Abstract
Fukuyama-type congenital muscular dystrophy (FCMD, MIM#253800) is an autosomal recessive disorder characterized by severe muscular dystrophy associated with brain malformations. FCMD is the second most common form of muscular dystrophy after Duchenne muscular dystrophy and one of the most common autosomal recessive diseases among the Japanese population, and yet few patients outside of Japan had been reported with this disorder. We report the first known Egyptian patient with FCMD, established by clinical features of generalized weakness, pseudohypertrophy of calf muscles, progressive joint contractures, severe scoliosis, elevated serum creatine kinase level, myopathic electrodiagnostic changes, brain MRI with cobblestone complex, and mutation in the fukutin gene. In addition, our patient displayed primary microcephaly, not previously reported associated with fukutin mutations. Our results expand the geographic and clinical spectrum of fukutin mutations.
Keywords: Congenital muscular dystrophy, Dystroglycanopathy, Fukutin, Cobblestone lissencephaly
1. Introduction
Alpha-dystroglycanopathies are a clinically and genetically distinct group of muscular dystrophies characterized by the reduced or absent glycosylation of the extracellular scaffolding protein alpha-dystroglycan (Buysse et al., 2013; Lefeber et al., 2009; Manzini et al., 2012; Muntoni et al., 2002; Riisager et al., 2013). The clinical manifestations of alpha-dystroglycanopathies are extremely variable, leading to a broad spectrum of phenotypes with limb-girdle muscular dystrophy (LGMD) without mental retardation delineating the milder end, and Walker–Warburg syndrome (WWS), muscle–eye–brain disease (MEB) and Fukuyama type congenital muscular dystrophy (FCMD) the severe end of the spectrum (Muntoni and Voit, 2004; Muntoni et al., 2011; Willer et al., 2012). FCMD, WWS and MEB diseases are clinically similar autosomal recessive disorders characterized by congenital muscular dystrophy (CMD), cobblestone lissencephaly, and anterior or posterior chamber ocular anomalies. FCMD patients typically survive beyond infancy, and ocular manifestations are rare and usually mild. WWS patients are severely affected from birth, and few live beyond infancy.
To date, mutations in thirteen genes have been identified in congenital muscle dystrophies (Sparks et al., 2001 (updated in 2012), eight of them are glycosyltransferase genes responsible for hypoglycosylation of α-dystroglycan though incriminated in dystroglycanopathies (Costa et al., 2013; Willer et al., 2012). Although each gene was originally described as associated with one syndrome, further genotype–phenotype correlations have broadened these associations to both more severe and less severe conditions. In fact, many of the genes originally linked to CMDs were later found to cause recessive forms of LGMD, and we now know that the dystroglycanopathies represent a broad spectrum of phenotypes with shared underlying molecular pathology (Muntoni, 2007; Riisager et al., 2013; Yoshioka, 2012).
Although FCMD is frequent only in Japan, WWS has been found in many different nationalities, whereas MEB has been observed mainly in Finland (Taniguchi et al., 2003; Toda et al., 2000). FCMD is the second most common form of muscular dystrophy in Japanese population after Duchenne muscular dystrophy, but is seen very rarely in other population. The incidence of FCMD is 6.2-11.9/100,000 or 5.6/100,000 (Fukuyama and Ohsawa, 1984; Takeshita et al., 1997; Toda et al., 2000), with carrier frequency of one in 80 and is one of the most common autosomal recessive disorder in Japan (Fukuyama and Ohsawa, 1984).
FKTN gene is located on chromosome 9q31, encoding a461 amino acid protein, and is broadly expressed in tissues (Fukuyama and Ohsawa, 1984; Kobayashi et al., 1998a). FKTN was the first human disease gene identified as mutated by ancient retrotransposon integration. Most FCMD-bearing chromosomes (87%) derive from a single ancestral founder, whose mutation consisted of a 3-kb retrotransposon insertion in the 3' noncoding region of the fukutin gene (Kobayashi et al., 1998a, 1998b). Since the original report, genotype–phenotype correlations indicate that mutations in FKTN can lead to three different forms of disease: a severe congenital form with brain and eye anomalies (type A4), WWS or MEB, a less severe congenital form without mental retardation (type B4) and a milder limb-girdle form (type C4) (Riisager et al., 2013; Yis et al., 2011; Yoshioka, 2009).
Fukuyama congenital muscular dystrophy is seen almost exclusively in Japan, but has been recently reported in other population with a very broad geographic origin with CMD (Costa et al., 2013; Vuillaumier-Barrot et al., 2009; Yis et al., 2011). Here we report on the first Egyptian patient with FCMD with a novel homozygous missense mutation. Our diagnosis is based initially on clinical phenotype, elevation of serum creatine kinase concentration; neuroimaging (MRI) of brain, electromyography (EMG) and confirmed by exome-based molecular genetic testing of FKTN.
2. Clinical report and results
A female patient from Family-1588, aged 5 2/12 years was referred to the outpatient clinic of the Clinical Genetics Department, with global developmental delay and muscle weakness. She was the only offspring of first cousin consanguineous marriage; the origin of both parents were from Kena Governorate in Upper Egypt. Family history showed three previous sibling relatives who died around 7 years old with global developmental delay. The father and mother were 32 and 29 years old, respectively at the time of the child's birth. During pregnancy, the mother noted sluggish fetal movement and serial 3D ultrasound showed no polyhydraminos but cessation of head growth around the 30th week's gestation till term. The patient was born full term by selective caesarian section. The birth weight was 2750 kg (−0.5 SD), the head circumference and length were not recorded although the parents noted a relatively small head with very small anterior fontanel. Hypotonia was noted since the early neonatal life. The patient developed physiological jaundice at the third day of birth and was incubated for 13 days for prolonged jaundice and weak suckling. Further, the patient gained weight slowly and had delayed physical and mental milestones. She was able to sit unsupported at 4 years of age and had well developed social skills with limited verbal skills. She developed early and progressive joint contracture particularly of knee joints and dorsolumbar kyphoscoliosis.
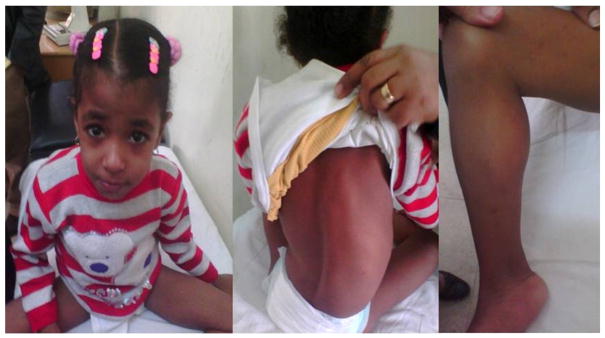
On examination, the patient was confined to a wheel chair, had myopathic facial appearance (Fig. 1), low forehead, bushy eyebrows, trichomegaly, prominent nasal root and tented upper lip. Chest, heart and abdominal examinations were within normal limits. Neurological examination revealed amyotrophy of both upper and lower limbs, with pseudohypertrophy of calf muscles, muscle weakness (grade 3 in upper limbs and grade 2 in lower limbs), marked hypotonia and hyporeflexia and intact sensation. The patient had limited extension of knee and ankle joints consistent with acquired arthrogryposis. Anthropometric measurements at the time of referral were; weight 13.5 kg (−1.9 SD), length 101 cm (−1.7 SD), and head circumference of 47.5 cm (−2.7 SD) and on following up at the age of 7; the head circumference was 47.7 cm (−3.1SD) while the weight was 15.3 kg (−1.7 SD) and the height was 110 cm (−1.8 SD). Investigations revealed normal electroencephalogram (EEG), fundus examination, electroretinogram, visual evoked potential and echocardiography. Creatine phosphokinase was highly elevated in several measurements reaching up to 15,970 U/L (normal up to 200 U/L). Electromyography showed clear but non-specific myopathic pattern in both upper and lower muscle groups, including rapid recruitment pattern, decreased maximum amplitude, increased number of low-amplitude short-duration motor unit potentials (MUPs), and increased number of polyphasic MUPs. The nerve conduction velocities were within normal limits. During psychological evaluation; the patient didn't respond to Wechsler Preschool and Primary Scale of Intelligence (WPPSI) and was assessed by Vineland social maturity scale that was 57 for social quotient (SQ).
Fig. 1.
Myopathic facial appearance of patient in this study, showing trichomegaly, prominent nasal root and tented upper lip, back showing scoliosis, pseudohypertrophy.
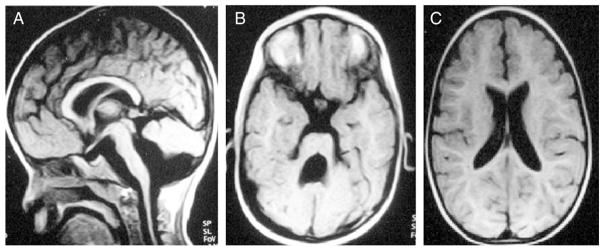
Brain MRI (Fig. 2) revealed abnormal gyral pattern in the form of mixed pachygyria and polymicrogyria in frontoparietal areas. The posterior fossa showed bilateral small cystic lesions within the cerebellar hemispheres, cerebellar vermian hypoplasia and brain stem hypoplasia, typical for cobblestone complex reported with FCMD (Beltran-Valero de Bernabe et al., 2003; Fukuyama et al., 1981; Vuillaumier-Barrot et al., 2009).
Fig. 2.
T1-weighted brain MRI (A) Midline sagittal, (B) Axial at the level of the midbrain–hindbrain junction, (C) Axial at the level of the genu of the corpus callosum. (A) Shows stretched corpus callosum as a result of ventriculomegaly, thin brainstem and misshapen cerebellum. (B) Shows large 4th ventricle and thickened superior cerebellar peduncles. (C) Shows ventriculomegaly.
In order to identify the genetic cause, we performed whole exome sequencing on the child. The study was approved by the IRB and the family provided informed consent for the study. Blood DNA was extracted by using Qiagen reagents, then subjected to exome capture with the Agilent SureSelect Human All Exome 50 Mb kit, sequenced on an Illumina HiSeq2000 instrument, resulting in ~91% recovery at > 10× coverage and 89% recovery at > 15× coverage. GATK (DePristo et al., 2011) was used for variant identification and intersected with identity-by-descent blocks identified by Homozygosity Mapper (See low et al., 2009) and then filtered for homozygous variants. There were a total of 6 rare variants, only one of which occurred in a gene known to give rise to a human disease-FKTN. This variant occurred at chromosome 9:108382302T>C and was a c.T1132C (in RefSeq clone NM_006731.2) leading to a p.W378R amino acid transversion in exon 10. The p.W378 residue was observed to be completely conserved in all sequenced species. Further, this variant was neither encountered in over 1000 Egyptian individuals sequenced to date, nor in any publicly available exome database. The mutation was confirmed by Sanger sequencing to segregate in the family in a recessive fashion.
3. Discussion
We report here a newly diagnosed non-Japanese patient with non-founder mutation, who clinically exhibited the classical FCMD phenotype according to the clinical classification (Kondo-Iida et al., 1999). Our patient also exhibits microcephaly, which has not been previously reported in patients with FCMD but is associated with other CMD gene mutations (Yanagisawa et al., 2007). As more patients are diagnosed, it will become clear if this trait is a new addition to the FCMD phenotype or a new finding in FCMD exclusive to Egyptian patients or this mutation.
FCMD was originally classified into three clinical groups according to maximum motor abilities displayed by the patient (Kondo-Iida et al., 1999): “typical” (patient is able to sit unassisted or to slide on buttocks); “mild” (patient could stand or walk with or without support) and “severe” (patient is only able to sit with support or had no head control). Most Japanese FCMD patients are homozygous for an ancestral founder mutation in FCMD gene (FKTN; MIM 607440), which arose from the insertion of a 3 kb retrotransposon element into the 3'untranslated region (UTR). Chromosome carrying the founder insertion in the non-coding region may produce a lower level of mature fukutin than normal and generate a “typical” FCMD phenotype (Kobayashi et al., 1998a, 1998b).
No Japanese FCMD patients have been identified with non-founder mutations on both alleles. Some patients are compound heterozygous, carrying another mutation in addition to the founder insertion, and leading to a more severe FCMD variant (Kobayashi et al., 1998a, 1998b; Kondo-Iida et al., 1999). Interestingly, compound heterozygous FKTN mutations (3-kb insertion plus new missense mutations p.Gln358Pro or p.Arg179Thr) have been recently reported in six Japanese patients showing dilated cardiomyopathy with no or minimal muscle involvement at a late age and normal intelligence (Murakami et al., 2006; Puckett et al., 2009). On the other hand, non-founder mutations, which include nonsense and missense mutations within the coding region, cause major structural changes in the fukutin protein and thus are likely to produce “severe” phenotypes (Beltran-Valero de Bernabe et al., 2003; Godfrey et al., 2007; Manzini et al., 2008; Silan et al., 2003).
The absence of patients with two non-founder mutations in Japan led to the hypothesis that this may be lethal, however since 2003 compound heterozygosity and homozygosity in at least 24 different non-founder FKTN mutations have been described in non-Japanese patients and phenotypes of these patients cover the entire range of alpha-dystroglycanopathies (Yis et al., 2011). Silan et al. (2003) described a Turkish patient with severe CMD brain and eye anomalies resembling WWS, with a homozygous 1 bp insertion mutation in FKTN exon 5. Another Turkish child with WWS displayed an exon 4 homozygous nonsense FKTN mutation (Beltran-Valero de Bernabe et al., 2003). Godfrey et al., 2007 reported seven additional FKTN-mutated patients with milder phenotypes: one with MEB-FCMD phenotype, one CMD and five LGMD2M patients without central nervous system involvement.
Manzini et al., 2008 studied a large cohort of 43 ethnically diverse patients with typical WWS, and found that all Ashkenazi Jewish individuals in their group shared an identical haplotype at the FKTN locus and the same homozygous mutation c.1167_1168insA in exon 9 suggesting a founder effect in this population. A striking difference was observed in the geographic distribution of mutations, as Middle East families were mostly carriers of POMT1 mutations while the most common cause of European/American cases was FKTN mutations. An additional four USA Ashkenazi Jewish families with WWS were found to carry this same founder FKTN mutation (Chang et al., 2009), suggesting that it may be useful for diagnostic testing and carrier screening in the Ashkenazi Jewish population.
Interestingly, the mutation described here is the first Fukutin mutation found in an Egyptian patient. It is a novel homozygous missense mutation. Similar phenotype and MRI findings were reported by Vuillaumier-Barrot et al. (2009) who described two Caucasian sisters of non-consanguineous marriage exhibiting CMD, mental retardation and cerebellar malformation with different FKTN mutations (p.Ala170Glu/p.Tyr371Cys).
Our results suggest that patients with FKTN gene mutations outside of Japan show a great variability of clinical presentation, and not so rare as initially supposed. Due to the high rates of consanguinity and limits in diagnostic molecular testing, no other Egyptian patients with FKTN mutations have emerged to date.
Abbreviations
- FCMD
Fukuyama-type congenital muscular dystrophy
- LGMD
limb-girdle muscular dystrophy
- WWS
Walker–Warburg syndrome
- MEB
Muscle–eye–brain disease
- CMD
congenital muscular dystrophy
- MRI
Magnetic Resonance Image
- EMG
electromyography
- EEG
electroencephalogram
- MUPs
motor unit potentials
- WPPSI
Wechsler Preschool and Primary Scale of Intelligence
- SQ
social quotient
- IRB
Institutional Review Board
- GATK
The Genome Analysis Toolkit
- UTR
untranslated region
Footnotes
Conflict of interest
There is no conflict of interest.
References
- Beltran-Valero de Bernabe D, et al. A homozygous nonsense mutation in the fukutin gene causes a Walker–Warburg syndrome phenotype. J Med Genet. 2003;40(11):845–848. doi: 10.1136/jmg.40.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse K, et al. Missense mutations in beta-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker–Warburg syndrome. Hum Mol Genet. 2013;22(9):1746–1754. doi: 10.1093/hmg/ddt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, et al. Founder Fukutin mutation causes Walker–Warburg syndrome in four Ashkenazi Jewish families. Prenat Diagn. 2009;29(6):560–569. doi: 10.1002/pd.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa C, et al. Portuguese case of Fukuyama congenital muscular dystrophy caused by a multi-exonic duplication in the fukutin gene. Neuromuscul Disord. 2013;23(7):557–561. doi: 10.1016/j.nmd.2013.03.005. [DOI] [PubMed] [Google Scholar]
- DePristo MA, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y, Ohsawa M. A genetic study of the Fukuyama type congenital muscular dystrophy. Brain Dev. 1984;6(4):373–390. doi: 10.1016/s0387-7604(84)80113-8. [DOI] [PubMed] [Google Scholar]
- Fukuyama Y, Osawa M, Suzuki H. Congenital progressive muscular dystrophy of the Fukuyama type — clinical, genetic and pathological considerations. Brain Dev. 1981;3(1):1–29. doi: 10.1016/s0387-7604(81)80002-2. [DOI] [PubMed] [Google Scholar]
- Godfrey C, et al. Refining genotype–phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130(10):2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998a;394(6691):388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, et al. Founder-haplotype analysis in Fukuyama-type congenital muscular dystrophy (FCMD) Hum Genet. 1998b;103(3):323–372. doi: 10.1007/s004390050824. [DOI] [PubMed] [Google Scholar]
- Kondo-Iida E, et al. Novel mutations and genotype–phenotype relationships in 107 families with Fukuyama-type congenital muscular dystrophy (FCMD) Hum Mol Genet. 1999;8(12):2303–2309. doi: 10.1093/hmg/8.12.2303. [DOI] [PubMed] [Google Scholar]
- Lefeber DJ, et al. Deficiency of Dol-PMan synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am J Hum Genet. 2009;85(1):76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, et al. Ethnically diverse causes of Walker–Warburg syndrome (WWS): FCMD mutations are a more common cause of WWS outside of the Middle East. Hum Mutat. 2008;29(11):E231–E241. doi: 10.1002/humu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini MC, et al. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker–Warburg syndrome. Am J Hum Genet. 2012;91(3):541–547. doi: 10.1016/j.ajhg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni F. Refining genotype–phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain. 2007;130(10):2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Voit T. The congenital muscular dystrophies in 2004: a century of exciting progress. Neuromuscul Disord. 2004;14(10):635–649. doi: 10.1016/j.nmd.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Brockington M, Blake DJ, Torelli S, Brown SC. Defective glycosylation in muscular dystrophy. Lancet. 2002;360:1419–1421. doi: 10.1016/S0140-6736(02)11397-3. [DOI] [PubMed] [Google Scholar]
- Muntoni F, Torelli S, Wells DJ, Brown SC. Muscular dystrophies due to glycosylation defects: diagnosis and therapeutic strategies. Curr Opin Neurol. 2011;24(5):437–442. doi: 10.1097/WCO.0b013e32834a95e3. [DOI] [PubMed] [Google Scholar]
- Murakami T, et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann Neurol. 2006;60(5):597–602. doi: 10.1002/ana.20973. [DOI] [PubMed] [Google Scholar]
- Puckett RL, et al. Further evidence of Fukutin mutations as a cause of childhood onset limb-girdle muscular dystrophy without mental retardation. Neuromuscul Disord. 2009;19(5):352–356. doi: 10.1016/j.nmd.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riisager M, Duno M, Hansen FJ, Krag TO, Vissing CR, Vissing J. A new mutation of the fukutin gene causing late-onset limb girdle muscular dystrophy. Neuromuscul Disord. 2013;23(7):562–567. doi: 10.1016/j.nmd.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Seelow D, Schuelke M, Hildebrandt F, Nürnberg P. Homozygosity Mapper—an interactive approach to homozygosity mapping. Nucleic Acids Res. 2009:W593–599. doi: 10.1093/nar/gkp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silan F, et al. A new mutation of the fukutin gene in a non-Japanese patient. Ann Neurol. 2003;53(3):392–396. doi: 10.1002/ana.10491. [DOI] [PubMed] [Google Scholar]
- Sparks S, et al. Gene Reviews™ [Internet] University of Washington; Seattle, Seattle (WA): 2001. Congenital muscular dystrophy overview; pp. 1993–2013. (updated 2012 Aug 23) [PubMed] [Google Scholar]
- Takeshita K, Yoshino K, Kitahara T, Nakashima T, Kato N. Survey of Duchenne type and congenital type of muscular dystrophy in Shimane, Japan. Jinrui Idengaku Zasshi. 1997;22:43–47. doi: 10.1007/BF01908284. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, et al. Worldwide distribution and broader clinical spectrum of muscle–eye–brain disease. Hum Mol Genet. 2003;12(5):527–534. doi: 10.1093/hmg/ddg043. [DOI] [PubMed] [Google Scholar]
- Toda T, Kobayashi K, Kondo-Lida E, Sasaki J, Nakamura Y. The Fukuyama congenital muscular dystrophy story. Neuromuscul Disord. 2000;10:153–159. doi: 10.1016/s0960-8966(99)00109-1. [DOI] [PubMed] [Google Scholar]
- Vuillaumier-Barrot S, et al. Four Caucasian patients with mutations in the fukutin gene and variable clinical phenotype. Neuromuscul Disord. 2009;19(3):182–188. doi: 10.1016/j.nmd.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Willer T, et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker–Warburg syndrome. Nat Genet. 2012;44(5):575–580. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa A, et al. New POMT2 mutations causing congenital muscular dystrophy: identification of a founder mutation. Neurology. 2007;69(12):1254–1260. doi: 10.1212/01.wnl.0000268489.60809.c4. [DOI] [PubMed] [Google Scholar]
- Yis U, et al. Fukutin mutations in non-Japanese patients with congenital muscular dystrophy: less severe mutations predominate in patients with a non-Walker–Warburg phenotype. Neuromuscul Disord. 2011;21(1):20–30. doi: 10.1016/j.nmd.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Yoshioka M. Phenotypic spectrum of Fukutinopathy: most severe phenotype of Fukutinopathy. Brain Dev. 2009;31(6):419–422. doi: 10.1016/j.braindev.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Yoshioka M. Alpha-Dystroglycanopathy, Muscular Dystrophy. In: Hegde Madhuri., editor. InTech; 2012. (Available from: http://www.intechopen.com/books/muscular-dystrophy/alphadystroglycanopathy) [Google Scholar]