Abstract
Introduction
Our goal was to prospectively compare the trajectories of depression symptoms through pregnancy and the postpartum between women who received normal prenatal screening results and those whose results indicated an increased risk for fetal aneuploidy.
Material and Methods
Women completed the Edinburgh Postnatal Depression Scale (EPDS) at four-week intervals between <26 weeks gestation and three months postpartum. We categorized women into four groups: 1) negative serum screening and ultrasound results (SS−/US−, n=103), 2) positive serum screening/negative ultrasound results (SS+/US−, n=42), 3) negative serum screening/positive ultrasound results (SS−/US+, n=19), or 4) positive serum screening and ultrasound results (SS+/US+, n=13), and compared EPDS scores between groups using Poisson regression.
Results
Women who received any positive prenatal screening result had significantly higher EPDS scores during pregnancy than SS−/US− women (p = 0.002), with SS−/US+ women having the highest scores. During the postpartum, any positive screening test result was only marginally significantly associated with EPDS scores (p=0.06), but women in the SS−/US+ group had significantly higher scores than women in the SS−/US− group (p = 0.05).
Conclusion
Our data suggest that different types of prenatal screening tests may have different effects on women’s moods, and that depression symptoms persist for women who have soft markers identified on ultrasound.
Keywords: Prenatal screening, soft markers, depression, mood, postpartum, pregnancy
Introduction
During pregnancy, women are routinely offered prenatal serum screening tests such as: serum integrated prenatal screening (SIPS), integrated prenatal screening (IPS), first trimester screening (FTS), non-invasive prenatal screening (NIPT) and ultrasound screening at 16–20 weeks gestation for soft markers (sonographic findings at the fetal anatomy scan that increase the chance of fetal aneuploidy; for example echogenic cardiac focus, echogenic bowel and increased nuchal fold) (1). These tests vary in their components, detection rates, eligibility criteria and timing of results. However, all aim to identify women whose fetuses are at increased risk of aneuploidy.
Shortly after receiving ‘positive’ screening test results (indicating increased risk for fetal aneuploidy), women often experience symptoms of depression and anxiety (2–10). What remains unknown however, is whether these symptoms persist through pregnancy or predispose women to postpartum depression, and whether different types of screening tests have different effects on mood.
We designed a prospective, longitudinal study to explore the proportions of women with different types of prenatal screening test results who experienced symptoms of depression during pregnancy and/or the postpartum, and to test the hypotheses that:
Women who received prenatal screening test results indicating increased risk for fetal aneuploidy (‘positive’) would have greater depression symptomatology during pregnancy than women whose test results showed no elevated risk for aneuploidy.
Women who received positive prenatal screening test results would have greater depression symptomatology during the postpartum period.
Material and Methods
Women were eligible to participate in the study if they were less than 26 weeks pregnant at the time of enrollment, able to read and respond to survey questions in English and had chosen to have some form of prenatal serum screening (for example, FTS, IPS, SIPS, NIPT). We recruited four groups of women, including those who had:
a negative serum screen and normal detailed ultrasound (SS−/US−),
a positive serum screen and a normal ultrasound result (SS+/US−),
a normal serum screen and an ultrasound which identified soft marker(s) (SS−/US+),
both a positive serum screen and ultrasound soft marker(s) (SS+/US+).
Women who ultimately received a diagnosis of fetal aneuploidy were not excluded from the study. All participants provided informed consent. Research ethics board approval was obtained from the University of British Columbia/Children’s & Women’s Health Centre of British Columbia Research Review Committee on May 15, 2012 (Approval number: H12-00331).
Women were recruited between August 2012 and July 2013 from various locations in and around Vancouver, Canada. Women with ‘normal’ prenatal test results (SS−/US−) were primarily recruited from physician, midwifery offices and ultrasound facilities, while the Departments of Medical Genetics in Vancouver and Victoria and the Diagnostic Ambulatory Program at British Columbia’s Women’s Hospital yielded most of the women with ‘positive’ prenatal test results (SS+/US−, SS−/US+, SS+/US+).
Given the paucity of data on which to draw for a power calculation, we opted for an exploratory approach, with a view of recruiting at least 10 participants in each of the four groups.
At enrollment (<26 weeks), eligible participants completed a demographic questionnaire including questions about their education, ethnicity, pregnancy, mental health history, as well as the type(s) of prenatal screening they had, their results and whether they elected to proceed with diagnostic testing (amniocentesis). Questions about prenatal screening results were repeated at subsequent time points to accommodate different timing of results (S1).
The Edinburgh Postnatal Depression Scale (EPDS) was administered at four-week intervals from enrollment to three months postpartum. The EPDS is a 10 item, self-report questionnaire that has been validated for use during both pregnancy and the postpartum (11–12). An EPDS score of 15 or greater in pregnancy has been validated in three studies with a sensitivity for major depression ranging from 57–100% and a specificity of 93–99% (13), and in the postpartum a score of 13 or greater has been validated in several studies with a sensitivity for major depression ranging from 34–100% and a specificity of 44–100% (13–15). Study data were collected and managed using REDCap (Research Electronic Data Capture) (16).
Data Analysis: All analyses were carried out in R v.3.1.0 (17). Analyses were conducted using only those EPDS surveys that were completed after 24 weeks of gestation (by which time women would have received their screening results). Data from women who completed more than one survey were included in analyses. All completed surveys were included in the analysis even if women were lost to follow-up. The mixed-effects models we used allowed for different numbers of time points (or surveys completed) per person and helped to mitigate bias due to excluding those lost to follow-up.
To test hypothesis one, we compared the EPDS scores across time and screening results using mixed-effects log-linear (Poisson) regression with repeated measures nested within individuals. The Poisson regression was better than a Gaussian model in this case as the EPDS scores were integers and were not normally distributed. The mixed-effects model takes into account the correlated nature of the measurements on the same woman while allowing for a test of whether there are overall differences in mean EPDS scores among the screening results groups, among the women who had a previous psychiatric diagnosis vs. those who did not, and if there is any directional trend in EPDS scores during pregnancy. In addition to the tests for main effects, we also tested for interactions between screening results and gestation. Post hoc tests were performed if necessary using the ‘glht’ function from the multcomp package (18). We also conducted a sub-analysis by repeating this test with two groups (after combining all women who received any positive screening result).
To test hypothesis two, we compared the EPDS scores across time, screening results and previous psychiatric diagnosis using Poisson regression with repeated measures nested within individuals. We also tested for an interaction between screening results and weeks postpartum. Post hoc tests were performed if necessary using the ‘glht’ function from the multcomp package (18). As above, we conducted a sub-analysis by repeating this test after combining women in the three positive screening test result categories into one. Women lost to follow-up during pregnancy were excluded from the post-partum analysis.
Results
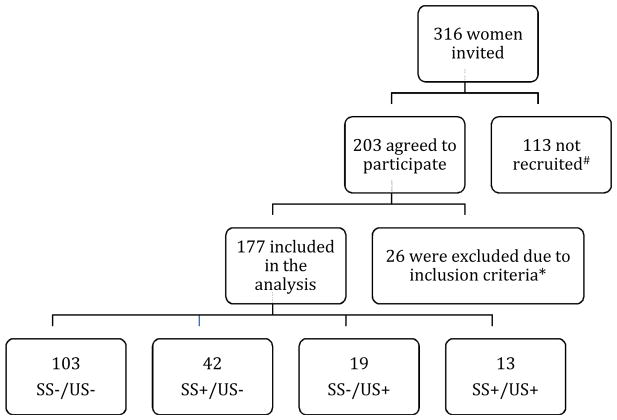
In total, 316 women were invited, 203 agreed to participate, and 177 were included in the analyses (as shown in Figure 1). Participants’ mean gestational age at the first study survey was 19.8 weeks, but as described above, we used data provided at 24 weeks gestation or later in analyses. The number of participants in each of the four screening result categories is shown in Table 1, together with demographic information. Women in the SS+/US− and SS+/US+ groups had an amniocentesis twice as often as women in the SS−/US+ group, and nine times more often than women in the SS−/US− group. Fetal chromosomal abnormalities were diagnosed in three women (see Table 1).
Figure 1. Participation flowchart.
Flow chart demonstrating the number of women who expressed interest in participating in the study, those who were recruited to participate and those that were included in the analysis.
#The 113 who were not recruited were those who: did not have screening, were over 26 weeks gestation, did not speak English, decided not to participate, lost the infant, or were not contactable.
*26 women were excluded from analyses due to ineligibility as a result of completing one or fewer time points after 24 weeks gestation. US = ultrasound, SS = serum screening.
Table 1.
Characteristics of the participants overall, and divided by screening test results during pregnancy. Mean (SD), n (%), US = ultrasound, SS = serum screening
| Variable | Overall N = 177 |
SS−/US− n = 103 |
SS+/US− n = 42 |
SS−/US+ n = 19 |
SS+/US+ n = 13 |
|---|---|---|---|---|---|
| Age | 34.3 (4.6) | 34.1 (4.6) | 35.4 (4.7) | 32.6 (4.0) | 35.4 (3.8) |
| In a relationship | 174 (98%) | 103 (100%) | 41 (98%) | 17 (89%) | 13 (100%) |
| Highest Educational Level Attained | |||||
| Elementary/middle | 2 (2%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| High school | 15 (5%) | 5 (5%) | 5 (12%) | 1 (5%) | 4 (31%) |
| Trade/technical | 22 (10%) | 10 (10%) | 7 (17%) | 3 (16%) | 2 (15%) |
| Undergraduate | 60 (36%) | 37 (36%) | 10 (24%) | 7 (37%) | 6 (46%) |
| Graduate/Professional | 78 (48%) | 49 (48%) | 20 (48%) | 8 (42%) | 1 (8%) |
| Annual Household Income | |||||
| < $20,000 | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) |
| $20,000–$49,999 | 22 (13%) | 12 (12%) | 6 (14%) | 3 (16%) | 1 (8%) |
| $50,000–$79,999 | 24 (14%) | 11 (11%) | 6 (14%) | 2 (11%) | 5 (38%) |
| $80,000–$99,999 | 26 (15%) | 10 (10%) | 10 (24%) | 5 (26%) | 1 (8%) |
| $100,000–$129,999 | 28 (16%) | 19 (19%) | 5 (12%) | 3 (16%) | 1 (8%) |
| ≥ $130,000 | 73 (42%) | 48 (48%) | 15 (36%) | 6 (32%) | 4 (31%) |
| Ethnicity | |||||
| Arab/West Asian | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Asian | 19 (11%) | 11 (11%) | 6 14%) | 1 (5%) | 1 (8%) |
| Caucasian | 127 (72%) | 77 (75%) | 30 (71%) | 14 (74%) | 6 (46%) |
| Filipino | 4 (2%) | 1 (1%) | 1 (2%) | 0 (0%) | 2 (15%) |
| First Nations | 4 (2%) | 2 (2%) | 1 (2%) | 0 (0%) | 1 (8%) |
| Latin American | 6 (3%) | 3 (3%) | 0 (0%) | 1 (5%) | 2 (15%) |
| South Asian | 10 (6%) | 4 (4%) | 4 10%) | 2 (11%) | 0 (0%) |
| Other | 6 (3%) | 4 (4%) | 0 (0%) | 1 (5%) | 1 (8%) |
| Number of times pregnant | |||||
| 1 | 73 (41%) | 47 (46%) | 13 (31%) | 9 (50%) | 4 (31%) |
| 2 | 52 (30%) | 32 (31%) | 12 (29%) | 4 (22%) | 4 (31%) |
| 3 | 27 (15%) | 11 (11%) | 10 (24%) | 3 (17%) | 3 (23%) |
| >3 | 24 (13%) | 13 (13%) | 7 (17%) | 2 (11%) | 2 (16%) |
| Number of children | |||||
| 0 | 94 (53%) | 60 (58%) | 18 (43%) | 10 (56%) | 6 (46%) |
| 1 | 66 (38%) | 36 (35%) | 17 (40%) | 7 (39%) | 6 (46%) |
| 2 | 12 (7%) | 4 (4%) | 6 (14%) | 1 (6%) | 1 (8%) |
| 3 | 4 (2%) | 3 (3%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Previous pregnancy where chromosome abnormality was suspected | 13 (7%) | 6 (6%) | 5 (12%) | 1 (5%) | 1 (8%) |
| Participants who had amniocentesis in this pregnancy | 33 (19%) | 5 (5%) | 18 (44%) | 4 (21%) | 6 (46%) |
| Diagnosed with a chromosomal abnormality | 3 (2%) | 1 (1%) | 0 (0%) | 2 (11%) | 0 (0%) |
The rates of pre-existing mental illness for women in each screening test result group is shown in Table 2, together with data about pregnancy and postpartum EDPS scores. The screening test result group that had the highest proportion of women scoring above the cut-off for depression was the SS−/US+ group, where almost a third, and just over a quarter of women scored above threshold during pregnancy and the postpartum, respectively.
Table 2.
History of mental illness diagnoses amongst participants, as well as data about mood immediately before and during study enrollment.
| Mean gestational age at enrollment@ | Prior to enrollment | Pregnancy | Postpartum | ||||
|---|---|---|---|---|---|---|---|
| Previous diagnosis of mental illness* n (%) | Symptoms of depression during 2 weeks before enrollment n (%)^ | Avg. EPDS Score (SD)$ | ≥15 on EPDS# n (%) | Avg. EPDS Score (SD)$ | ≥13 on EPDS# n (%) | ||
| SS−/US− (n=103) | 19 | 9 (9) | 13 (13) | 4.8 (2.7) | 7 (7) | 4.7 (2.7) | 15 (15) |
| SS+/US− (n=42) | 20 | 5 (12) | 11 (26) | 6.1 (2.9) | 7 (17) | 5.7 (2.7) | 7 (17) |
| SS−/US+ (n=19) | 22 | 1 (5) | 6 (32) | 7.9 (3.1) | 6 (32) | 7.0 (2.4) | 5 (26) |
| SS+/US+ (n=13) | 23 | 1 (8) | 3 (23) | 6.7 (3.1) | 4 (31) | 3.4 (1.5) | 1 (8) |
| Any positive screening test (SS+/US− SS−/US+ and SS+/US+ combined) (n=74) | 21 | 7 (9) | 20 (27) | 6.7 (3.6) | 10 (14) | 5.6 (3.0) | 13 (19) |
Psychiatric diagnoses included depression (n= 8), schizophrenia (n=1), anxiety (n=5), attention deficit disorder (n=1) and depression and obsessive-compulsive disorder (n=1).
Number of women with at least one EPDS score above the threshold indicated.
The SDs provided take into account the structure in the data (for example, the multiple measures per person)
These data are based on responses to this question: ‘During this pregnancy, have you experienced any symptoms of depression (low mood, loss of interest in things you enjoy) or anxiety that lasted for longer than 2 weeks?’
Women in the positive screen groups were primarily recruited following the receipt of their results in Medical Genetics, thus they enrolled at a later gestational age as compared to the other groups.
Women who received some form of positive prenatal test result reported depression symptoms more frequently in the two weeks before enrollment as compared to the SS−/US− group (Table 2).
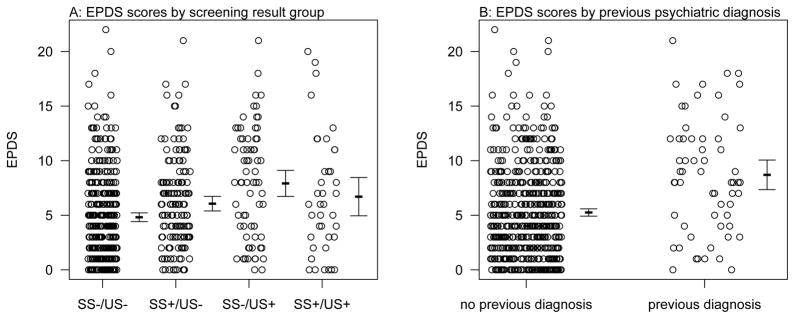
During pregnancy, participants completed an average of 3.6 surveys (range = one to six). Women in the SS−/US+ group had the greatest average EPDS scores during pregnancy (Table 2). There was no significant interaction term between screening results and weeks of gestation (p = 0.24) so it was removed from the model for subsequent estimation. There was no significant association between gestational age and EPDS score (p = 0.71), but there was a significant association between screening results and EPDS score (p = 0.007), and between EPDS score and previous psychiatric diagnosis (p=0.002; Table 3). Post-hoc Tukey tests suggest that the only screening result group comparison that was significant was between SS−/US− and SS−/US+ groups (p = 0.006), with the SS−/US+ group having scores that were 1.8 times higher on average when weeks of gestation and previous psychiatric diagnosis were held constant. None of the other pairwise tests between individual groups showed significant differences, after correction for multiple testing (all p > 0.05, Figure 2). The coefficients also suggest that having a previous psychiatric diagnosis increased the EPDS scores by about 1.8 on average (Figure 2).
Table 3.
Mixed-effects poisson regression results for EPDS scores during pregnancy and postpartum. Shown are both the coefficients (SE) as well as the Incident Rate Ratios (IRR), which are the antilogs of the coefficients. P-values are from Likelihood ratio tests.
| Variable | Coefficient (SE) | IRR (SE) | p-value | |
|---|---|---|---|---|
| Pregnancy: | ||||
| Screening results1 | 0.007 | |||
| SS+/US− | 0.28 (0.14) | 1.32 (1.15) | ||
| SS−/US+ | 0.61 (0.19) | 1.83 (1.20) | ||
| SS+/US+ | 0.27 (0.23) | 1.31 (1.25) | ||
| Previous psychiatric diagnosis | 0.60 (0.19) | 1.82 (1.21) | 0.002 | |
| Weeks of gestation | 0.001 (0.004) | 1.00 (1.00) | 0.71 | |
| Postpartum: | ||||
| Screening results1 | 0.01 | |||
| SS+/US− | 0.29 (0.16) | 1.33 (1.18) | ||
| SS−/US+ | 0.57 (0.22) | 1.77 (1.25) | ||
| SS+/US+ | −0.37 (0.29) | 0.69 (1.34) | ||
| Previous psychiatric diagnosis | 0.62 (0.24) | 1.86 (1.27) | 0.01 | |
| Weeks postpartum | −0.01 (0.004) | 0.99 (1.00) | 0.001 | |
The reference category is all negative screening results
Figure 2. Prenatal EPDS Scores by screening result and previous psychiatric diagnosis.
Shown are the raw data offset slightly along the x-axis for clarity, as well as the means and 95% CI to the right of each group.
When the positive screening groups were combined (SS−/US+, SS+/US−, and SS+/US+), there was still a significant association between EPDS scores and screening results (p = 0.002) with women with any positive screening result having EPDS scores on average 1.5 times higher than women with SS−/US− results. There was also a significant relationship between EPDS scores and having a previous psychiatric diagnosis (p=0.003), with women who had a previous diagnosis having EPDS score on average 1.8 times higher than those without a diagnosis.
During the postpartum period, there were 168 women with results that were included in this analysis. Women filled out an average of 3.5 surveys in the postpartum period (range = one to six). The SS−/US+ group had the greatest average EPDS score in the postpartum (Table 2).
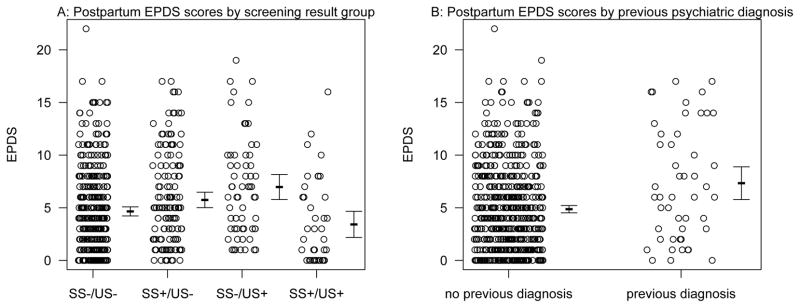
There was no significant interaction term between screening results and weeks postpartum (p = 0.55) so it was removed from the model for subsequent estimation. There was a significant association between screening results and EPDS score (p = 0.01), a significant association between previous psychiatric diagnosis and EPDS score (p = 0.01), and a significant association between EPDS score and weeks postpartum (p = 0.001; Table 2). The negative coefficient for weeks postpartum suggests that EPDS scores declined slightly over time with every week postpartum resulting in an estimated EPDS of 0.99 times the EPDS score in the previous week on average. Post-hoc Tukey tests suggest that the comparisons that were significant were between the SS+/US+ group and the SS−/US+ group (p = 0.03, with the SS−/US+ group having scores that were 2.6 times higher on average), and between the SS−/US− group and the SS−/US+ group (p = 0.05, with the SS−/US+ group having EPDS scores that were 1.8 times higher on average). None of the other pairwise tests were significant after correction for multiple testing (all p > 0.05; Figure 3). Finally, the coefficients also suggest that having a previous psychiatric diagnosis increased EPDS scores by about 1.9 on average as well (Figure 3).
Figure 3. Postpartum EPDS scores by screening group and previous psychiatric diagnosis.
Shown are the raw data offset slightly along the x-axis for clarity, as well as the means and 95% CI to the right of each group.
When the positive screening results categories were combined (SS−/US+, SS+/US−, and SS+/US+), there was only a marginal association of EPDS scores with screening results (p = 0.06). However, there was still a relationship between EPDS scores and previous psychiatric diagnosis (p = 0.002), and a strong negative relationship with weeks postpartum (p = 0.001).
Discussion
Our prospective study supports previous findings demonstrating that after receiving positive prenatal screening results (serum and ultrasound soft markers) indicating increased risk for fetal aneuploidy, women experience more depression symptomatology than women who receive negative screening results. We identified only a marginal association between having received any positive screening result and depression symptoms in the postpartum period. However, women in the SS−/US+ group experienced significantly more symptoms of depression both during pregnancy and the postpartum period than women in the SS−/US− group.
In interpreting our findings, contextual understanding of women’s experiences is important. Specifically, women in the SS−/US+ group may be unprepared to learn about the detection of soft markers, especially if they are approaching the ultrasound simply as a chance “to see the baby.” Not all guidelines include reference to the importance of talking about soft markers during prenatal visits (19), and studies have shown that women often have little knowledge about soft markers prior to their ultrasound (20,1). Perhaps the very nature of soft markers (directly observable features) results in their being perceived as more “real”, and producing stronger negative emotional reactions than serum screen results, which are perhaps less tangible. On the other hand, the counseling women receive prior to prenatal serum screening may explain why women in the SS+/US− and SS+/US+ groups did not have significantly more depression symptomatology than women in the SS−/US− group (19,21,22).
Our data also showed that women in the SS−/US+ group underwent invasive diagnostic testing less often than those in the SS+/US− and SS+/US+ groups (Table 1). This may be because their adjusted risk for fetal aneuploidy did not meet the criteria for diagnostic testing at their particular center. Without reassurance from the diagnostic testing (23), women who have soft markers identified may have lingering concerns into the postpartum period.
Monitoring and treating depression during pregnancy and the postpartum have been shown to improve maternal and child health in women with a history of a psychiatric disorder (24–27). Our data suggest that routine screening for depression may also be beneficial for women who have soft markers identified (28). Proactively addressing mental health can help reduce the stigma associated with mental illness (25), and importantly, treatments are available and can help prevent potential negative impacts (25,29–30).
Our study limitations are as follows; our study participants were predominantly Caucasian, well educated, of higher socioeconomic status. As the majority of the participants with positive screening results were referred to the Department of Medical Genetics, it may be that they had higher serum screening risk estimates and/or increased anxiety as compared to women who were seen by their community healthcare providers. It is unlikely that differences in depression symptoms between groups could be attributed solely to the chromosomal aneuploidy diagnoses, given the small numbers. We did not collect the nature of which soft marker(s) were identified and though counseling for each type of soft marker finding is likely to be broadly consistent between centers, counseling for an echogenic focus is different than a thickened nuchal fold (for example). Future studies exploring these issues could be considered. The EPDS tool is a screening tool, and our data should be considered in this light. Last, every time point was not completed for each participant, rendering it possible that there were more symptoms of depression across all groups than reported.
In conclusion, our data support previous findings that women with prenatal screening results that indicate an increased risk of fetal aneuploidy have greater vulnerability to experiencing depression symptomatology during pregnancy. Furthermore, women who have soft markers identified on ultrasound may have the greatest risk for depression symptoms in pregnancy and postpartum, as compared to women receiving other types of prenatal screening test results. Future larger scale studies could seek to understand these relationships and their clinical significance more fully. However, our data supports a rationale for healthcare providers to assess and monitor the mood of women who receive ‘positive’ prenatal screening results, especially those with soft markers identified. Pretest counseling prior to the detailed ultrasound may be beneficial. Our results also raise the question of whether to offer diagnostic prenatal testing to women who may not meet criteria but have symptoms of depression and soft markers identified.
Supplementary Material
Supporting Information 1: Study Questionnaire: The demographic questionnaire provided to women at enrolment. This demographic questionnaire includes the Edinburgh Postnatal Depression Scale.
Key Message.
Our data suggest that different types of prenatal screening tests may have different effects on women’s moods. Furthermore, our study shows that depression symptoms persist for women who have soft markers identified on ultrasound.
Acknowledgments
The authors thank the volunteers, physicians and genetic counselors who helped with recruitment, the Canadian Mental Health Association (who provided information for participants about perinatal depression and tokens of appreciation), and the Translational Psychiatric Genetics Group for their support.
Funding Statement: This study was funded by the Public Health Special Interest Group of the National Society of Genetic Counselors, the University of British Columbia’s Department of Medical Genetics, and the Jane Engelberg Memorial Fellowship Student Award Program. JA was supported by the Canada Research Chairs Program, and BC Mental Health and Substance Use Services. CH and AA were supported by the Women’s Health Research Institute.
Abbreviations
- SIPS
Serum integrated prenatal screening
- IPS
Integrated prenatal screening
- FTS
First trimester screening
- NIPT
non invasive prenatal screening
- SS
serum screen
- US
ultrasound
- EPDS
Edinburgh Postnatal Depression Scale
Footnotes
Conflict of interest statement: The authors have no financial or personal relationships relevant to the content of this manuscript that could present as potential conflict of interest.
References
- 1.Roshanai AH, Ingvoldstad C, Lindgren P. Fetal ultrasound examination and assessment of genetic soft markers in Sweden: Are ethical principles respected? Acta Obstet Gynecol Scand. 2014;94:141–7. doi: 10.1111/aogs.12554. [DOI] [PubMed] [Google Scholar]
- 2.Abuelo DN, Hopmann MR, Barsel-Bowers G, Goldstein A. Anxiety in women with low maternal serum alpha-fetoprotein screening results. Prenat Diagn. 1991;11:381–5. doi: 10.1002/pd.1970110607. [DOI] [PubMed] [Google Scholar]
- 3.Santalahti P, Latikka AM, Ryynanen M, Hemminki E. Women’s experiences of prenatal serum screening. Birth. 1996;23:101–7. doi: 10.1111/j.1523-536x.1996.tb00837.x. [DOI] [PubMed] [Google Scholar]
- 4.Santalahti P, Hemminki E, Latikka AM, Ryynanen M. Women’s decision-making in prenatal screening. Soc Sci Med. 1998;46:1067–76. doi: 10.1016/s0277-9536(97)10038-7. [DOI] [PubMed] [Google Scholar]
- 5.Statham H, Green J. Serum screening for Down’s syndrome: some women’s experiences. BMJ. 1993;307:174–6. doi: 10.1136/bmj.307.6897.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinans MJ, Huijssoon AM, Tymstra T, Gerrits MC, Bekhuis JR, Mantingh A. How women deal with the results of serum screening for Down syndrome in the second trimester of pregnancy. Prenat Diagn. 2000;20:705–8. doi: 10.1002/1097-0223(200009)20:9<705::aid-pd904>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Hippman C, Oberlander TF, Honer WG, Misri S, Austin JC. Depression during pregnancy: the potential impact of increased risk for fetal aneuploidy on maternal mood. Clin Genet. 2009;75:30–6. doi: 10.1111/j.1399-0004.2008.01056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgsson Ohman S, Saltvedt S, Grunewald C, Waldenstrom U. Does fetal screening affect women’s worries about the health of their baby? A randomized controlled trial of ultrasound screening for Down’s syndrome versus routine ultrasound screening. Acta Obstet Gynecol Scand. 2004;83:634–40. doi: 10.1111/j.0001-6349.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 9.Georgsson Ohman S, Waldenstrom U. Second-trimester routine ultrasound screening: expectations and experiences in a nationwide Swedish sample. Ultrasound Obstet Gynecol. 2008;32:15–22. doi: 10.1002/uog.5273. [DOI] [PubMed] [Google Scholar]
- 10.Georgsson Ohman S, Grunewald C, Waldenstrom U. Perception of risk in relation to ultrasound screening for Down’s syndrome during pregnancy. Midwifery. 2009;25:264–76. doi: 10.1016/j.midw.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. Br J Psychiatry. 1993;163:27–31. doi: 10.1192/bjp.163.1.27. [DOI] [PubMed] [Google Scholar]
- 12.Murray L, Carothers AD. The validation of the Edinburgh Post-Natal Depression Scale on a community sample. Br J Psychiatry. 1990;157:288–90. doi: 10.1192/bjp.157.2.288. [DOI] [PubMed] [Google Scholar]
- 13.Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand. 2009;119:350–64. doi: 10.1111/j.1600-0447.2009.01363.x. [DOI] [PubMed] [Google Scholar]
- 14.Toreki A, Ando B, Dudas RB, Dweik D, Janka Z, Kozinszky Z, Kereszturi A. Validation of the Edinburgh Postnatal Depression Scale as a screening tool for postpartum depression in a clinical sample in Hungary. Midwifery. 2014;30:911–8. doi: 10.1016/j.midw.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Santos IS, Matijasevich A, Tavares BF, Barros AJD, Botelho IP, Lapolli C. Validation of the Edinburgh Postnatal Depression Scale (EPDS) in a sample of mothers from the 2004 Pelotas Birth Cohort Study. Cad Saude Publica. 2007;23:2577–88. doi: 10.1590/s0102-311x2007001100005. [DOI] [PubMed] [Google Scholar]
- 16.Harris P, Taylor R, Theilke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal Biomedical Information. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.R Core Team. A language and environment for statistical computing. 2014. [Google Scholar]
- 18.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biometrical Journal. 2008;50:346–63. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 19.Cartier L, Murphy-Kaulbeck L Genetics Committee. Counseling considerations for prenatal genetic screening. J Obstet Gynaecol Can. 2012;34:489–93. doi: 10.1016/S1701-2163(16)35248-3. [DOI] [PubMed] [Google Scholar]
- 20.Cash R, Manogaran M, Sroka H, Okun N. An assessment of women’s knowledge of and views on the reporting of ultrasound soft markers during the routine anatomy ultrasound examination. J Obstet Gynaecol Can. 2010;32:120–5. doi: 10.1016/S1701-2163(16)34425-5. [DOI] [PubMed] [Google Scholar]
- 21.Hwa HL, Huang LH, Hsieh FJ, Chow SN. Informed consent for antenatal serum screening for Down syndrome. Taiwan J Obstet Gynecol. 2010;49:50–6. doi: 10.1016/S1028-4559(10)60009-5. [DOI] [PubMed] [Google Scholar]
- 22.Keenan KL, Basso D, Goldkrand J, Butler WJ. Low level of maternal serum alpha-fetoprotein: its associated anxiety and the effects of genetic counseling. Am J Obstet Gynecol. 1991;164:54–6. doi: 10.1016/0002-9378(91)90624-z. [DOI] [PubMed] [Google Scholar]
- 23.El-Hage W, Leger J, Delcuze A, Guraudeau B, Perrotin F. Amniocentesis, maternal psychopathology and prenatal representations of attachment: a prospective comparative study. PLoS One. 2012;7:e41777. doi: 10.1371/journal.pone.0041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grote NK, Bridge JA, Gavin AR, Melvile JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–24. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcus SM. Depression during pregnancy: Rates, risks and consequences--motherisk update 2008. Can J Clin Pharmacol. 2009;16:e15–22. [PubMed] [Google Scholar]
- 26.Loomans EM, van Dijk AE, Vrijkotte TG, van Eijsden M, Stronks K, Gemke RJ, et al. Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi-ethnic community-based birth cohort. Eur J Public Health. 2013;23:485–91. doi: 10.1093/eurpub/cks097. [DOI] [PubMed] [Google Scholar]
- 27.Smith MV, Shao L, Howell H, Lin H, Yonkers KA. Perinatal depression and birth outcomes in a healthy start project. Matern Child Health J. 2011;15(3):401–9. doi: 10.1007/s10995-010-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams J, Cadario B, Li D. BC Reproductive Mental Health Program, Perinatal Screening BC. Best practice guidelines for mental health disorders in the perinatal period. 2014:1–120. [Google Scholar]
- 29.Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160:555–62. doi: 10.1176/appi.ajp.160.3.555. [DOI] [PubMed] [Google Scholar]
- 30.O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1: Study Questionnaire: The demographic questionnaire provided to women at enrolment. This demographic questionnaire includes the Edinburgh Postnatal Depression Scale.