Abstract
Objective
Hippocampal atrophy has been associated with mild cognitive impairment (MCI) in Parkinson's disease (PD). However, literature on how hippocampal atrophy affects the pathophysiology of cognitive impairment in PD has been limited. Previous studies assessed the hippocampus as an entire entity instead of their individual subregions. We studied the progression of cognitive status in PD subjects over 18 in relation to hippocampal subfields atrophy.
Methods
65 PD subjects were included. Using the MDS task force criteria, PD subjects were classified as either having no cognitive impairment (PD-NCI) or PD-MCI. We extended the study by investigating the hippocampal subfields atrophy patterns in those who converted from PD-NCI to PD-MCI (PD-converters) compared to those who remained cognitively stable (PD-stable) over 18 months. Freesurfer 6.0 was used to perform the automated segmentation of the hippocampus into thirteen subregions.
Results
PD-MCI showed lower baseline volumes in the left fimbria, right CA1, and right HATA; and lower global cognition scores compared to PD-NCI. Baseline right CA1 was also correlated with baseline attention. Over 18 months, decline in volumes of CA2–3 and episodic memory were also seen in PD-converters compared to PD-stable. Baseline volumes of GC-DG, right CA4, left parasubiculum, and left HATA were predictive of the conversion from PD-NCI to PD-MCI.
Conclusion
The findings from this study add to the anatomical knowledge of hippocampal subregions in PD, allowing us to understand the unique functional contribution of each subfield. Structural changes in the hippocampus subfields could be early biomarkers to detect cognitive impairment in PD.
Abbreviations: CA, cornu ammonis; HATA, hippocampal-amygdaloid transition region
Keywords: Parkinson's disease, Hippocampal atrophy, Volumetry, Cognitive impairment
Highlights
-
•
Hippocampal subfields atrophy could detect cognitive impairment in PD.
-
•
Each hippocampal subfields has a unique functional contribution.
-
•
Baseline hippocampal subfields volumes predicted conversion to from NCI to MCI.
1. Introduction
Parkinson's disease (PD), the second most common neurodegenerative disorder, is characterized by motor and non-motor symptoms (Hanganu et al., 2014). Cognitive impairment is an important feature of non-motor symptoms in PD affecting around 25% of newly diagnosed patients (Ibarretxe-Bilbao et al., 2012) and it can be observed in the early stages of the disease (Pereira et al., 2013). Up to 80% of PD patients without cognitive impairment, eventually develop mild cognitive impairment (PD-MCI) and dementia (Aarsland et al., 2003). Consequentially, this has important implications in disease management, which underscores the need to establish biomarkers that could allow for the early identification of PD patients at risk of developing dementia.
The neuropathological mechanisms underlying cognitive deficits in PD remain to be clearly understood. In the recent years, emerging data have altered the traditional view that the hippocampus is not implicated in PD, except for its possible implications in dementia, to the hypothesis that suggests a pivotal role of hippocampal synaptic plasticity in memory in PD patients (Pereira et al., 2013, Calabresi et al., 2013). While there is a general agreement that hippocampal atrophy is associated with impaired memory in PD (Calabresi et al., 2013, Brück et al., 2004, Kandiah et al., 2014, Camicioli et al., 2003), previous studies investigated hippocampus as a single structure. However, the hippocampus is not a homogeneous structure but consist of several subfields with distinct histological characteristics and specific pathway that interplay to affect the memory (Mueller et al., 2010). This implies that the cytoarchitecture of the individual hippocampal subfields have different effects on the memory and it may be a significant predictor in the progression of cognitive impairment and dementia in PD. Therefore, studying hippocampal subfields may be a significant biomarker to detect cognitive impairment in PD. Moreover, previous studies investigating the effects of hippocampal atrophy on PD patients were mainly cross-sectional measurement, which precludes the ability of these studies to detect subtle changes in the hippocampus due to between-subject variance. Therefore, in this present study, we seek to obviate these issues by investigating the hippocampus in their distinct subfields using serial imaging, which could allow us to study the progression of atrophy in the hippocampal subfields in PD.
In this study, we investigated the baseline characteristics and hippocampal subfields volumes in PD-NCI and PD-MCI subjects. Subsequently, we extended the study to examine the progression of hippocampal subfields atrophy in PD-NCI subjects who either remained cognitively stable (PD-stable) or those who converted into PD-MCI (PD-converters) over 18 months. We further examined the predictive utility of baseline hippocampal subfield volumes in determining the conversion of cognitive status from PD-NCI to PD-MCI. Correlations between the atrophy of hippocampal subfields and cognitive and motor decline were also investigated. Based on a previous cross-sectional study investigating hippocampal subfields in Alzheimer's disease using FreeSurfer, which demonstrated that MCI patients showed smaller CA1 and CA2 regions compared to controls (Mueller et al., 2010), we postulate that specific hippocampal subfields rather than whole hippocampal volume may be more useful in predicting cognitive impairment in PD subjects. As such, we hypothesize that PD-MCI would be characterized by specific baseline reduction of hippocampal subfields compared to PD-NCI; and that specific baseline volumes of hippocampal subfields would also be useful in predicting the conversion from PD-NCI to PD-MCI over 18 months.
2. Methods
2.1. Subjects
Between August 2011 and March 2012, 85 subjects from a tertiary neurology centre were recruited and followed up for 18 months. Over the course of the study duration, 19 subjects withdrew and one deceased. Only subjects (n = 65) with baseline and follow-up were included in this study. Parkinson's disease was diagnosed by movement disorder specialists according to the National Institute of Neurological Disorders and Stroke (NINDS) criteria (Gelb et al., 1999). Inclusion criteria included literate subjects with mild PD, defined by Hoehn and Yahr staging of < 3, aged between 50 and 90 years. Subjects with dementia, or serious medical and psychiatric co-morbidities were excluded from the study. The study was approved by the Singhealth institutional ethics review board and informed consent was obtained for all subjects.
2.2. Assessments
Subjects underwent clinical and neuropsychological evaluations as well as MRI imaging at baseline and month 18. Clinical evaluations included medical history, Unified PD Rating Scale (UPDRS) to ascertain subjects' motor and functional abilities, and Hoehn and Yahr to determine the stage of the disease.
Neuropsychological assessments of global cognition included the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) and Montreal Cognitive Assessment (MoCA) (Dalrymple-Alford et al., 2010). In addition, in accordance with MDS Task Force recommendations (Litvan et al., 2012), five cognitive domains were assessed. Episodic memory was assessed with word-list delayed and recognition recall from the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-Cog); executive function was assessed with the 10-point clock drawing and frontal assessment battery (FAB); attention was assessed with digit span and colour trails 1; visuospatial function was assessed with figure copy test and number of errors made on a Maze test in ADAS-Cog; and language was assessed with comprehension of test instructions from ADAS-Cog and fluency assessment (Saumier et al., 2009). Performance on individual tasks was transformed into z-scores and a composite summary index for each cognitive domain was then derived from the corresponding averages of the individual neuropsychological tests. Changes in memory domains were calculated using the following formula: (memory domainfollow-up – memory domainbaseline).
Subjects were classified as PD-MCI with Movement Disorder Society (MDS) level 2 criteria if they were impaired (1.5 SD) below the normative means on at least two cognitive tests as represented by either impairment of 2 tests in one domain or impairment of one test in two different domains. Subjects who did not fulfill the above criteria were classified as PD-NCI. Using the same criteria as aforementioned, we further classified PD-NCI subjects into: those who converted to PD-MCI (PD-converters) and those who remained cognitively stable (PD-stable) over 18 months.
2.3. Image acquisition
All subjects underwent both baseline and repeat MRI with a mean 18-month interval on a 3 Tesla GE scanner system. A high-resolution T1-weighted MPRAGE (axial acquisition, 176 slices, matrix size = 256 × 256, voxel size = 1.0 × 1.0 × 1.0 mm3, TE = 3.2 ms, TR = 7 ms, TI = 850 ms, flip angle 8°, field of view 256 × 256 mm2) was acquired for all subjects.
2.4. 2.4 image analysis
Cortical reconstruction and volumetric segmentation were performed using FreeSurfer 6.0 image analysis suit (http://surfer.nmr.mgh.harvard.edu/). The technical details have been described previously (Fischl and Dale, 2000). In summary, image processing procedures include the following steps: motion correction and average of multiple volumetric T1-weighted images, removal of non-brain tissue, automated Talairach transformation, intensity normalization, segmentation of the subcortical white matter and deep grey matter volumetric structures, tessellation of the grey matter and white matter boundary, automated topology correction, and surface deformation to optimally place the grey/white and grey/cerebrospinal fluid boundaries (Reuter et al., 2012). All surface models were inspected for accuracy and manual corrections were performed in the event of tissue misclassification and/or white matter errors while blinded to the diagnostic information.
For longitudinal processing, an unbiased within-subject template space (Reuter and Fischl, 2011) and image was created using robust, inverse consistent registration (Reuter et al., 2012). The following steps, which included skull stripping, Talairach transformations, atlas registration, spherical surface maps and parcellation to initialize with common information from within-subject template, were taken to significantly increase reliability and statistical power. The cortical thickness was calculated as the closest distance from the grey/white matter boundary to the grey/CSF boundary at each vertex while total grey matter volume includes both surface-based volume calculations and voxel counts.
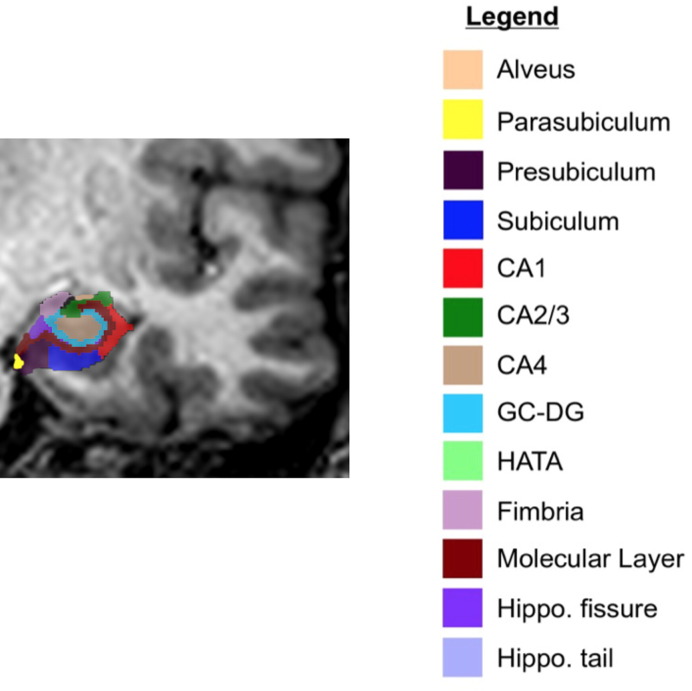
Total hippocampal volumes were obtained from the automated pipeline for volumetric segmentation of subcortical structures implemented in FreeSurfer for both baseline and follow-up. Subsequently, the hippocampal subfields were segmented using a Bayesian inference approach and a novel atlas algorithm of the hippocampal formations built primarily upon ultra-high resolution (~ 0.1 mm isotropic) ex vivo MRI data from autopsy brains (Iglesias et al., 2015). The left and right hippocampi were segmented into thirteen subfields: alveus, parasubiculum, presubiculum, subiculum, CA1, CA2–3, CA4, granule cell layer of dentate gyrus (GC-DG), hippocampus-amygdala-transition-area (HATA), fimbria, molecular layer, hippocampal fissure, hippocampal tail (Fig. 1). This presented atlas was more sensitive than the previous version (FreeSurfer 5.3) that used in vivo atlas for segmenting hippocampal subfields as it allowed for greater accuracy in the delineation of the boundaries within the subfields (Iglesias et al., 2015). Subsequently, percentage changes in the volumes between two time points in hippocampal longitudinal changes were calculated using the following formula: [(volumefollow-up − volumebaseline)/volumebaseline] * 100%.
Fig. 1.
Hippocampal subfields in a coronal view of a PD subject.
2.5. Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS, Inc.; Chicago, IL, USA). Skweness-Kurtosis and visual inspection of histogram were used to examine normality for continuous variables. Demographic differences between the two groups were examined using independent samples t-test or Mann-Whitney U test depending on the normality of distributions; and chi-square test for categorical variables. Comparisons of hippocampal subfields were assessed using analysis of covariance (ANCOVA) followed by post-hoc pairwise Bonferroni tests. Additionally, we performed partial correlations to investigate the associations between hippocampal subfields and memory functions. Logistic regression was also performed to investigate the predictive utility of baseline hippocampal subfield volumes in the conversion from PD-NCI to PD-MCI over 18 months. All volumetric analyses in this study were corrected for age, gender, education, and intracranial volumes. Results with a two-tailed probability value of < 0.05 were considered significant.
3. Results
3.1. Baseline subject characteristics and hippocampal subfields volumes of PD-NCI and PD-MCI
Table 1 summarizes the baseline clinical, neuropsychological, and neuroimaging information of PD-NCI and PD-MCI. 65 subjects were classified as follow: 54 PD-NCI and 11 PD-MCI. The subjects were well matched in their baseline demographic and clinical characteristics, except that subjects with PD-MCI were significantly older than PD-NCI. PD-NCI and PD-MCI also showed no significant differences in baseline cortical thickness and grey matter volumes. PD-MCI showed lower baseline global cognition scores compared to PD-NCI. More significantly, at baseline, hippocampal subfields volumes revealed that PD-MCI had lower volumes in the left fimbria, right CA1, and right HATA regions compared to PD-NCI. Baseline right CA1 volume also showed partial correlations with baseline attention (r = 0.273, p = 0.038, df = 56).
Table 1.
Baseline subject characteristics and hippocampal subfields volumes of PD-NCI and PD-MCI.
| PD-NCI (N = 54) |
PD-MCI (N = 11) |
p-Value | |
|---|---|---|---|
| Subjects' baseline characteristics | |||
| Ageb | 63.39 ± 6.86 | 69.45 ± 10.19 | 0.017 |
| Gender (Male, %)a | 41 (75.9%) | 6 (54.55%) | χ2 = 2.086, p = 0.149 |
| Educationc | 10.93 ± 3.40 | 9.73 ± 2.97 | 0.653 |
| Disease durationb | 4.74 ± 3.02 | 4.09 ± 3.39 | 0.431 |
| Scan intervalb | 1.31 ± 0.14 | 1.39 ± 0.22 | 0.625 |
| APOE4a (Yes, %) | 11 (20.37%) | 2 (18.18%) | χ2 = 1.609, p = 0.447 |
| Diabetesa (Yes, %) | 8 (14.81%) | 3 (27.27%) | χ2 = 1.009, p = 0.379 |
| Hypertensiona (Yes, %) | 20 (37.04%) | 4 (36.36%) | χ2 = 0.002, p = 0.966 |
| Hyperlipidemiaa (Yes, %) | 24 (44.44%) | 7 (63.64) | χ2 = 1.349, p = 0.327 |
| Smoking historya (Yes, %) | 19 (35.19%) | 3 (27.27%) | χ2 = 0.256, p = 0.737 |
| LEDDc | 621.20 ± 447.38 | 633.88 ± 452.27 | 0.803 |
| Motor characteristics (Baseline) | |||
| Hoehn and Yahrc | 2.08 ± 0.41 | 2.00 ± 0.59 | 0.762 |
| UPDRS IIIc | 18.95 ± 8.96 | 15.27 ± 5.26 | 0.667 |
| Neuroimaging characteristics | |||
| Cortical thickness LHb | 2.19 ± 0.10 | 2.19 ± 0.11 | 0.878 |
| Cortical thickness RHb | 2.18 ± 0.10 | 2.18 ± 0.11 | 0.856 |
| Total GM volb | 518.85 ± 37.56 | 509.46 ± 48.11 | 0.474 |
| Memory domains (Baseline) | |||
| Global cognitionc | 0.10 ± 0.90 | − 0.62 ± 1.11 | 0.013 |
| Episodic Memoryc | 0.14 ± 0.88 | − 0.06 ± 0.47 | 0.121 |
| Executivec | 0.15 ± 0.67 | − 0.37 ± 1.02 | 0.130 |
| Attentionb | − 0.04 ± 0.64 | 0.28 ± 0.63 | 0.140 |
| Visuospatialc | 0.09 ± 0.58 | − 0.48 ± 1.40 | 0.210 |
| Languagec | 0.09 ± 0.77 | − 0.29 ± 0.89 | 0.074 |
| Hippocampal subfields LH (Baseline)d | |||
| Hippocampal tail | 0.50 ± 0.06 | 0.50 ± 0.08 | 0.227 |
| Subiculum | 0.41 ± 0.05 | 0.41 ± 0.07 | 0.265 |
| CA1 | 0.57 ± 0.07 | 0.59 ± 0.10 | 0.066 |
| Hippocampal fissure | 0.16 ± 0.05 | 0.16 ± 0.02 | 0.861 |
| Presubiculum | 0.29 ± 0.04 | 0.29 ± 0.05 | 0.332 |
| Parasubiculum | 0.06 ± 0.03 | 0.05 ± 0.01 | 0.503 |
| Molecular Layer | 0.52 ± 0.08 | 0.53 ± 0.08 | 0.220 |
| GC-DG | 0.28 ± 0.04 | 0.27 ± 0.04 | 0.874 |
| CA2–3 | 0.20 ± 0.03 | 0.20 ± 0.03 | 0.858 |
| CA4 | 0.24 ± 0.03 | 0.23 ± 0.03 | 0.861 |
| Fimbria | 0.09 ± 0.02 | 0.07 ± 0.01 | 0.048 |
| HATA | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.870 |
| Whole Hippocampus | 3.24 ± 0.31 | 3.21 ± 0.50 | 0.221 |
| Hippocampal subfields RH (Baseline)d | |||
| Hippocampal tail | 0.57 ± 0.33 | 0.51 ± 0.09 | 0.777 |
| Subiculum | 0.42 ± 0.05 | 0.42 ± 0.06 | 0.174 |
| CA1 | 0.63 ± 0.09 | 0.60 ± 0.07 | 0.031 |
| Hippocampal fissure | 0.18 ± 0.06 | 0.18 ± 0.03 | 0.908 |
| Presubiculum | 0.28 ± 0.04 | 0.28 ± 0.05 | 0.207 |
| Parasubiculum | 0.06 ± 0.03 | 0.05 ± 0.01 | 0.409 |
| Molecular Layer | 0.54 ± 0.08 | 0.56 ± 0.07 | 0.108 |
| GC-DG | 0.30 ± 0.04 | 0.30 ± 0.04 | 0.356 |
| CA2–3 | 0.22 ± 0.03 | 0.22 ± 0.03 | 0.402 |
| CA4 | 0.25 ± 0.03 | 0.25 ± 0.03 | 0.181 |
| Fimbria | 0.08 ± 0.03 | 0.06 ± 0.01 | 0.130 |
| HATA | 0.06 ± 0.01 | 0.05 ± 0.005 | 0.011 |
| Whole Hippocampus | 3.32 ± 0.54 | 3.37 ± 0.45 | 0.186 |
Values expressed as Mean ± SD.
Cognitive scores were Z-scored.
Abbreviations: APOE, Apolipoprotein E; LEDD indicates levodopa equivalent dose; UPDRS, Unified Parkinson's Disease Rating Scale; LH, Left hemisphere; RH, Right hemisphere; CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampus-amygdala-transition-area.
χ2 (df = 1).
Indepedent samples t-test (df = 63).
Mann-Whitney test.
ANCOVA (df = 1) controlling for age, gender, education, intracranial volume with post-hoc Bonferroni pairwise test.
3.2. Longitudinal subject characteristics and hippocampal subfields volumes of PD-stable and PD-converters
Over 18 months, within the PD-NCI group (n = 54) at baseline, 42 subjects remained cognitively stable and were classified as PD-stable while 12 subjects developed MCI and were classified as PD-converters. Table 2 shows the change in clinical, neuropsychological, and neuroimaging information of these subjects. The groups were well matched in their demographic and clinical characteristics. PD-converters, however, showed greater decline in episodic memory over 18 months. PD-converters also showed greater atrophy in the right CA2–3 overtime compared to PD-stable (Table 2, p < 0.05).
Table 2.
Longitudinal subject characteristics and hippocampal subfields volumes of PD-stable and PD-converters.
| PD-stable (N = 42) |
PD-converters (N = 12) |
p-Value | |
|---|---|---|---|
| Subjects' baseline characteristics | |||
| Ageb | 63.19 ± 7.21 | 64.08 ± 5.65 | 0.696 |
| Gender (Male, %)a | 32 (76.19%) | 9 (75.0%) | χ2 = 0.007, p = 0.932 |
| Educationc | 10.83 ± 3.41 | 11.25 ± 3.49 | 0.712 |
| Disease durationb | 4.57 ± 3.01 | 5.36 ± 3.10 | 0.431 |
| Scan intervalb | 1.31 ± 0.14 | 1.29 ± 0.14 | 0.625 |
| APOE4a (Yes, %) | 8 (19.05%) | 3 (25%) | χ2 = 0.463, p = 0.793 |
| Diabetesa (Yes, %) | 8 (19.05%) | 0 (0%) | χ2 = 2.683, p = 0.176 |
| Hypertensiona (Yes, %) | 18 (42.86%) | 2 (16.67%) | χ2 = 2.745, p = 0.098 |
| Hyperlipidemiaa (Yes, %) | 20 (47.62%) | 4 (33.33%) | χ2 = 0.771, p = 0.515 |
| Smoking historya (Yes, %) | 14 (33.33%) | 5 (41.67%) | χ2 = 0.284, p = 0.734 |
| LEDDc | 621.20 ± 447.37 | 633.88 ± 452.27 | 0.803 |
| Motor characteristics (Change) | |||
| Hoehn and Yahrc | − 0.05 ± 0.35 | − 0.11 ± 0.18 | 0.606 |
| UPDRS IIIc | 0.26 ± 0.92 | 0.28 ± 0.55 | 0.289 |
| Memory domains (Change) | |||
| Global cognition | 0.51 ± 0.34 | 0.02 ± 0.31 | 0.632 |
| Epsodic Memoryc | 0.20 ± 0.69 | − 0.70 ± 0.69 | 0.013 |
| Executivec | 0.12 ± 0.89 | − 0.56 ± 1.02 | 0.055 |
| Attentionb | − 0.02 ± 0.66 | 0.12 ± 0.58 | 0.105 |
| Visuospatialc | − 0.16 ± 1.13 | − 0.16 ± 0.70 | 0.934 |
| Languagec | − 0.15 ± 1.27 | 0.11 ± 0.43 | 0.269 |
| Neuroimaging characteristics (Change) | |||
| Cortical thickness LHb | − 0.42 ± 1.89 | 0.12 ± 1.49 | 0.369 |
| Cortical thickness RHb | 0.29 ± 2.13 | 0.27 ± 1.47 | 0.978 |
| Total GM volb | − 0.60 ± 1.83 | − 1.04 ± 0.85 | 0.577 |
| Hippocampal subfields LH (Change)d | |||
| Hippocampal tail | − 0.70 ± 4.62 | − 1.94 ± 4.34 | 0.714 |
| Subiculum | − 1.99 ± 2.48 | − 1.82 ± 1.70 | 0.952 |
| CA1 | − 1.33 ± 4.35 | − 2.78 ± 2.49 | 0.418 |
| Hippocampal fissure | − 0.29 ± 7.56 | − 2.05 ± 5.94 | 0.501 |
| Presubiculum | − 1.71 ± 4.11 | − 2.46 ± 3.59 | 0.729 |
| Parasubiculum | − 0.99 ± 8.28 | − 1.55 ± 5.17 | 0.932 |
| Molecular layer | − 2.11 ± 2.80 | − 1.76 ± 2.36 | 0.534 |
| GC-DG | − 1.89 ± 4.05 | − 2.61 ± 3.07 | 0.808 |
| CA2–3 | − 1.31 ± 5.22 | − 4.52 ± 3.40 | 0.121 |
| CA4 | − 1.83 ± 4.30 | − 2.59 ± 2.83 | 0.802 |
| Fimbria | − 1.21 ± 4.98 | 0.98 ± 6.42 | 0.095 |
| HATA | − 0.61 ± 5.48 | − 0.64 ± 10.32 | 0.944 |
| Whole hippocampus | − 1.87 ± 2.40 | − 2.63 ± 1.99 | 0.545 |
| Hippocampal subfields RH (Change)d | |||
| Hippocampal tail | − 1.35 ± 4.45 | − 0.98 ± 1.67 | 0.631 |
| Subiculum | − 1.41 ± 3.23 | − 1.05 ± 4.09 | 0.976 |
| CA1 | − 1.24 ± 3.89 | − 2.19 ± 2.17 | 0.553 |
| Hippocampal fissure | − 1.83 ± 5.83 | − 1.06 ± 8.19 | 0.752 |
| Presubiculum | − 0.98 ± 3.48 | − 0.44 ± 3.21 | 0.759 |
| Parasubiculum | − 0.52 ± 4.72 | − 1.11 ± 3.76 | 0.766 |
| Molecular layer | − 1.54 ± 3.07 | 0.04 ± 2.68 | 0.062 |
| GC-DG | − 0.15 ± 4.04 | − 0.21 ± 3.25 | 0.755 |
| CA2–3 | 0.33 ± 5.15 | − 3.81 ± 3.69 | 0.029 |
| CA4 | 0.37 ± 3.85 | − 0.96 ± 4.17 | 0.490 |
| Fimbria | − 5.50 ± 10.42 | − 6.01 ± 15.80 | 0.683 |
| HATA | − 3.95 ± 7.90 | − 3.94 ± 6.38 | 0.929 |
| Whole hippocampus | − 1.45 ± 2.69 | − 1.70 ± 1.53 | 0.904 |
Values expressed as Mean ± SD.
Cognitive scores were Z-scored.
Abbreviations: APOE, Apolipoprotein E; LEDD indicates levodopa equivalent dose; UPDRS, Unified Parkinson's Disease Rating Scale; LH, Left hemisphere; RH, Right hemisphere; CA, cornu ammonis; GC-DG, granule cell layer of dentate gyrus; HATA, hippocampus-amygdala-transition-area.
χ2 (df = 1).
Indepedent samples t-test (df = 63).
Mann-Whitney test.
ANCOVA (df = 1) controlling for age, gender, education, intracranial volume with post-hoc Bonferroni pairwise tests.
Change in memory domains = follow-up visit − baseline; Change in imaging data = [(volumefollow-up − volumebaseline) / volumebaseline] * 100%.
Additional logistic regression analyses also showed that decreasing baseline volumes of GC-DG (left p = 0.049; right p = 0.040), right CA4 (p = 0.026), left parasubiculum (p = 0.023), and left HATA (p = 0.022) increased the likelihood of the conversion from PD-NCI to PD-MCI over 18 months, after controlling for age, education, gender, and ICV.
4. Discussion
The main findings of this study were: (i) At baseline, PD-MCI showed lower volumes in the left fimbria, right CA1, and right HATA together with lower global cognition scores compared to PD-NCI; (ii) Baseline right CA1 was significantly associated with baseline attention scores; (iii) PD-converters showed greater atrophy in the CA2–3 and episodic memory deficits compared to PD-stable over 18 months; (iv) Baseline volumes of GC-DG, right CA4, left parasubiculum, and left HATA were predictive of the conversion from PD-NCI to PD-MCI over 18 months.
To date, only a few studies have investigated the role of regional hippocampal atrophy in PD; as such the neuropathological mechanisms of hippocampus in modulating cognition in PD, have not been well elucidated. A previous neuropathological study has shown that the extent of deposition of Lewy bodies and Lewy neuritis in the hippocampus was associated to the severity of cognitive impairment in PD, implying that hippocampus structures play an important role in cognition in PD (Churchyard and Lees, 1997). Previous neuroimaging studies using volumetric analyses have also supported the association between cognitive impairment and hippocampal atrophy in PD (Ibarretxe-Bilbao et al., 2012, Carlesimo et al., 2012). However, a study by Apostolova et al. (2010) did not find any hippocampal contribution to cognition in PD after correcting for age (Apostolova et al., 2010). The heterogeneity in findings could be due in part to the variability of methods used to examine the hippocampus. Furthermore, within the paucity of hippocampal literature in PD, only Beyer et al. (2013) and Pereira et al. (2013) have assessed precise measurements of the specific subfields within the hippocampus in PD subjects. The former showed associations between delayed recall and subfield areas of CA1, CA3 and subiculum, and short-term recall with CA1 and subiculum; whereas the latter demonstrated that CA2–3 and CA4-DG subfields had greater volume loss in the PD group compared to controls. Although these studies specifically assessed structures within the hippocampus compared to studies assessing regional hippocampal atrophy, they were however limited by their cross-sectional designs.
Hippocampus is a complex structure where the neuroanatomical organization and interconnections between the subfields could influence the pathophysiological and cognitive processes in PD subjects. Considering the functional specialization of the histologically distinct hippocampal subregions, local analyses of the hippocampus subregions may be a viable method of characterizing the involvement of cytoarchitecture of these areas in the pathologenesis of PD and cognitive impairment (Mak et al., 2016). In light of this, we first explored the baseline volumes of hippocampal subfields in PD-NCI and PD-MCI; we then examined the cognitive progression in PD-NCI (PD-stable or PD-converters) in affecting the longitudinal impact of atrophy in the individual subfields of the hippocampus.
PD-MCI showed reduced volumes in CA1 at baseline compared to PD-NCI and correlated significantly with baseline attention scores. This study was consistent with previous studies showing that CA1 was one of the earliest sites within the hippocampus to be affected, which was associated with increased risk for conversion from MCI to dementia (Beyer et al., 2013). Similarly, previous post-mortem and imaging studies have showed that degenerations within CA1 and subiculum were more severe in MCI than in dementia (Braak and Braak, 1997, Lim et al., 2013). In addition, at a molecular level, animal studies have shown that alterations in the glutamatergic transmission might cause the defect in CA1 hippocampal long-term potentiation, which could in turn contribute to dopamine-dependent impairment and thereby, resulting in cognitive deficits in PD (Calabresi et al., 2013). This implies that the interplay of glutamatergic and dopaminergic deficiencies in CA1 could cause cognitive decline in PD-MCI. Furthermore, a review paper by Muzzio et al. (2009) reported that due to the neuroanatomical position of CA1 region that renders its ability to compare information that is processed through the trisynaptic loop with new information arriving directly from the cortex, it has been posited that the CA1 region may serve as an attentional gate. Taken together, atrophy in CA1 could exacerbate cognitive decline, specifically attentional deficits, in PD-MCI; and that progressive atrophy could potentially promote the conversion of MCI to dementia in PD.
At baseline, PD-MCI also showed reduced volumes in fimbria, a white matter structure that extends from the alveus and eventually forms the fornix, and HATA, which lies in the medial region of the hippocampus and is superior to the other subfields. Several animal studies have postulated that the projection of fimbria-fornix to the dorsal part of basal nucleus in amygdala plays an important role in mediating the amygdala-hippocampus interactions (Gary-Bobo and Bonvallet, 1977, Jas et al., 2000). Lesions to either the fimbria or the amygdala could disrupt the network and eventually result in visuospatial and object discrimination dysfunction (Chai and White, 2004, Wible et al., 1992). These animal studies could potentially translate to human studies; therefore, the reduction of volumes in fimbria and HATA in this study could be an early biomarker to visuospatial dysfunction in PD-MCI.
PD-converters had greater pathological alterations in CA2–3 over 18 months compared to PD-stable; they also showed that baseline volumes of GC-DG, CA4, parasubiculum, and HATA were predictive of the conversion from PD-NCI to PD-MCI over 18 months. Previous imaging studies, including postmortem evidence showed specific degeneration in the CA2–3 hippocampal subregion in both demented and non-demented PD subjects (Pereira et al., 2013, Mattila et al., 1999, Harding and Halliday, 2001). This implies that atrophy of CA2–3 hippocampal subfield could be a significant hallmark across the different neurodegenerative stages of PD. Additionally, the results from PD-converters could be explained by two major pathways: mossy fibers pathway and the hippocampal-amygdala connections. Any disruption to the mossy fibers pathway – where mossy fibers within GC-DG make numerous synapses en passage with the dendrites in CA4 and then in CA3 subfields – could lead to a deregulation of glutamate release in the mossy fibers (Acsády et al., 1998, Amaral et al., 2007). Evidence has also demonstrated that altered glutamatergic neurotransmission and neuronal metabolic dysfunction could result in pathophysiological motor and cognitive dysfunction in PD (Cenci, 2014). In turn, baseline volumes of GC-DG and CA4 might be able to predict greater glutamatergic deafferentation in the conversion of PD-NCI to PD-MCI. Moreover, animal studies have shown that axonal projections from the lateral and basolateral nuclei of amygdaloid complex to the entorhinal cortex to the ventral part of subiculum and the parasubiculum could modulate information processing within the hippocampal formation (Pikkarainen et al., 1999). Consequently, it is postulated that in humans, any atrophy within the parasubiculum and HATA might disrupt the integrity of the hippocampal-amygdala network affecting the information processed. Collectively, disruptions to the mossy fibers pathway and hippocampal-amygdala pathway due to reduction of volumes in CA3, GC-DG, CA4, parasubiculum, or HATA could promote cognitive dysfunctions and serve as early indicators and potential biomarkers for the conversion from PD-NCI to PD-MCI.
Limitations in this study should be acknowledged. Firstly, this study has a relatively short follow-up of 18 months, which did not allow us to study progression to dementia. Moreover, this short follow-up has also created variability in the study as many of these PD subjects may not experience atrophy in a linear way or are at a high risk but have not yet reached a significant rapid decline of cognition. Additionally, the relatively short follow-up of 18 months inhibited us from studying the progression of the PD subjects to dementia. However, as the cohort is currently being followed-up, impact of hippocampal subfields atrophy on progression to PD-dementia will be investigated at a later time point. Secondly, this study has a relatively small sample size. Hence, results from this study needs to be reproduced in larger studies. Moreover, due to the lack of control subjects, we could not make comparisons between the changes found in PD subjects and the physiological ageing. As such, these findings need to be confirmed in larger prospective studies with an additional group of well-matched healthy controls. Lastly, due to the lack of T2 sequence, only MPRAGE sequences were used. MPRAGE lacks the relevant landmarks for the precise subfield boundaries and hence the segmentation process is purely inferential.
In addition, there are limitations of the atlas used in FreeSurfer 6.0. The Bayesian inferential method deployed on FreeSurfer 6.0 has not been robustly validated against a gold standard method, such as manual segmentation (Wolf et al., 2015). Furthermore, even with ultra-high resolution MRI, there are boundaries such as the interfaces between the CA fields along the pyramidal layer of the hippocampus or the CA4/GC-DG interface that cannot be seen. However, it is imperative to note that this remains as a widely known problem in the hippocampal subregion MRI literature wherein remains the discrepancy and variability in subregion definitions, especially when comparing the heterogeneous manual annotations used in previous voxel-based morphometry studies (Iglesias et al., 2015). Therefore, volumetric results from the individual subregions must be interpreted with caution. Despite so, automatic segmentation using FreeSufer still provides highly useful information of the hippocampus as the ex vivo atlas significantly outperforms the in vivo atlas in the segmentation of the whole hippocampus. This allow us to detect distinct subtle changes within the hippocampus in PD and to understand how the architecture and interconnections of the hippocampus in affecting the pathophysiology of PD. Moreover, by using an automated segmentation, it allows for a more reproducible and automatic fashion of segmenting the hippocampus into its subregions instead of the typical labor intensive and biased method of manual hippocampus segmentation.
It is important to note that as clinical criteria for PD-MCI do not differentiate whether cognitive impairment is due to PD or Alzheimer's disease (AD) pathological process, these hippocampal subfield changes suggest a possible underlying AD pathology among PD patients. This is especially so when the hippocampus has shown to be an early area to be affected in AD. Future studies should investigate the underlying pathological processes behind hippocampal subfields atrophy in PD patients as this will allow for better disease management.
Another strength in this study includes the novelty of tracking the progression of hippocampal atrophy and to investigate the association between atrophic subregions and cognition in the PD subjects overtime. These allowed us to understand the pathophysiological mechanisms that underlie cognitive impairment in PD and potentially use specific hippocampal subfields as biomarkers in predicting cognitive impairment in PD.
5. Conclusions
Current literature does not fully incorporate the intricately connected hippocampal subfields networks due to the complexity of its architecture; however, such anatomical knowledge is imperative for understanding the functional contribution of each subfield. In this present study, PD-MCI showed significant atrophy in specific reduction of hippocampal subfield volumes. More importantly, baseline volume of GC-DG, CA4, parasubiculum, and HATA could be important biomarkers in the prediction of conversion from PD-NCI to PD-MCI. Consistent with emerging data showing the pivotal role of hippocampus in cognition in PD, this study provides strong evidence that structural changes in the hippocampus could result in cognitive deficits. Therefore, the hippocampus could aid in early detection of cognitive impairment in PD subjects.
Funding
This study was supported by Ministry of Health, National Medical Research Council, Singapore [grant number NMRC/NIG/1043/2011].
Disclosures
Heidi Foo, Elijah Mak, Yong Ting Ting, Wen Ming Ching, Russell Jude Chander, and Au Wing Lok report no disclosures. Nagaendran Kandiah has received honorarium and CME spon- sorship from Lundbeck, Novartis, Pfizer and Eisai. He has also received research funding from Singhealth Foundation, Media Development Authority of Singapore and the National Medical Research Council of Singapore. Louis CS Tan has received research support from Singapore Millenium Foundation and funding for conference travel from GlaxoSmithKline Pte Ltd.
Acknowledgements
Heidi Foo formulated the research question, performed the statistical analyses, interpreted the results, and wrote the article. Elijah Mak assisted with the interpretation of results and provided suggestions for the revision of the draft. Russell Jude Chander and Aloysius Ng administered the neuropsychological assessments. Wing Lok Au and Louis Tan assisted with the recruitment of study subjects and reviewed the article. Yih Yian Sitoh designed the imaging protocol, undertook routine quality assurance on the MR system, and reviewed the article. Nagaendran Kandiah assisted with the recruitment of study subjects, assisted with the interpretation of results, and provided suggestions for the revision of the draft. All authors approved the final article.
References
- Aarsland D., Andersen K., Larsen J.P., Lolk A., Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch. Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- Acsády L., Kamondi A., Sík A., Freund T., Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J. Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D.G., Scharfman H.E., Lavenex P. The dentate gyrus: fundamental neuroanatomical organization (dentate gyrus for dummies) Prog. Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova L.G., Beyer M., Green A.E., Hwang K.S., Morra J.H., Chou Y.Y., Avedissian C., Aarsland D., Janvin C.C., Larsen J.P., Cummings J.L., Thompson P.M. Hippocampal, caudate, and ventricular changes in Parkinson's disease with and without dementia. Mov. Disord. 2010;25:687–695. doi: 10.1002/mds.22799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer M.K., Bronnick K.S., Hwang K.S., Bergsland N., Tysnes O.B., Larsen J.P., Thompson P.M., Somme J.H., Apostolova L.G. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2013;84:23–28. doi: 10.1136/jnnp-2012-303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak E., Braak H. Alzheimer's disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon's horn. Acta Neuropathol. 1997;93:323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- Brück A., Kurki T., Kaasinen V., Vahlberg T., Rinne J.O. Hippocampal and prefrontal atrophy in patients with early non-demented Parkinson's disease is related to cognitive impairment. J. Neurol. Neurosurg. Psychiatry. 2004;75:1467–1469. doi: 10.1136/jnnp.2003.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P., Castrioto A., Di Filippo M., Picconi B. New experimental and clinical links between the hippocampus and the dopaminergic system in Parkinson's disease. Lancet Neurol. 2013;12:811–821. doi: 10.1016/S1474-4422(13)70118-2. [DOI] [PubMed] [Google Scholar]
- Camicioli R., Moore M.M., Kinney A., Corbridge E., Glassberg K., Kaye J.A. Parkinson's disease is associated with hippocampal atrophy. Mov. Dis. 2003;18:784–790. doi: 10.1002/mds.10444. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Piras F., Assogna F., Pontieri F.E., Caltagirone C., Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- Cenci M.A. Glutamatergic pathways as a target for the treatment of dyskinesias in Parkinson's disease. Biochem. Soc. Trans. 2014;42:600–604. doi: 10.1042/BST20140006. [DOI] [PubMed] [Google Scholar]
- Chai S.C., White N.M. Effects of fimbria-fornix, hippocampus, and amygdala lesions on discrimination between proximal locations. Behav. Neurosci. 2004;118:770–784. doi: 10.1037/0735-7044.118.4.770. [DOI] [PubMed] [Google Scholar]
- Churchyard A., Lees A.J. The relationship between dementia and direct involvement of the hippocampus and amygdala in Parkinson's disease. Neurology. 1997;49:1570–1576. doi: 10.1212/wnl.49.6.1570. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford J.C., MacAskill M.R., Nakas C.T., Livingston L., Graham C., Crucian G.P., Melzer T.R., Kirwan J., Keenan R., Wells S., Porter R.J., Watts R., Anderson T.J. The MoCA: well- suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state: a practical method for grading the cognitive state of patients for the clinicians. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gary-Bobo E., Bonvallet M. Commissural projection to the amygdala through the fimbria fornix system in the cat. Exp. Brain Res. 1977;27:61–70. doi: 10.1007/BF00234825. [DOI] [PubMed] [Google Scholar]
- Gelb D.J., Oliver E., Gilman S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- Hanganu A., Beditti C., Degroot C., Mejia-Constain B., Lafontaine A.L., Soland V., Chouinard S., Bruneau M.A., Mellah S., Belleville S., Monchi O. Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson's disease longitudinally. Brain. 2014;137:1120–1129. doi: 10.1093/brain/awu036. [DOI] [PubMed] [Google Scholar]
- Harding A.J., Halliday G.M. Cortical lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001;102:355–363. doi: 10.1007/s004010100390. [DOI] [PubMed] [Google Scholar]
- Ibarretxe-Bilbao N., Junque C., Segura B., Baggio H.C., Marti M.J., Valldeoriola F., Bargallo N., Tolosa E. Progression of cortical thinning in Parkinson's disease. Mov. Disord. 2012;27:1746–1754. doi: 10.1002/mds.25240. [DOI] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M., Roy N., Frosch M.P., McKee A.C., Wald L.L., Fischl B., Van Leemput K.A. Computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jas J., Almaguer W., Frey J.U., Bergado J. Lesioning the fimbria-fornix impairs basolateral amygdala induced reinforcement of LTP in the dentate gyrus. Brain Res. 2000;861:186–189. doi: 10.1016/s0006-8993(00)02037-0. [DOI] [PubMed] [Google Scholar]
- Kandiah N., Zainal N.H., Narasimhalu K., Chander R.J., Ng A., Mak E., Au W.L., Sitoh Y.Y., Nadkarni N., Tan L.C. Hippocampal volume and white matter disease in the prediction of dementia in Parkinson's disease. Parkinsonism Rel. Disord. 2014;20:1203–1208. doi: 10.1016/j.parkreldis.2014.08.024. [DOI] [PubMed] [Google Scholar]
- Lim H.K., Hong S.C., Jung W.S., Ahn K.J., Won W.Y., Hahn C., Kim I.S., Lee C.U. Automated segmentation of hippocampal subfields in drug-naïve patients with Alzheimer disease. Am. J. Neuroradiol. 2013;34:747–751. doi: 10.3174/ajnr.A3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I., Goldman J.G., Tröster A.I., Schmand B.A., Weintraub D., Petersen R.C., Mollenhauer B., Adler C.H., Marder K., Williams-Gray C.H., Aarsland D., Kulisevsky J., Rodrigues-Oroz M.C., Burn D.J., Barker R.A., Emre M. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement disorder society task force guidelines. Mov. Disord. 2012;27:349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak E., Li S., Williams G., Watson R., Firbank M., Blamire A., O'brien J. Differential atrophy of hippocampal subfields: a comparative study of dementia with Lewy bodies and Alzheimer's disease. Am. J. Geriatr. Psychiatry. 2016;24:136–143. doi: 10.1016/j.jagp.2015.06.006. [DOI] [PubMed] [Google Scholar]
- Mattila P.M., Rinne J.O., Helenius H., Roytta M. Neuritic degen- eration in the hippocampus and amygdala in Parkinson's disease in relation to alzheimer pathology. Acta Neuropathol. 1999;98:157–164. doi: 10.1007/s004010051064. [DOI] [PubMed] [Google Scholar]
- Mueller S.G., Schuff N., Yaffe K., Madison C., Miller B., Weiner M.W. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio I.A., Kentros C., Kandel E. What is remembered? Role of attention on encoding and retrieval of hippocampal representations. J. Physiol. 2009;587:2837–2854. doi: 10.1113/jphysiol.2009.172445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J.B., Junqué C., Bartrés-Faz D., Ramírez-Ruiz B., Marti M.J., Tolosa E. Hippocampus. 2013;23:720–728. doi: 10.1002/hipo.22131. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M., Rönkkö S., Savander V., Insausti R., Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J. Comp. Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumier D., Duong A., Haine D., Garceau D., Sampalis J. Domain-specific cognitive effects of Tramiprosate in patients with mild to moderate Alzheimer's disease: ADAS-Cog subscale results from the alphase study. J. Nutr. Health Aging. 2009;13:808–812. doi: 10.1007/s12603-009-0217-4. [DOI] [PubMed] [Google Scholar]
- Wible C.G., Shiber J.R., Olton D.S. Hippocampus, fimbria-fornix, amygdala, and memory: object discriminations in rats. Behav. Neurosci. 1992;106:751–761. doi: 10.1037//0735-7044.106.5.751. [DOI] [PubMed] [Google Scholar]
- Wolf D., Fischer F.U., de Flores R., Chételat G., Fellgiebel A. Differential associations of age with volume and microstructure of hippocampal subfields in healthy older adults. Hum. Brain Mapp. 2015;10:3819–3831. doi: 10.1002/hbm.22880. [DOI] [PMC free article] [PubMed] [Google Scholar]