both sustained in response to NGF versus EGF within the same timeframe that its targets are preferentially expressed. These data thus provide evidence that Egr1 contributes to the transcriptional program activated by sustained ERK signaling in response to NGF, specifically by contributing to the preferential expression of its target genes identified here.
Introduction
PC12 rat pheochromocytoma cells are an established model to study molecular events associated with neuronal differentiation. Following treatment with nerve growth factor (NGF), PC12 cells differentiate into neuron-like cells [1], characterized by cessation of cell proliferation, up-regulation of neuronal genes, neurite outgrowth, and development of electrical excitability. NGF initiates these effects through activation of its receptor TrkA, which in turn stimulates multiple signaling cascades including the Ras/Raf/MEK/ERK, PI 3-kinase/Akt, and phospholipase C pathways [2]. ERK signaling plays a central role in PC12 differentiation, indicated by experiments demonstrating that inhibition of ERK signaling pharmacologically or via expression of dominant-negative Ras, Raf, MEK, or ERK blocks differentiation in response to NGF [3–5], whereas expression of constitutively active forms of Ras, Raf, MEK, or ERK is sufficient to induce differentiation [6]. The mechanism through which ERK signaling induces PC12 differentiation, however, is not fully understood.
One key mechanistic link between ERK signaling and PC12 differentiation is the duration for which ERK remains active following stimulation. Stimulation with NGF induces rapid ERK activation that peaks within minutes and then gradually declines to basal levels through 3–4 h. Sustained ERK signaling through this timeframe is essential for differentiation, demonstrated in part by observation that some growth factors induce more transient ERK signaling and do not cause differentiation. The most well studied example is epidermal growth factor (EGF), which similarly stimulates ERK activation within minutes, but activity declines to basal levels more rapidly within 30–60 min. Rather than inducing differentiation, this more transient ERK signal promotes proliferation [7].
One potential basis for the distinct biological response of PC12 cells to NGF is that NGF elicits a transcriptional program that is dependent on sustained ERK signaling through 2–4 h after stimulation. To investigate this, DNA microarray analysis was used to compare global gene expression profiles of PC12 cells treated with either NGF or EGF for 2 or 4 hours, which identified a set of 69 genes preferentially up-regulated in response to NGF at those time points (of which many have known roles in neuronal differentiation and/or function) [8]. Subsequent experiments indicated that transcription factors AP-1 and CREB contribute to the preferential expression of several genes within the set. Here, we tested the hypothesis that Egr transcription factors likewise contribute to the preferential expression of the gene set, which was based on the presence of putative Egr binding sites upstream of 21 genes within the set.
The Egr family comprises five members—Egr1-4 and Wilms Tumor 1 (WT1)—which share highly homologous DNA-binding domains at their C-termini composed of three zinc finger motifs that bind similar GC-rich, 9-nucleotide response elements [9–15]. Their N-termini exhibit greater variability, but similarly contain activation domains through which they transactivate target genes [10, 12, 16–19]. Egr1-3 also contain an R1 domain that binds coregulators NAB1 and 2 [20–22]; NABs are best characterized as corepressors [23], but can also act as coactivators [24]. Egr4 and WT1 lack an R1 domain, however, WT1 also exhibits trans-activation and -repression activities in a target gene-specific manner [19, 25–29]. The molecular details that dictate the effect of Egr1-3 and WT1 on target gene expression—activation or repression—are not fully understood.
Egr proteins are also immediate early genes (IEGs) induced rapidly and transiently in response to several stimuli including growth factors, cytokines, neurotransmitters, and multiple cellular stressors (e.g., hypoxia and oxidative stress) [30–32]. Upon induction, Egr proteins contribute to several cell behaviors including proliferation, apoptosis, and differentiation in a cell type- and stimulus-specific manner [11, 33–35]. Roles for Egr1-3 are particularly well-documented in the nervous system, where Egr1 and 3 play important roles in learning and memory through regulation of genes that contribute to synaptic plasticity and long-term potentiation [36–50]. Egr2 is expressed in Schwann cells, where it promotes peripheral nerve myelination through, at least in part, activation of the gene encoding myelin protein zero [51–56].
A role for Egr proteins in PC12 differentiation is also established. Levkovitz et al. (2001) determined that NGF-induced neurite outgrowth is inhibited by transfection with a truncated Egr3 consisting of its DNA-binding domain that acts as a dominant-negative on Egr-mediated transactivation [57]. Transfection with Egr1 siRNA by Ravni et al. (2008) similarly inhibited neurite outgrowth induced by PACAP (pituitary adenylate cyclase-activating polypeptide) [58]. Identification of Egr transcriptional targets that contribute to PC12 differentiation has been limited to the gene encoding p35, which activates Cdk5 kinase activity via protein-protein interactions [59]. Harada et al. (2001) demonstrated that Egr1-mediated induction of p35 expression is necessary for Cdk5 activation and neurite outgrowth in response to NGF. The present study provides evidence that Egr1 contributes to PC12 differentiation via regulation of several additional transcriptional targets.
Materials and Methods
Cell culture
PC12 cells [3] were maintained in growth medium consisting of Dulbecco’s modification of Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 5% horse serum (HS) at 37°C, 10% CO2. For gene expression and Western blot experiments, 106 cells were plated per 60-mm dish in growth medium and incubated for 24 h before medium was replaced with reduced serum medium (DMEM + 0.5% HS). For ChIP experiments, 6 x 106 cells were plated per 150-mm dish in growth medium and incubated for 72 h before medium was replaced with reduced serum medium. After placing in reduced serum medium, cells were incubated another 24 h before treatment with 50 ng/ml NGF (EMD Millipore, 480275) or 25 ng/ml EGF (EMD Millipore, 324831).
Real-time reverse transcriptase polymerase chain reaction (RT-PCR)
For gene expression experiments, total RNA was extracted using TRIzol Reagent (Thermo Fisher Scientific, 15596026) as follows. Cell cutlures were washed once with PBS and lysed in 500 μl of TRIzol, 100 μl of chloroform were added to each lysate, and samples then incubated for 3 min at room temperature. Samples were subjected to centrifugation at 12,000 x g for 15 min at 4°C and the aqueous phase collected. Two hundred μl of 2-propanol were added to each sample and samples incubated for 10 min at room temperature. Samples were subjected to centrifugation at 16,000 x g for 10 min at 4°C and the supernatants removed. RNA pellets were washed by adding 1 ml of 70% ethanol, vortexed briefly, and then subjected to centrifugation at 16,000 x g for 15 min at 4°C. The supernatants were removed and RNA pellets air dried for approximately 10 min. RNA pellets were then suspended in 50 μl of nuclease-free water and incubated at 60°C for 10 min before spectrophotometry to determine nucleic acid concentrations.
Reverse transcription reactions were conducted on 900 ng of nucleic acids from each sample using TaqMan Reverse Transcription kit (Life Technologies, N8080234), according to the manufacturer’s instructions. Real-time PCR reactions where then conducted using SYBR Select Mater Mix (Life Technologies, 4472908), according to the manufacturer’s instructions. Primer sequences used for real-time PCR are listed in S1 Table.
Immunoblot
Following treatment with NGF or EGF, cells were washed twice with phosphate-buffered saline (PBS), lysed directly in 300 μl of 2X Laemmli buffer, and heated to 95–100°C for 5 min. Twenty μl of each lysate were loaded and electrophoresed through SDS-polyacrylamide gels (10% for Egr1 immunoblots, 12% for Egr2-4, WT1, and β-actin immunoblots), transferred to nitrocellulose membrane, and immunoblot conducted to detect Egr1 (Santa Cruz, sc-110, 1:1000 dilution), Egr2 (Covance, PRB-236P, 1:500 dilution), Egr3 (Cell Signaling, 2559, 1:1000 dilution; Santa Cruz, sc-191, 1:500 dilution; Sigma, SAB2104196, 1 μg/ml working concentration), Egr4 (Santa Cruz, sc-19868, 1:500 dilution), WT1 (Santa Cruz, sc-68880, 1:500 dilution) and β-actin (Sigma, A5441, 1:20,000 dilution).
Membranes were blocked in 5% nonfat dried milk in TBS + 0.2% Tween-20 (blocking buffer) for 30 min at room temperature and then incubated with primary antibody diluted in blocking buffer for 12–18 h at 4°C. Membranes were then washed three times for 15 min in TBS + 0.2% Tween-20 and incubated with secondary antibody (goat anti-rabbit HRP-conjugate, Bio-Rad, 172–1019, 1:3000 dilution; goat anti-mouse HRP-conjugate, Bio-Rad, 170–6516, 1:3000 dilution) diluted in blocking buffer for 1–2 h at room temperature. Membranes were washed three times for 15 min in TBS + 0.2% Tween-20 after which bands were detected by chemiluminesce (Western Lightning Plus-ECL, Enhanced Chemiluminescence Substrate, Perkin Elmer, NEL105001).
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as previously described [60]. For each experimental condition, cells from three 15-cm plates were scrapped, pooled, and crosslinked in 1% formaldehyde for 10 min at 37°C. Cells were then subjected to centrifugation at 800 x g for 4 min at 25°C and resulting cell pellets were washed twice with 15 ml of cold PBS supplemented with protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml pepstatin A). Cells were lysed in 4 ml of PIPES lysis buffer (5 mM PIPES, 85 mM KCl, 0.5% NP-40, pH 8.1) supplemented with protease inhibitors, subjected to centrifugation at 800 x g for 5 min at 4°C, and resulting nuclear pellets washed twice with 15 ml cold PBS supplemented with protease inhibitors. Pellets were resuspended in 1.2 ml of PBS lysis buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS in PBS) supplemented with protease inhibitors and subjected to sonication using a Branson Sonifier S-250D Digital Ultrasonic Cell Disrupter with four 30-sec pulses at 25% amplitude. Samples were subjected to centrifugation at 16,000 x g for 15 min at 4°C and supernatants collected. One hundred μl of the supernatant were transferred to a clean tube, combined with 200 μl high salt lysis buffer, and stored at -20°C for use as “input fractions” during subsequent real-time PCR. Remaining volumes from sonication were precleared by incubation with 80 μl of protein A agarose 50% slurry for 30 min at 4°C with mixing followed by centrifugation at 16,000 x g for 30–60 sec at 4°C. Supernatants were collected and separated into 350 μl volumes for immunoprecipitation (IP) using 5 μg of the following antibodies: Egr1 (Santa Cruz, sc-110), Egr2 (Covance, PRB-236P), Egr3 (Santa Cruz, sc-191), Egr4 (Santa Cruz, sc-19868), and WT1 (Santa Cruz, sc-68880). Samples were incubated with antibody for 12–18 h at 4°C after which 60 μl of protein A agarose 50% slurry were added and incubated for 2 h at 4°C. Samples were subjected to centrifugation at 16,000 x g for 30–60 sec at 4°C, supernatant discarded, and protein A agarose beads subjected to five sequential washes with 1 ml of the following buffers: [1] low salt wash buffer (20 mM Tris, 150 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton X-100, pH 8.1), [2] high salt wash buffer (20 mM Tris, 500 mM NaCl, 2 mM EDTA, 0.1% SDS, 1% Triton X-100, pH 8.1), [3] LiCl wash buffer (10 mM Tris, 1mM EDTA, 250 mM LiCl, 1% IGEPAL-Ca 630, 1% deoxycholic acid, pH 8.10, [4] and [5] Tris-EDTA, pH 8.0. Antigens were then eluted from protein A agarose by two sequentional incubations in 150 μl elution buffer (1% SDS, 100 mM sodium bicarbonate) for 15 min at room temperature, which were pooled into one 300 μl eluate for each sample/experimental condition. Each eluate and input fraction was incubated with 200 mM NaCl for 12–18 h at 65°C to reverse crosslinks, after which DNA was purfied using QIAquick Gel Extraction Kit (Qiagen, 28706); 900 μl Buffer QG and 300 μl 2-propanol were added to each sample for step 1, after which the procedure was conducted according to the manufacturer’s protocol. Real-time PCR was then conducted on 1:40 dilutions of input fraction DNA and 1:10 dilutions of IP fraction DNA using using SYBR Select Mater Mix (Life Technologies, 4472908) according to the manufacturer’s instructions and primers that anneal within 250 bp of respective predicted transcription factor binding sites (see S2 Table). Recovery of each binding site during IP was quantified as % input.
Results
Expression analysis of Egr family members in PC12 cells following NGF treatment
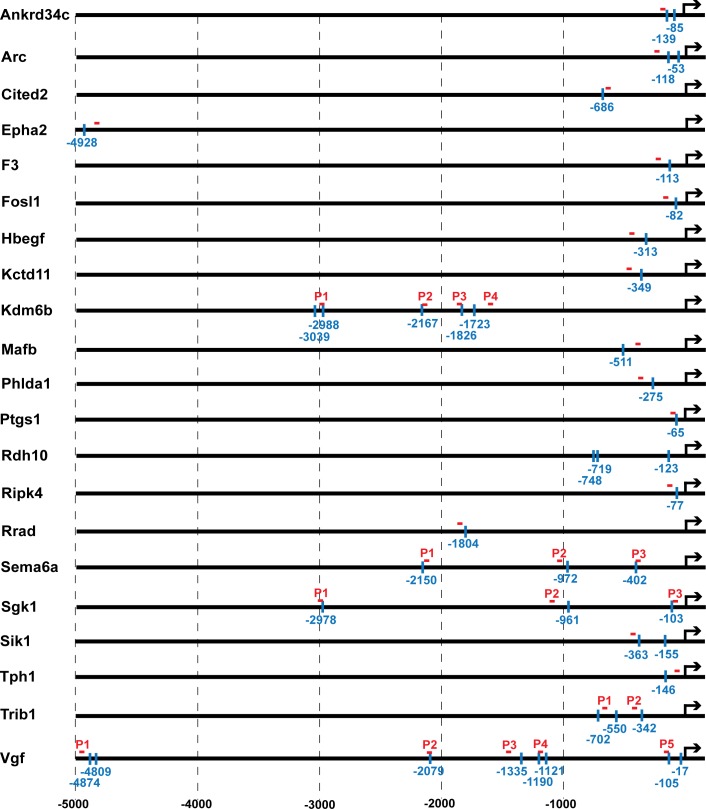
Mullenbrock et al. (2011) identified 69 genes preferentially up-regulated in PC12 cells 2–4 h after NGF treatment as compared to EGF treatment; 21 of those genes contained putative Egr binding sites (identified using the V$KROX_Q6 position weight matrix) within 5 kb upstream of their transcription start sites that were conserved in human, mouse and rat (Fig 1 and S2 Table). Given the homology within their DNA-binding domains and the similarity of response elements to which they bind, all five Egr family members were examined as potential regulators of this gene set in response to NGF.
Fig 1. Predicted Egr binding sites and ChIP primer locations upstream of genes preferentially expressed during sustained ERK signaling in response to NGF.
Of the 69 genes that Mullenbrock et al. (2011) determined are preferentially expressed during sustained ERK signaling in response to NGF, 21 contained putative Egr binding sites within 5 kb upstream of their transcription start site (TSS). The locations of each Egr binding site are denoted by the vertical blue lines. The red horizontal lines denote the relative locations of primer sets used for real time PCR to detect Egr binding to nearby Egr binding site(s). For genes with multiple dispersed Egr binding sites, multiple primers sets were designed (denoted P1, P2, etc.) to detect Egr binding to the nearest predicted Egr binding site.
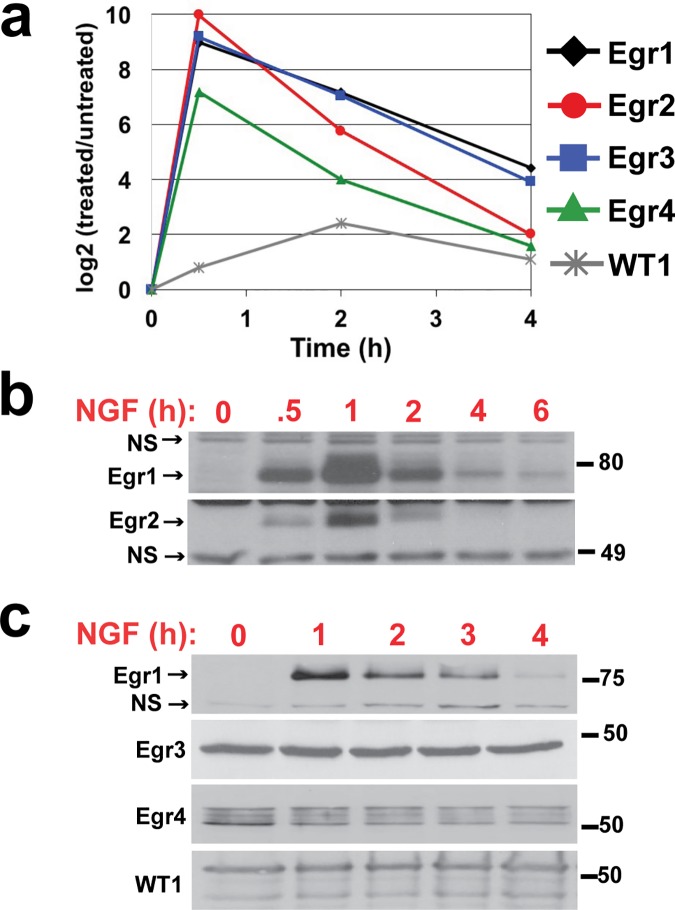
Effects of NGF on Egr expression levels were evaluated first. Consistent with their characterization as IEGs, NGF induced a robust increase in Egr1-4 transcripts at 30 min post-treatment, followed by a gradual decline through 4 h (Fig 2A). Transcript levels of WT1 increased more modestly and slowly, peaking at 2 h, then declining through 4 h. Egr1 and 2 protein levels were affected similarly to their transcripts; Western blot for both proteins detected bands near their predicted molecular weights (82 kD predicted for Egr1, 50 kD predicted for Egr2) that rapidly accumulated through 1 h of NGF treatment and then gradually declined to near basal levels through 6 h (Fig 2B). Western blot analysis for Egr3, Egr4, and WT1 detected prominent bands near their predicted molecular weights (50 kD predicted for Egr3 and 4, 49 kD predicted for WT1) in untreated cells, all of which remained largely unchanged through 4 h of NGF treatment (Fig 2C). For Egr3 in particular, three different antibodies were used to evaluate its levels, all of which detected bands in the range of its predicted molecular weight and none of which exhibited a clear or consistent change in response to NGF (results from Sigma, SAB2104196 are shown in Fig 2C; data not shown from Cell Signaling, 2559 and Santa Cruz, sc-191). This expression analysis altogether demonstrates that NGF causes transient induction of mRNAs for all Egr family members and a corresponding induction of Egr1 and 2 protein; corresponding changes in Egr3, Egr4, and WT1 protein levels, on the other hand, were not detected.
Fig 2. Time course analysis of Egr family expression following NGF treatment.
PC12 cell cultures were treated with 50 ng/ml NGF for 0–4 h before harvest. (a) Total RNA was extracted and subjected to real-time RT-PCR to quantify changes in mRNA levels or (b) total cell lysates were prepared and subjected to SDS-PAGE and Western blot analysis to evaluate changes in protein levels. NS, nonspecific band.
Our data for Egr1 are consistent with previous studies that also demonstrated induction of Egr1 transcript and protein in PC12 cells with near identical kinetics following NGF treatment [59, 61]. Induction of Egr2-4 transcripts by NGF in PC12 cells has also been shown, however, these studies did not evaluate protein levels [10, 62, 63]. To our knowledge, effects of NGF on WT1 expression in PC12 cells has not been previously described. Several studies have documented corresponding transcript and protein inductions for Egr family members in cells other than PC12 in response to a variety of growth factors [59, 64–69]; however, previous examples of Egr transcript induction without concomitant protein induction (as shown here for Egr3, Egr4, and WT1) have not been previously documented.
Egr1 binds upstream of several predicted target genes following NGF treatment
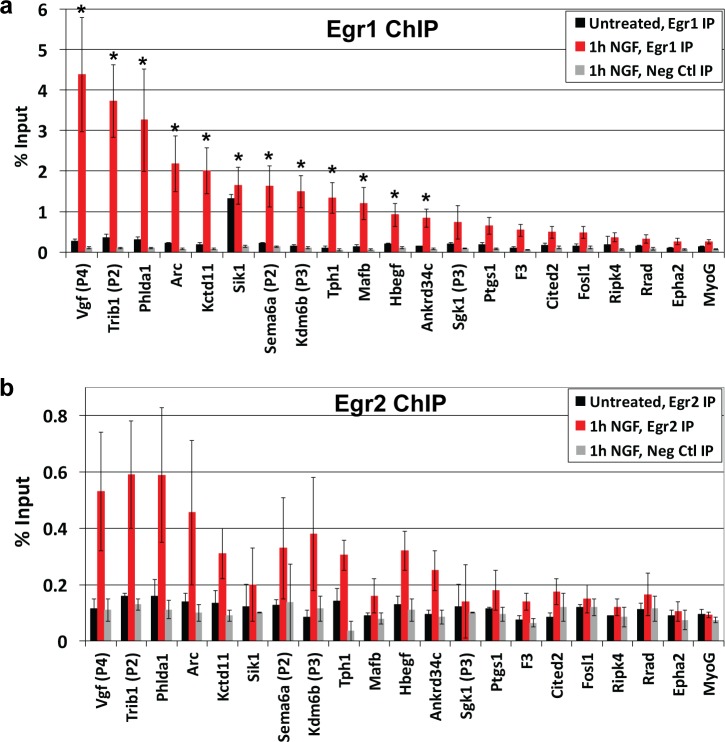
Binding of Egr family members to upstream regions of genes with putative Egr binding sites was examined next in PC12 cells by ChIP. Primers were designed within 250 bp of each predicted Egr binding site to quantify their recovery by real-time PCR, except for the three putative sites upstream of Rdh10 for which primer design was limited due to high GC content (see Fig 1 and S2 Table for primer locations and sequences). Several genes (Vgf, Kdm6b/Jmjd3, Trib1, Sema6a, and Sgk1) contained multiple dispersed Egr binding site candidates, for which multiple primer sets were used (denoted P1, P2, etc. for each target gene) to evaluate Egr binding within the respective regions. Primers to amplify a region approximately 100 bp upstream of the Myog gene, which lacks an Egr binding site, were used as a negative control for Egr binding.
Binding of Egr1 was examined first due to its established role in PC12 differentiation [58, 59]. Since the data in Fig 2B show that Egr1 levels peak at 1 h post-NGF treatment, cells were treated with NGF for 0 or 1 h and subjected to ChIP with either anti-Egr1 antibody or non-immune IgG as a negative control; real-time PCR was then conducted on input and IP fractions to quantify recovery during IP as % input. As shown in Fig 3A, % input values for most predicted Egr targets were similar to Myog following Egr1 ChIP from untreated cells, whereas % input for Sik1/Snf1lk was 7-fold greater, indicating Egr1 is bound upstream of only Sik1/Snf1lk in the absence of NGF. After 1 h of NGF treatment, % input values increased well above that of Myog for several targets. Of the 20 genes examined, 15 exhibited average values greater than 2-fold above Myog, of which 12 were statistically significant (p < 0.05) and 2 approached significance (p-values for Ptgs1 and F3 from a one-tailed Student’s t test were 0.056 and 0.053, respectively). To our knowledge, 10 of these genes (Trib1, Phlda1, Sema6, Kctd11, Sik1/Snf1lk, Kdm6b/Jmjd3, Mafb, Hbegf, Ankrd34c, and Ptgs1) are novel Egr1 targets, whereas 5 (Vgf, Arc, Tph1, Sgk1, and F3) were previously identified targets [70–74]. For genes that were tested with multiple primer sets, data for the set with highest % input value are included in Fig 3; results from the remaining primer sets are provided in S1 Fig. These data indicate that Egr1 induction by NGF results in its binding upstream of approximately 17% of the genes that are preferentially expressed during sustained ERK signaling, whereas Egr1 is constitutively bound to Sik1/Snf1lk. The molecular explanation for Egr1’s differential binding to Sik1/Snf1lk versus the other targets was not examined.
Fig 3. ChIP analysis to evaluate binding of Egr1 and Egr2 upstream of genes with predicted Egr binding sites.
PC12 cultures were treated with or without 50 ng/ml NGF for 1 h and subjected to ChIP assay using antibodies against Egr1, Egr2, or an IgG control. Real-time PCR was then conducted on the immunoprecipitated DNA using primers within 250 bp of each predicted Egr binding site (see Fig 1 and S2 Table for primer locations and sequences). For genes with multiple dispersed Egr binding sites, multiple primer sets (denoted P1, P2, etc.) were used; only data for the set that detected highest level of binding are included here (see S1 Fig for data from the remaining primer sets). Primers to amplify a region approximately 100 bp upstream of the Myog gene were used as a negative control for Egr1 binding. (a) Data from Egr1 ChIP are plotted as % input and are averages from three to four independent experiments ± S.E. *, One-tailed Student’s t tests were conducted comparing the % input value for Myog after Egr1 IP to the % input values for each predicted Egr target after Egr1 IP, which yielded p values ≤ 0.05 for 12 predicted Egr targets. (b) Data from Egr2 ChIP are plotted as % input and are averages from two independent experiments ± S.E.
ChIP with anti-Egr2 antibody yielded similar results to Egr1, but with notable differences (Fig 3B). In untreated cells, Egr2 binding was not detected for any targets, including Sik1/Snf1lk. After NGF treatment, 11 targets exhibited % input values greater than 2-fold above Myog, which were the same targets bound by Egr1 after NGF treatment excluding Mafb. However, values for those 11 targets were 3- to 8-fold lower than % input values from Egr1 ChIP. Whether these differences reflect differential binding of Egr1 versus Egr2 upstream of target genes or differential effectiveness of the respective antibodies in ChIP was not determined. ChIP with anti-Egr3, -Egr4 or -WT1 antibodies did not demonstrate binding of these family members upstream of any putative targets before or after NGF treatment (data not shown).
Altogether, these ChIP data demonstrate that NGF induces Egr1 binding upstream of several genes that are preferentially up-regulated during sustained ERK signaling, indicating it may contribute to their expression at 2–4 h. The data provide more modest evidence for Egr2 binding, suggesting it may also play a role. Given the more robust data for Egr1, additional experiments focused on further examining its role in the network.
NGF induces sustained Egr1 expression and binding to target genes associated with PC12 neuronal differentiation
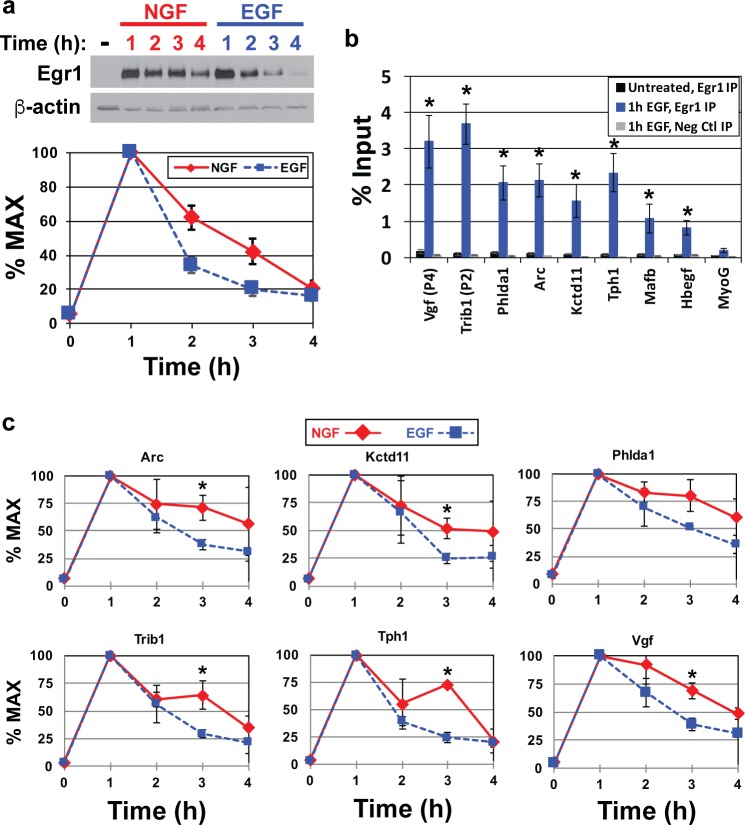
To begin examining how Egr1 might contribute to the preferential expression of its target genes 2–4 h post-NGF versus -EGF treatment, Western blot analysis and densitometry were conducted to compare its levels 0–4 h after treatment with each growth factor. Both NGF and EGF induced strong up-regulation of Egr1 at 1h followed by a decline to near basal levels through 2–4 h (Fig 4A); however, Egr1 declined more rapidly after 1 h of EGF treatment. This is consistent with a previous study by Harada et al. (2001), who also demonstrated sustained Egr1 expression through 3 h of NGF treatment compared to EGF; moreover, the authors showed that sustained Egr1 levels caused sustained expression of p35, which in turn promoted differentiation via sustained activation of Cdk5 [59]. These observations together with those from Fig 3A suggest that sustained Egr1 levels in response to NGF may result in sustained binding and regulation of its target genes.
Fig 4. Sustained Egr1 expression and binding upstream of target genes in response to NGF versus EGF.
(a) PC12 cell cultures were treated with 50 ng/ml NGF or 25 ng/ml EGF for 0–4 h. Total cell lysates were harvested and subjected to SDS-PAGE and Western blot for Egr1. Egr1 levels were quantified by densitometry and converted to % maximum levels for ten independent experiments and plotted (average ± S.E.). (b) PC12 cultures were treated with or without 25 ng/ml EGF for 1 h and subjected to ChIP assay using antibodies against Egr1 or an IgG control. Real-time PCR was then conducted on the immunoprecipitated DNA using primers within 250 bp of predicted Egr binding sites (see Fig 1 and S2 Table for primer locations and sequences). Primers to amplify a region approximately 100 bp upstream of the Myog gene were used as a negative control for Egr1 binding. Data are plotted as % input and are averages from three to six independent experiments ± S.E. *, One-tailed Student’s t tests were conducted comparing the % input value for Myog after Egr1 IP to the % input values for each predicted Egr target after Egr1 IP, which yielded p values ≤ 0.05. (c) PC12 cell cultures were treated with 50 ng/ml NGF or 25 ng/ml EGF for 0–4 h and subjected to ChIP with anti-Egr1 antibody. Immunoprecipitated DNA was then subjected to real-time PCR with primers to detect Egr1 binding to a subset of its target genes, which was quantified as % input. Percent input values were then converted into % maximum values, which were plotted. *, One-tailed Student’s t tests were conducted comparing the % input values at each time point after NGF versus EGF treatment, which yielded p values ≤ 0.05 at the 3-hour time point for Arc, Kctd11, Trib1, Tph1, and Vgf.
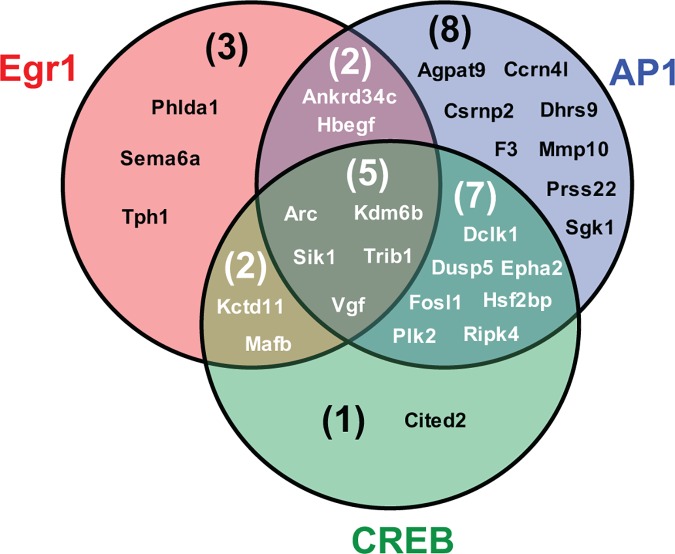
To test this, ChIP was conducted to first determine if Egr1 similarly binds a subset of predicted targets in response to EGF when at peak levels 1 h after treatment (Fig 4B). As expected, % input values for all tested targets were similar to Myog after Egr1 IP from untreated cells, whereas values increased above Myog after EGF treatment. Comparison of % input values for each gene independently after 1 h NGF versus 1 h EGF treatment showed no significant difference (S3 Table), indicating Egr1 binds its targets at similar levels after 1 h of treatment with either growth factor. Next, maintenance of Egr1 binding upstream several targets at 1–4 h post-treatment was evaluated by ChIP. For these experiments, the time point with highest % input value was defined as maximum binding and values for remaining time points were converted to % maximum. Similar to its protein levels, Egr1 binding to all targets peaked at 1 h post-treatment with either NGF or EGF and then gradually declined through 4 h (Fig 4C). However, binding was comparatively sustained after NGF treatment, specifically at the 2 and 3 h time points, which mirrors changes in Egr1 protein levels. These data confirm that NGF induces sustained expression and binding of Egr1 to several target genes that are preferentially expressed during sustained ERK signaling and more broadly supports the hypothesis that Egr1 acts cooperatively with CREB and AP-1 to activate a transcriptional program that is associated with sustained ERK signaling in response to NGF (Fig 5).
Fig 5. AP-1, CREB, and Egr1 cooperatively regulate 28 genes during their preferential expression in response to NGF and sustained ERK signaling.
The Venn diagram summarizes genes bound by AP-1, CREB, and/or Egr1 during their preferentially expression in response to NGF and sustained ERK signaling, as detected by Mullenbrock et al. (2011) and the present study.
Discussion
The experiments described here extend a previous study aimed at understanding how differences in ERK signaling duration cause distinct behavioral responses in PC12 cells; more specifically, how sustained ERK signaling lasting 3–4 h in response to NGF induces neuronal differentiation, whereas more transient ERK signaling lasting 30–60 min in response to EGF promotes proliferation [8]. The previous study identified a transcriptional program that is activated by sustained ERK signaling in response to NGF, in which CREB and AP-1 act as part of a transcription factor network that preferentially up-regulates a set of 69 genes 2–4 h after treatment with NGF versus EGF. The present study tested the hypothesis that Egr proteins (Egr1-4 and WT1) act cooperatively with CREB and AP-1 to activate this gene set, which is based on computational analysis that identified putative Egr binding sites upstream of 21 genes within the set of 69. Of the five Egr family members, our data provides strongest evidence that Egr1 contributes to this transcriptional program.
As expected based on its previous characterizations as an IEG, NGF induced strong, but transient Egr1 expression; protein levels peaked 1 h after treatment and then declined to near basal levels through 6 h. ChIP experiments demonstrated that 1 h of NGF treatment induced Egr1 binding upstream of 11 of the 21 genes with predicted Egr binding sites, whereas Egr1 was bound upstream of one target, Sik1/Snf1lk, at similar levels in untreated and NGF-treated cells. To our knowledge, nine of these 12 genes (Trib1, Phlda1, Sema6, Kctd11, Sik1/Snf1lk, Kdm6b/Jmjd3, Mafb, Hbegf, and Ankrd34c) are novel Egr1 targets and three (Vgf, Arc, and Tph1) were previously identified. The kinetics of Egr1 binding to a subset of these targets were examined 0–4 h after NGF treatment; consistent with its expression pattern, binding peaked 1 h after NGF treatment and then gradually declined through 4 h. EGF induced similar effects on Egr1 expression and binding upstream of target genes, but with notable differences. Both Egr1 protein levels and binding upstream of targets peaked 1 h after EGF treatment; however, both then declined more rapidly through 2–3 h compared to NGF treatment. These data collectively demonstrate that, compared to EGF, NGF induces sustained Egr1 expression and binding upstream of several target genes during the same timeframe in which those genes are preferentially expressed, which supports our hypothesis that Egr1 contributes to the transcriptional program activated by sustained ERK signaling. Given Egr1’s transactivation activity, the simplest extrapolation is that Egr1 contributes to the program through sustained transactivation of its target genes. However, since Egr1 can also act as a transcriptional repressor, a more complex mechanism for Egr1’s contribution cannot be ruled out.
The Venn diagram in Fig 5 summarizes our data and model for the cooperative roles of AP-1, CREB, and Egr1 in regulation of transcriptional targets during PC12 differentiation. Of the 69 genes preferentially up-regulated during sustained ERK signaling in response to NGF, 28 are bound by one or more of these three transcription factors within the same timeframe. AP-1 binds the largest subset (21 genes), of which 16 are also bound by Egr1 and/or CREB. Of the 12 Egr1 targets identified here, nine are similarly bound by AP-1 and/or CREB; and of the 15 CREB targets, all but one are also bound by AP-1 and/or Egr1.
One surprising result from the Egr1 ChIP experiments discussed here was the selective binding of Egr1 upstream of only Sik1/Snf1lk in the untreated cells, especially given its low expression levels compared to 1 h post-NGF treatment. AP-1, whose expression and binding upstream of target genes are induced by NGF similarly to Egr1 [8], also binds upstream of Sik1/Snf1lk. However, in contrast to Egr1, AP-1 binding to Sik1/Snf1lk was induced similarly to AP-1 binding to its other targets. Nevertheless, selective binding of transcription factors to specific target genes when at basal levels is not unprecedented. For example, transcription factors c-Myc, REST/NRSF, and Ste12, have been shown to selectively bind target genes when at basal levels [75–77]. Enrichment of CpG islands in the vicinity of binding sites has been proposed as a factor that promotes selective binding of transcription factors to sites via epigenetic modifications [76]; however, comparison of sequences surrounding the Egr1 binding sites identified in this study did not reveal a unique enrichment of CpG islands in the vicinity of the two putative Egr binding sites upstream of Sik1/Snf1lk.
Of the 69 genes preferentially induced by NGF, approximately 50% have been implicated in neuronal development and/or function [8], which is consistent with the Egr1 targets identified here. Of the 12 Egr1 targets, eight have previously characterized roles in the nervous system. Vgf and Hbegf both encode neurotrophic factors expressed throughout the nervous system and have been implicated in several aspects of its development and maintenance [71, 78–81]. Kdm6b/Jmjd3 encodes an H3K27 demethylase and Kctd11 encodes an adaptor for the ubiquitin ligase Cullin3, both which contribute to differentiation of embryonic and neural stem cells to neurons [82–86]. Sema6a encodes the transmembrane protein semaphorin6a, which regulates axon guidance and dendritic growth during development [87–89], whereas Arc encodes a post-synaptic protein that plays important roles in synaptic plasticity associated with learning and memory [90, 91]. Mafb encodes a transcription factor that controls hindbrain segmentation and development at least in part via regulation of Hoxa3 and Hoxb3 expression [92–94]. Tph1 encodes tryptophan hydroxylase-1, which catalyzes the rate-limiting step in serotonin synthesis; it is expressed in mouse brain during late stages of development and then restricted to the pineal gland in adulthood. Polymorphisms within the Tph1 gene have been implicated in multiple psychiatric conditions [95–97].
Examination of Egr family members other than Egr1 provided more modest evidence for contribution of Egr2 to the transcriptional program. Egr2 transcript and protein levels mirrored those of Egr1 in response to NGF. ChIP experiments suggested induction of Egr2 binding to predicted targets after NGF treatment with an overall pattern similar to Egr1. Target genes that yielded highest % input values from Egr1 ChIP after 1 h of NGF treatment (e.g., Vgf, Trib1, and Phlda1) also yielded highest values from Egr2 ChIP. However, the Egr2 ChIP values were overall much lower (3- to 8-fold) than those from Egr1 ChIP, which could be due either to lower levels of Egr2 binding to the Egr binding sites or lower efficiency of the Egr2 antibody for ChIP in general. Given the modesty of the Egr2 ChIP results, additional experiments focused on Egr1.
Altogether, the experiments described here extend the study by Mullenbrock et al. (2011) that characterized a transcriptional program in PC12 cells whose activity is associated with sustained ERK signaling and neuronal differentiation. Mullenbrock et al. identified a set of 69 genes preferentially up-regulated during sustained ERK signaling and provided evidence that transcription factors AP-1 and CREB cooperatively contribute to the preferential expression of 25 genes within the set. Data shown here provide evidence that transcription factor Egr1 also plays a cooperative role through regulation of 12 genes within the set, nine of which are also targeted by AP-1 and/or CREB during sustained ERK signaling (see Fig 5); we also identify nine novel targets of Egr1 regulation in general. Identification of additional transcription factors that contribute to the transcriptional program associated with PC12 neuronal differentiation awaits future experiments.
Supporting Information
PC12 cultures were treated with or without 50 ng/ml NGF for 1 h and subjected to ChIP assay using antibodies against Egr1, Egr2, or an IgG control. Real-time PCR was then conducted on the immunoprecipitated DNA using primers within 250 bp of each predicted Egr binding site (see Fig 1 and S2 Table for primer locations and sequences). For genes with multiple dispersed Egr binding sites, multiple primer sets (denoted P1, P2, etc.) were used (see S1 Fig for data from the remaining primer sets). Primers to amplify a region approximately 100 bp upstream of the Myog gene were used as a negative control for Egr1 binding. (a) Data from Egr1 ChIP are plotted as % input and are averages from three to four independent experiments ± S.E. *, Student’s t tests were conducted comparing the % input value for Myog after Egr1 IP to the % input values for each predicted Egr target after Egr1 IP, which yielded p values ≤ 0.05 for several primer sets. (b) Data from Egr2 ChIP are plotted as % input and are averages from two independent experiments ± S.E.
(TIFF)
Transcript levels of Egr1-4 and WT1 were evaluated by real-time RT-PCR using the forward and reverse primers listed within this table.
(XLSX)
This table provides the locations and sequences of putative Egr binding sites identified within the rat genome. Locations and sequences for forward and reverse primers used in real-time PCR following ChIP are also listed, alongside their Figs 1 and 3 and S1 Fig designations.
(XLSX)
Percent input values for several Egr1 target genes after Egr1 ChIP from cells treated with 50 ng/ul NGF or 25 ng/ul EGF for 1 h are listed and compared by Student’s t test. p values for all Egr1 targets were ≥ 0.25.
(XLSX)
Acknowledgments
This work was supported primarily by Grant RO1 CA18689 from the National Institutes of Health, but also by undergraduate research grants provided by the Adrian Tinsley Program for Undergraduate Research at Bridgewater State University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The majority of funding for this program was provided by grant RO1 CA18689 from the National Institutes of Health (https://grants.nih.gov/grants/oer.htm). Additional funding was provided through several undergraduate research grants provided by the Adrian Tinsley Program for Undergraduate Research at Brigewater State University (https://my.bridgew.edu/departments/OUR/SitePages/Funding%20and%20Presentation%20Opportunities.aspx). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America. 1976;73(7):2424–8. Epub 1976/07/01. PubMed Central PMCID: PMC430592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annual review of neuroscience. 1996;19:463–89. 10.1146/annurev.ne.19.030196.002335 [DOI] [PubMed] [Google Scholar]
- 3.Szeberenyi J, Cai H, Cooper GM. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Molecular and cellular biology. 1990;10(10):5324–32. PubMed Central PMCID: PMC361225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klesse LJ, Meyers KA, Marshall CJ, Parada LF. Nerve growth factor induces survival and differentiation through two distinct signaling cascades in PC12 cells. Oncogene. 1999;18(12):2055–68. 10.1038/sj.onc.1202524 [DOI] [PubMed] [Google Scholar]
- 5.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. The Journal of biological chemistry. 1995;270(23):13585–8. [DOI] [PubMed] [Google Scholar]
- 6.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77(6):841–52. [DOI] [PubMed] [Google Scholar]
- 7.Huff K, End D, Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. The Journal of cell biology. 1981;88(1):189–98. PubMed Central PMCID: PMC2111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mullenbrock S, Shah J, Cooper GM. Global expression analysis identified a preferentially nerve growth factor-induced transcriptional program regulated by sustained mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) and AP-1 protein activation during PC12 cell differentiation. The Journal of biological chemistry. 2011;286(52):45131–45. Epub 2011/11/09. PubMed Central PMCID: PMC3248011. 10.1074/jbc.M111.274076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao XM, Koski RA, Gashler A, McKiernan M, Morris CF, Gaffney R, et al. Identification and characterization of the Egr-1 gene product, a DNA-binding zinc finger protein induced by differentiation and growth signals. Molecular and cellular biology. 1990;10(5):1931–9. PubMed Central PMCID: PMCPMC360539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosby SD, Puetz JJ, Simburger KS, Fahrner TJ, Milbrandt J. The early response gene NGFI-C encodes a zinc finger transcriptional activator and is a member of the GCGGGGGCG (GSG) element-binding protein family. Molecular and cellular biology. 1991;11(8):3835–41. PubMed Central PMCID: PMCPMC361165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton TB, Borel F, Romaniuk PJ. Comparison of the DNA binding characteristics of the related zinc finger proteins WT1 and EGR1. Biochemistry. 1998;37(7):2051–8. 10.1021/bi9717993 [DOI] [PubMed] [Google Scholar]
- 12.Lemaire P, Vesque C, Schmitt J, Stunnenberg H, Frank R, Charnay P. The serum-inducible mouse gene Krox-24 encodes a sequence-specific transcriptional activator. Molecular and cellular biology. 1990;10(7):3456–67. PubMed Central PMCID: PMCPMC360781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rauscher FJ 3rd, Morris JF, Tournay OE, Cook DM, Curran T. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990;250(4985):1259–62. [DOI] [PubMed] [Google Scholar]
- 14.Swirnoff AH, Milbrandt J. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Molecular and cellular biology. 1995;15(4):2275–87. PubMed Central PMCID: PMCPMC230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christy B, Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(22):8737–41. PubMed Central PMCID: PMCPMC298363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Molecular and cellular biology. 1993;13(8):4556–71. PubMed Central PMCID: PMCPMC360074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patwardhan S, Gashler A, Siegel MG, Chang LC, Joseph LJ, Shows TB, et al. EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene. 1991;6(6):917–28. [PubMed] [Google Scholar]
- 18.Vesque C, Charnay P. Mapping functional regions of the segment-specific transcription factor Krox-20. Nucleic Acids Res. 1992;20(10):2485–92. PubMed Central PMCID: PMCPMC312382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang ZY, Qiu QQ, Deuel TF. The Wilms' tumor gene product WT1 activates or suppresses transcription through separate functional domains. The Journal of biological chemistry. 1993;268(13):9172–5. [PubMed] [Google Scholar]
- 20.Russo MW, Matheny C, Milbrandt J. Transcriptional activity of the zinc finger protein NGFI-A is influenced by its interaction with a cellular factor. Molecular and cellular biology. 1993;13(11):6858–65. PubMed Central PMCID: PMCPMC364748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo MW, Sevetson BR, Milbrandt J. Identification of NAB1, a repressor of NGFI-A- and Krox20-mediated transcription. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(15):6873–7. PubMed Central PMCID: PMCPMC41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Molecular and cellular biology. 1996;16(7):3545–53. PubMed Central PMCID: PMCPMC231349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swirnoff AH, Apel ED, Svaren J, Sevetson BR, Zimonjic DB, Popescu NC, et al. Nab1, a corepressor of NGFI-A (Egr-1), contains an active transcriptional repression domain. Molecular and cellular biology. 1998;18(1):512–24. PubMed Central PMCID: PMCPMC115883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sevetson BR, Svaren J, Milbrandt J. A novel activation function for NAB proteins in EGR-dependent transcription of the luteinizing hormone beta gene. The Journal of biological chemistry. 2000;275(13):9749–57. [DOI] [PubMed] [Google Scholar]
- 25.Drummond IA, Madden SL, Rohwer-Nutter P, Bell GI, Sukhatme VP, Rauscher FJ 3rd. Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992;257(5070):674–8. [DOI] [PubMed] [Google Scholar]
- 26.Gashler AL, Bonthron DT, Madden SL, Rauscher FJ 3rd, Collins T, Sukhatme VP. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(22):10984–8. PubMed Central PMCID: PMCPMC50467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madden SL, Cook DM, Morris JF, Gashler A, Sukhatme VP, Rauscher FJ 3rd. Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991;253(5027):1550–3. [DOI] [PubMed] [Google Scholar]
- 28.Maheswaran S, Park S, Bernard A, Morris JF, Rauscher FJ 3rd, Hill DE, et al. Physical and functional interaction between WT1 and p53 proteins. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(11):5100–4. PubMed Central PMCID: PMCPMC46662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang ZY, Madden SL, Deuel TF, Rauscher FJ 3rd. The Wilms' tumor gene product, WT1, represses transcription of the platelet-derived growth factor A-chain gene. The Journal of biological chemistry. 1992;267(31):21999–2002. [PubMed] [Google Scholar]
- 30.O'Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22(4):167–73. [DOI] [PubMed] [Google Scholar]
- 31.Pagel JI, Deindl E. Disease progression mediated by egr-1 associated signaling in response to oxidative stress. Int J Mol Sci. 2012;13(10):13104–17. PubMed Central PMCID: PMCPMC3497314. 10.3390/ijms131013104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiel G, Muller I, Rossler OG. Expression, signaling and function of Egr transcription factors in pancreatic beta-cells and insulin-responsive tissues. Mol Cell Endocrinol. 2014;388(1–2):10–9. 10.1016/j.mce.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 33.Khachigian LM, Anderson KR, Halnon NJ, Gimbrone MA Jr., Resnick N, Collins T. Egr-1 is activated in endothelial cells exposed to fluid shear stress and interacts with a novel shear-stress-response element in the PDGF A-chain promoter. Arterioscler Thromb Vasc Biol. 1997;17(10):2280–6. [DOI] [PubMed] [Google Scholar]
- 34.Lee CG, Cho SJ, Kang MJ, Chapoval SP, Lee PJ, Noble PW, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200(3):377–89. PubMed Central PMCID: PMC2211975. 10.1084/jem.20040104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagel JI, Ziegelhoeffer T, Heil M, Fischer S, Fernandez B, Schaper W, et al. Role of early growth response 1 in arteriogenesis: impact on vascular cell proliferation and leukocyte recruitment in vivo. Thromb Haemost. 2012;107(3):562–74. 10.1160/TH11-07-0490 [DOI] [PubMed] [Google Scholar]
- 36.Cheval H, Chagneau C, Levasseur G, Veyrac A, Faucon-Biguet N, Laroche S, et al. Distinctive features of Egr transcription factor regulation and DNA binding activity in CA1 of the hippocampus in synaptic plasticity and consolidation and reconsolidation of fear memory. Hippocampus. 2012;22(3):631–42. 10.1002/hipo.20926 [DOI] [PubMed] [Google Scholar]
- 37.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340(6233):474–6. 10.1038/340474a0 [DOI] [PubMed] [Google Scholar]
- 38.Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, et al. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148(3):633–43. PubMed Central PMCID: PMCPMC2597331. 10.1016/j.neuroscience.2007.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han S, Hong S, Mo J, Lee D, Choi E, Choi JS, et al. Impaired extinction of learned contextual fear memory in early growth response 1 knockout mice. Mol Cells. 2014;37(1):24–30. PubMed Central PMCID: PMCPMC3907009. 10.14348/molcells.2014.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–96. 10.1038/85138 [DOI] [PubMed] [Google Scholar]
- 41.Li L, Yun SH, Keblesh J, Trommer BL, Xiong H, Radulovic J, et al. Egr3, a synaptic activity regulated transcription factor that is essential for learning and memory. Mol Cell Neurosci. 2007;35(1):76–88. PubMed Central PMCID: PMCPMC2683345. 10.1016/j.mcn.2007.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonergan ME, Gafford GM, Jarome TJ, Helmstetter FJ. Time-dependent expression of Arc and zif268 after acquisition of fear conditioning. Neural Plast. 2010;2010:139891 PubMed Central PMCID: PMCPMC2877205. 10.1155/2010/139891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maddox SA, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learn Mem. 2011;18(1):24–38. PubMed Central PMCID: PMCPMC3023969. 10.1101/lm.1980211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mokin M, Keifer J. Expression of the immediate-early gene-encoded protein Egr-1 (zif268) during in vitro classical conditioning. Learn Mem. 2005;12(2):144–9. PubMed Central PMCID: PMCPMC1074332. 10.1101/lm.87305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin X, Jiang Y, Tse YC, Wang Y, Wong TP, Paudel HK. Early Growth Response 1 (Egr-1) Regulates N-Methyl-d-aspartate Receptor (NMDAR)-dependent Transcription of PSD-95 and alpha-Amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptor (AMPAR) Trafficking in Hippocampal Primary Neurons. The Journal of biological chemistry. 2015;290(49):29603–16. PubMed Central PMCID: PMCPMC4705959. 10.1074/jbc.M115.668889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei F, Xu ZC, Qu Z, Milbrandt J, Zhuo M. Role of EGR1 in hippocampal synaptic enhancement induced by tetanic stimulation and amputation. The Journal of cell biology. 2000;149(7):1325–34. PubMed Central PMCID: PMCPMC2175137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams JM, Beckmann AM, Mason-Parker SE, Abraham WC, Wilce PA, Tate WP. Sequential increase in Egr-1 and AP-1 DNA binding activity in the dentate gyrus following the induction of long-term potentiation. Brain Res Mol Brain Res. 2000;77(2):258–66. [DOI] [PubMed] [Google Scholar]
- 48.Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, et al. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990;4(4):603–14. [DOI] [PubMed] [Google Scholar]
- 49.Worley PF, Christy BA, Nakabeppu Y, Bhat RV, Cole AJ, Baraban JM. Constitutive expression of zif268 in neocortex is regulated by synaptic activity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(12):5106–10. PubMed Central PMCID: PMCPMC51820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, et al. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn Mem. 1994;1(2):140–52. [PubMed] [Google Scholar]
- 51.Decker L, Desmarquet-Trin-Dinh C, Taillebourg E, Ghislain J, Vallat JM, Charnay P. Peripheral myelin maintenance is a dynamic process requiring constant Krox20 expression. J Neurosci. 2006;26(38):9771–9. 10.1523/JNEUROSCI.0716-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jang SW, LeBlanc SE, Roopra A, Wrabetz L, Svaren J. In vivo detection of Egr2 binding to target genes during peripheral nerve myelination. J Neurochem. 2006;98(5):1678–87. 10.1111/j.1471-4159.2006.04069.x [DOI] [PubMed] [Google Scholar]
- 53.Jang SW, Srinivasan R, Jones EA, Sun G, Keles S, Krueger C, et al. Locus-wide identification of Egr2/Krox20 regulatory targets in myelin genes. J Neurochem. 2010;115(6):1409–20. PubMed Central PMCID: PMCPMC3260055. 10.1111/j.1471-4159.2010.07045.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeBlanc SE, Jang SW, Ward RM, Wrabetz L, Svaren J. Direct regulation of myelin protein zero expression by the Egr2 transactivator. The Journal of biological chemistry. 2006;281(9):5453–60. 10.1074/jbc.M512159200 [DOI] [PubMed] [Google Scholar]
- 55.Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371(6500):796–9. 10.1038/371796a0 [DOI] [PubMed] [Google Scholar]
- 56.Zorick TS, Syroid DE, Arroyo E, Scherer SS, Lemke G. The transcription factors SCIP and Krox-20 mark distinct stages and cell fates in Schwann cell differentiation. Mol Cell Neurosci. 1996;8(2–3):129–45. 10.1006/mcne.1996.0052 [DOI] [PubMed] [Google Scholar]
- 57.Levkovitz Y, O'Donovan KJ, Baraban JM. Blockade of NGF-induced neurite outgrowth by a dominant-negative inhibitor of the egr family of transcription regulatory factors. J Neurosci. 2001;21(1):45–52. Epub 2001/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ravni A, Vaudry D, Gerdin MJ, Eiden MV, Falluel-Morel A, Gonzalez BJ, et al. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol. 2008;73(6):1688–708. Epub 2008/03/26. PubMed Central PMCID: PMC4188547. 10.1124/mol.107.044792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3(5):453–9. Epub 2001/05/02. 10.1038/35074516 [DOI] [PubMed] [Google Scholar]
- 60.Tullai JW, Schaffer ME, Mullenbrock S, Kasif S, Cooper GM. Identification of transcription factor binding sites upstream of human genes regulated by the phosphatidylinositol 3-kinase and MEK/ERK signaling pathways. The Journal of biological chemistry. 2004;279(19):20167–77. 10.1074/jbc.M309260200 [DOI] [PubMed] [Google Scholar]
- 61.Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238(4828):797–9. [DOI] [PubMed] [Google Scholar]
- 62.Lonn P, Zaia K, Israelsson C, Althini S, Usoskin D, Kylberg A, et al. BMP enhances transcriptional responses to NGF during PC12 cell differentiation. Neurochem Res. 2005;30(6–7):753–65. 10.1007/s11064-005-6868-6 [DOI] [PubMed] [Google Scholar]
- 63.Joseph LJ, Le Beau MM, Jamieson GA Jr., Acharya S, Shows TB, Rowley JD, et al. Molecular cloning, sequencing, and mapping of EGR2, a human early growth response gene encoding a protein with "zinc-binding finger" structure. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(19):7164–8. PubMed Central PMCID: PMCPMC282144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. The Journal of biological chemistry. 2013;288(28):20488–98. PubMed Central PMCID: PMCPMC3711314. 10.1074/jbc.M112.447250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang F, Ooka K, Bhattacharyya S, Wei J, Wu M, Du P, et al. The early growth response gene Egr2 (Alias Krox20) is a novel transcriptional target of transforming growth factor-beta that is up-regulated in systemic sclerosis and mediates profibrotic responses. Am J Pathol. 2011;178(5):2077–90. PubMed Central PMCID: PMCPMC3081194. 10.1016/j.ajpath.2011.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. The Journal of biological chemistry. 2006;281(40):29431–5. 10.1074/jbc.C600167200 [DOI] [PubMed] [Google Scholar]
- 67.Suehiro J, Hamakubo T, Kodama T, Aird WC, Minami T. Vascular endothelial growth factor activation of endothelial cells is mediated by early growth response-3. Blood. 2010;115(12):2520–32. PubMed Central PMCID: PMCPMC2845904. 10.1182/blood-2009-07-233478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarfstein R, Werner H. The WT1 Wilms' tumor suppressor gene is a downstream target for insulin-like growth factor-I (IGF-I) action in PC12 cells. J Neurochem. 2006;99(3):818–26. 10.1111/j.1471-4159.2006.04119.x [DOI] [PubMed] [Google Scholar]
- 69.Schabbauer G, Schweighofer B, Mechtcheriakova D, Lucerna M, Binder BR, Hofer E. Nuclear factor of activated T cells and early growth response-1 cooperate to mediate tissue factor gene induction by vascular endothelial growth factor in endothelial cells. Thromb Haemost. 2007;97(6):988–97. PubMed Central PMCID: PMCPMC2879320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cui MZ, Parry GC, Oeth P, Larson H, Smith M, Huang RP, et al. Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR-1. The Journal of biological chemistry. 1996;271(5):2731–9. [DOI] [PubMed] [Google Scholar]
- 71.D'Arcangelo G, Habas R, Wang S, Halegoua S, Salton SR. Activation of codependent transcription factors is required for transcriptional induction of the vgf gene by nerve growth factor and Ras. Molecular and cellular biology. 1996;16(9):4621–31. PubMed Central PMCID: PMCPMC231461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grasberger H, Chang L, Shih W, Presson AP, Sayuk GS, Newberry RD, et al. Identification of a functional TPH1 polymorphism associated with irritable bowel syndrome bowel habit subtypes. Am J Gastroenterol. 2013;108(11):1766–74. PubMed Central PMCID: PMCPMC4067697. 10.1038/ajg.2013.304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.James AB, Conway AM, Morris BJ. Regulation of the neuronal proteasome by Zif268 (Egr1). J Neurosci. 2006;26(5):1624–34. 10.1523/JNEUROSCI.4199-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Molecular and cellular biology. 2005;25(23):10286–300. PubMed Central PMCID: PMCPMC1291244. 10.1128/MCB.25.23.10286-10300.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bruce AW, Donaldson IJ, Wood IC, Yerbury SA, Sadowski MI, Chapman M, et al. Genome-wide analysis of repressor element 1 silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) target genes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(28):10458–63. PubMed Central PMCID: PMCPMC478591. 10.1073/pnas.0401827101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, et al. Genomic targets of the human c-Myc protein. Genes Dev. 2003;17(9):1115–29. PubMed Central PMCID: PMCPMC196049. 10.1101/gad.1067003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeitlinger J, Simon I, Harbison CT, Hannett NM, Volkert TL, Fink GR, et al. Program-specific distribution of a transcription factor dependent on partner transcription factor and MAPK signaling. Cell. 2003;113(3):395–404. [DOI] [PubMed] [Google Scholar]
- 78.Jin K, Mao XO, Sun Y, Xie L, Jin L, Nishi E, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22(13):5365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levi A, Ferri GL, Watson E, Possenti R, Salton SR. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol. 2004;24(4):517–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewis JE, Brameld JM, Jethwa PH. Neuroendocrine Role for VGF. Front Endocrinol (Lausanne). 2015;6:3. PubMed Central PMCID: PMCPMC4313783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oyagi A, Hara H. Essential roles of heparin-binding epidermal growth factor-like growth factor in the brain. CNS Neurosci Ther. 2012;18(10):803–10. 10.1111/j.1755-5949.2012.00371.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgold T, Spreafico F, De Santa F, Totaro MG, Prosperini E, Natoli G, et al. The histone H3 lysine 27-specific demethylase Jmjd3 is required for neural commitment. PLoS One. 2008;3(8):e3034 PubMed Central PMCID: PMCPMC2515638. 10.1371/journal.pone.0003034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Canettieri G, Di Marcotullio L, Greco A, Coni S, Antonucci L, Infante P, et al. Histone deacetylase and Cullin3-REN(KCTD11) ubiquitin ligase interplay regulates Hedgehog signalling through Gli acetylation. Nat Cell Biol. 2010;12(2):132–42. 10.1038/ncb2013 [DOI] [PubMed] [Google Scholar]
- 84.Gallo R, Zazzeroni F, Alesse E, Mincione C, Borello U, Buanne P, et al. REN: a novel, developmentally regulated gene that promotes neural cell differentiation. The Journal of cell biology. 2002;158(4):731–40. PubMed Central PMCID: PMCPMC2174014. 10.1083/jcb.200202024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450(7168):415–9. 10.1038/nature06270 [DOI] [PubMed] [Google Scholar]
- 86.Lee S, Lee SK. Crucial roles of histone-modifying enzymes in mediating neural cell-type specification. Curr Opin Neurobiol. 2010;20(1):29–36. PubMed Central PMCID: PMCPMC2848506. 10.1016/j.conb.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leighton PA, Mitchell KJ, Goodrich LV, Lu X, Pinson K, Scherz P, et al. Defining brain wiring patterns and mechanisms through gene trapping in mice. Nature. 2001;410(6825):174–9. 10.1038/35065539 [DOI] [PubMed] [Google Scholar]
- 88.Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, et al. Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53(4):535–47. 10.1016/j.neuron.2007.01.028 [DOI] [PubMed] [Google Scholar]
- 89.Zhuang B, Su YS, Sockanathan S. FARP1 promotes the dendritic growth of spinal motor neuron subtypes through transmembrane Semaphorin6A and PlexinA4 signaling. Neuron. 2009;61(3):359–72. PubMed Central PMCID: PMCPMC2654783. 10.1016/j.neuron.2008.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28(46):11760–7. PubMed Central PMCID: PMCPMC2615463. 10.1523/JNEUROSCI.3864-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morin JP, Guzman-Ramos K, Bermudez-Rattoni F. New Insights on Retrieval-Induced and Ongoing Memory Consolidation: Lessons from Arc. Neural Plast. 2015;2015:184083 PubMed Central PMCID: PMCPMC4561316. 10.1155/2015/184083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manzanares M, Cordes S, Ariza-McNaughton L, Sadl V, Maruthainar K, Barsh G, et al. Conserved and distinct roles of kreisler in regulation of the paralogous Hoxa3 and Hoxb3 genes. Development. 1999;126(4):759–69. [DOI] [PubMed] [Google Scholar]
- 93.Manzanares M, Cordes S, Kwan CT, Sham MH, Barsh GS, Krumlauf R. Segmental regulation of Hoxb-3 by kreisler. Nature. 1997;387(6629):191–5. 10.1038/387191a0 [DOI] [PubMed] [Google Scholar]
- 94.Manzanares M, Trainor PA, Nonchev S, Ariza-McNaughton L, Brodie J, Gould A, et al. The role of kreisler in segmentation during hindbrain development. Dev Biol. 1999;211(2):220–37. 10.1006/dbio.1999.9318 [DOI] [PubMed] [Google Scholar]
- 95.Malek ZS, Dardente H, Pevet P, Raison S. Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. Eur J Neurosci. 2005;22(4):895–901. 10.1111/j.1460-9568.2005.04264.x [DOI] [PubMed] [Google Scholar]
- 96.Nakamura K, Hasegawa H. Developmental role of tryptophan hydroxylase in the nervous system. Mol Neurobiol. 2007;35(1):45–54. [DOI] [PubMed] [Google Scholar]
- 97.Nakamura K, Sugawara Y, Sawabe K, Ohashi A, Tsurui H, Xiu Y, et al. Late developmental stage-specific role of tryptophan hydroxylase 1 in brain serotonin levels. J Neurosci. 2006;26(2):530–4. 10.1523/JNEUROSCI.1835-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PC12 cultures were treated with or without 50 ng/ml NGF for 1 h and subjected to ChIP assay using antibodies against Egr1, Egr2, or an IgG control. Real-time PCR was then conducted on the immunoprecipitated DNA using primers within 250 bp of each predicted Egr binding site (see Fig 1 and S2 Table for primer locations and sequences). For genes with multiple dispersed Egr binding sites, multiple primer sets (denoted P1, P2, etc.) were used (see S1 Fig for data from the remaining primer sets). Primers to amplify a region approximately 100 bp upstream of the Myog gene were used as a negative control for Egr1 binding. (a) Data from Egr1 ChIP are plotted as % input and are averages from three to four independent experiments ± S.E. *, Student’s t tests were conducted comparing the % input value for Myog after Egr1 IP to the % input values for each predicted Egr target after Egr1 IP, which yielded p values ≤ 0.05 for several primer sets. (b) Data from Egr2 ChIP are plotted as % input and are averages from two independent experiments ± S.E.
(TIFF)
Transcript levels of Egr1-4 and WT1 were evaluated by real-time RT-PCR using the forward and reverse primers listed within this table.
(XLSX)
This table provides the locations and sequences of putative Egr binding sites identified within the rat genome. Locations and sequences for forward and reverse primers used in real-time PCR following ChIP are also listed, alongside their Figs 1 and 3 and S1 Fig designations.
(XLSX)
Percent input values for several Egr1 target genes after Egr1 ChIP from cells treated with 50 ng/ul NGF or 25 ng/ul EGF for 1 h are listed and compared by Student’s t test. p values for all Egr1 targets were ≥ 0.25.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.