Abstract
BACKGROUND AND PURPOSE
Distinct injuries to various limbic white matter pathways have been reported to be associated with different aspects of cognitive dysfunction in multiple sclerosis (MS). Diffusion tensor imaging (DTI) offers a noninvasive method to map tissue microstructural organization. We utilized quantitative magnetic resonance imaging methods to analyze the main limbic system—white matter structures in MS patients with cognitive impairment (CI).
METHODS
Ten cognitively nonimpaired MS (MSNI) patients and 36 patients with diagnosed CI (MSCI) underwent the minimal assessment of Cognitive Function in MS (MACFIMS) battery. DTI measures of fornix, cingulum, uncinate fasciculus (UF) included tract volume and corresponding fractional anisotropy (FA), mean (MD), axial (AD), and radial (AD) diffusivities. These were statistically analyzed for associations with CI after adjusting for the confounders.
RESULTS
Fornix FA and RD, left cingulum FA, MD, and RD, right cingulum FA, MD, and RD, and left UF FA showed significant differences between MSNI and MSCI (P < .001). Fornix FA (r = −.6) and RD (r = .52), and right cingulum FA (r = −.54) and RD (r = .5) correlated significantly with CI in regression analyses.
CONCLUSIONS
The extent of disruption of microstructural disorganization in the main limbic pathways using DTI impacts the extent of CI seen in subjects with MS.
Keywords: multiple sclerosis, diffusion tensor imaging, cingulum, fornix, uncinate fasciculus, cognitive impairment
Introduction
The human brain limbic system white matter connections play crucial roles in aspects of cognition. Fornix and mamillothalamic tracts are responsible for memory and spatial orientation. The uncinate fasciculi play roles in multimodal sensory integration and are associated with behavioral inhibition, reward-pleasure system, and memory for visual information. On the other hand, the cingula are associated with attention, response selection and action monitoring, self-knowledge, person perception, reasoning, and empathy.1–3
Many patients with multiple sclerosis (MS) develop cognitive impairment (CI) manifested as disturbances in memory, attention, verbal fluency, conceptual reasoning, information processing speed, and visuospatial perception.4 Damage to white matter structures leading to disconnection between the cortical and subcortical regions responsible for cognition underlies the cognitive symptomatology of MS.5–7 Distinct pathologies in various limbic white matter pathways have been previously reported using diffusion tensor imaging (DTI) methods to be related with different aspects of cognition in MS8–10 and in other conditions such as Alzheimer’s disease11 and Parkinson’s disease.12
In this study, we utilized quantitative magnetic resonance imaging (qMRI) methods including DTI,13–15 fluid-attenuated inversion recovery (FLAIR), dual echo sequences, and lesion mapping to analyze the main limbic system white matter structures in MS patients without and with CI. We determined the correlations of CI as derived from the minimal assessment of cognitive function in MS (MACFIMS) with DTI-derived attributes of fornix, cingulum, and uncinate fasciculus (UF) tracts.
Materials and Methods
Ten non-cognitively impaired MS patients (MSNI) and 36 patients with diagnosed CI (MSCI) were enrolled (39 relapsing remitting [RR], and 7 secondary progressive [SP]) age 40.80 ± 11.26 (range 18–58) years, education 14.17 ± 2.34 years, disease duration 13.29 ± 9.21 years, and EDSS 3.51 ± 2.03 (0–7). Table 1 provides the demographics of the cohort. Written informed consent was obtained from each subject following our Institutional Review Board approval of the research protocol. All patients underwent the MACFIMS battery. Exclusion criteria included history of psychiatric disorders, recent history of drug or alcohol abuse, history of depression within 3 months of enrollment, history of allergy to gadolinium, history of other brain pathology, claustrophobia, or positive urine pregnancy test prior to MRI.
Table 1.
Summary of the Mean ± Standard Deviation of Volume (vol) Intracranial Volume (ICV)-Normalized Volume (Nvol), Fractional Anisotropy (FA), Mean (MD), Axial (AD), and Radial (RD) Diffusivities of Fornix, Cingulum (Cing), Uncinated Fasciculus (UF) with the Demographics in Whole Cohort, Cognitively Normal (MSNI), and Impaired (MSCI) Groups. Our Cohort Includes Subjects with Relapsing-Remitting (RRMS) and Secondary Progressive Multiple Sclerosis (SPMS).
| Entire Cohort | MSNI | MSCI | |
|---|---|---|---|
| Number of subjects | 46 | 10 | 29 |
| Total lesion volume (mL)** | 19.85 ± 19.17 | 7.57 ± 7.10 | 27.01 ± 20.55 |
| Age (years) | 40.80 ± 11.26 | 36.7 ± 10.64 | 43.1 ± 11.05 |
| Gender | 36 F, 10 M | 6 F, 4M | 21 F, 6M |
| Education in years* | 14.17 ± 2.34 | 16 ± 2.49 | 13.79 ± 2.09 |
| Disease duration in years | 13.29 ± 9.02 | 9.65 ± 8.19 | 15.1 ± 9.89 |
| Expanded Disability Status Scale (EDSS)*** | 3.51 ± .3 (0–7) | 1.3 ± .78 (0–3) | 4.47 ± 1.75 (1.5–7) |
| Cognitive Impairment (CI) Index | 0–7 | 0–.25 | .35–1 |
| MS subtypes | 39 RRMS, 7 SPMS | 10 RRMS | 22 RRMS, 7 SPMS |
| Fornix- Vol | 3.14 ± 1.34 | 4.19 ± 1.17 | 2.71 ± 1.18 |
| Fornix-Nvol* | 2.38 ± 1.01 | 2.99 ± .82 | 2.08 ± .92 |
| Fornix FA*** | .33 ± .03 | .36 ± .02 | .31 ± .03 |
| Fornix-MD** | 1.33 ± .11 | 1.24 ± .08 | 1.37 ± .11 |
| Fornix-AD | 1.85 ± .14 | 1.80 ± .13 | 1.89 ± .14 |
| Fornix-RD*** | 1.07 ± .10 | .97 ± .06 | 1.11 ± .10 |
| Left Cing-Vol | 10.99 ± 2.32 | 11.84 ± 1.99 | 10.92 ± 2.25 |
| Left Cing-Nvol | 8.28 ± 1.58 | 8.45 ± 1.54 | 8.27 ± 1.54 |
| Left Cing-FA*** | .36 ± .02 | .37 ± .02 | .35 ± 0.02 |
| Left Cing-MD*** | .81 ± .05 | .76 ± .02 | .82 ± .02 |
| Left Cing-AD* | 1.15 ± .05 | 1.12 ± .02 | 1.16 ± .05 |
| Left Cing-RD*** | .64 ± .05 | .59 ± .02 | .65 ± .05 |
| Right Cing-Vol | 10.24 ± 3.06 | 11.76 ± 3.36 | 10.23 ± 2.91 |
| Right Cing-Nvol | 7.70 ± 2.04 | 8.36 ± 2.35 | 7.74 ± 1.98 |
| Right Cing-FA*** | .34 ± .02 | .36 ± .02 | .33 ± .02 |
| Right Cing-MD*** | .80 ± .05 | .76 ± .02 | .81 ± .05 |
| Right Cing-AD* | 1.12 ± .05 | 1.09 ± .03 | 1.13 ± .05 |
| Right Cing-RD*** | .64 ± .05 | .59 ± .02 | .65 ± .05 |
| Left UF-Vol | 4.69 ± 2.67 | 5.38 ± 2.69 | 4.43 ± 2.78 |
| Left UF-Nvol | 3.52 ± 1.91 | 3.76 ± 1.81 | 3.36 ± 1.99 |
| Left UF-FA*** | .36 ± .06 | .39 ± .02 | .34 ± .07 |
| Left UF-MD* | .83 ± .14 | .81 ± .04 | .83 ± .17 |
| Left UF-AD | 1.19 ± .19 | 1.19 ± .05 | 1.19 ± .25 |
| Left UF-RD** | .65 ± .11 | .62 ± .03 | .66 ± .14 |
| Right UF-Vol | 4.65 ± 2.04 | 6.11 ± 1.76 | 3.94 ± 1.71 |
| Right UF-Nvol** | 3.5 ± 1.48 | 4.29 ± .92 | 3.00 ± 1.34 |
| Right UF-FA* | .35 ± .03 | .37 ± .02 | .34 ± .03 |
| Right UF-MD** | .84 ± .06 | .80 ± .02 | .86 ± .06 |
| Right UF-AD | 1.19 ± .07 | 1.16 ± .02 | 1.20 ± .07 |
| Right UF-RD** | .66 ± .06 | .63 ± .03 | .68 ± .06 |
Mann-Whitney U-test was used for group comparisons and for significance level, *represents P < .05,
P < .01, and
P < .001. Units: Volume = mL or cm3, FA = μ ± σ, MD, AD, and RD= 10−3 mm2 s−1. ICV defined as total brain volume that is equal to sum of cerebrum, cerebellum, and all cerebrospinal fluid and blood within.
Behavioral Laboratory Measures
The MACFIMS is designed to quantify cognitive function with psychometric testing,16 and includes the following assessments: processing speed and working memory assessed by Paced Auditory Serial Addition Test (PASAT) and Symbol Digit Modality Test (SDMT), memory and learning evaluated by California Verbal Learning Test Second Edition (CVLT-II) and Brief Visuospatial Memory Test-Revised (BVMT-R), executive function using the Delis-Kaplan Executive Function System (D-KEFS) sorting test, visual perception/spatial processing using Judgment of Line Orientation test (JLO), and verbal fluency measured by the controlled oral word association test (COWAT).
MACFIMS Scoring
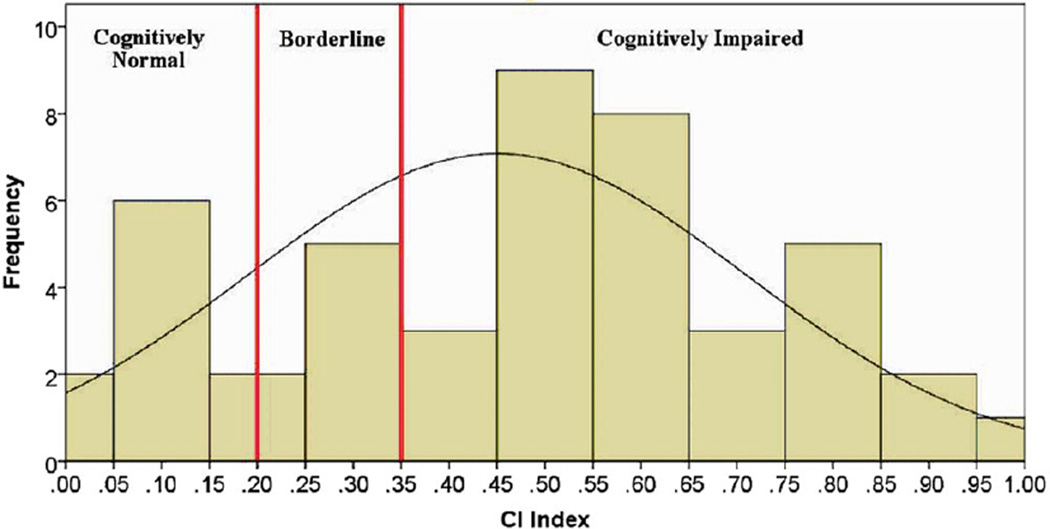
In order to determine whether to classify patients as MSCI or MSNI, an impairment index (ii) methodology was applied to the MACFIMS data. Twenty MACFIMS parameters were identified in an a priori manner by literature review as most pertinent in the measurement of MS-related cognitive deficits.16,17 Based on previously validated methodology,18 a CI index was derived. In this cohort, patients were classified as cognitively impaired if their performance was more than one standard deviation below the mean on at least 40% of the 20 preidentified parameters (ie, 8 of 20 parameters), for an ii of >.35. MS patients were classified as MSNI if performance was less than 40% impaired (ie, more than 1 standard deviation below the mean) of the parameters (ie, less than 8 of 20 parameters, for an impairment index of <.2). Patients with ii scores between .2 and .35 were considered borderline CI and excluded from group analysis (see Fig 1).19
Fig 1.
Distribution of cognitive impairment (CI) index scores in entire cohort.
Magnetic Resonance Imaging Data Acquisition
Whole brain MRI data were acquired on a Philips 3.0 T Intera scanner using a SENSE receive head coil. The MRI protocol included conventional and nonconventional MRI sequences (dual echo turbo spin echo, fluid attenuation by inversion recovery (FLAIR), and 3-dimensional T1-weightedmagnetization prepared rapid acquisition of gradient echo [MPRAGE]). The T1-weighted sequence spatial resolution was 1 mm × 1 mm × 1 mm, TR/TE:8,000/80 and field-of-view (FOV) = 256 mm × 256 mm. Slice thickness/gap/number of slices = 1 mm/0 mm/180. Dual fast spin-echo (FSE) images were acquired with echo and repetition times of TR/TE1/TE2 = 6,800/8.2/90 ms and a FLAIR sequence with TE/TI/TR = 80/2,500/8,000 ms, and for both sequences; slice thickness/gap/number of slices = 3 mm/0 mm/44. DWI data were acquired axially from the same graphically prescribed conventional MRI volumes using a single-shot multislice 2-dimensional spin-echo diffusion sensitized and fat-suppressed echo planar imaging (EPI) sequence, with the balanced Icosa21 tensor encoding scheme.20,21 The b-factor = 1,000 s mm−2, TR/TE = 7,100/65 ms, FOV = 256 mm × 256 mm, and slice thickness/gap/number of slices = 3 mm/0 mm/44. The EPI phase encoding used a SENSE k-space undersampling factor of 2, with an effective k-space matrix of 128 × 128, and an image matrix after zero-filling of 256 × 256. The constructed image spatial resolution for the DWI data was 1 mm × 1 mm × 3 mm.
Lesion Volume Segmentation Using Conventional MRI
Whole brain lesion volume was quantified in all patients using the coregistered multispectral dual FSE and the FLAIR volumes as described elsewhere.22 The individual lesion volumes were saved as binary masks to enable fusion with other multimodal volumes and fiber tracts acquired from the same subject as described below.
Diffusion Tensor Fiber Tractography
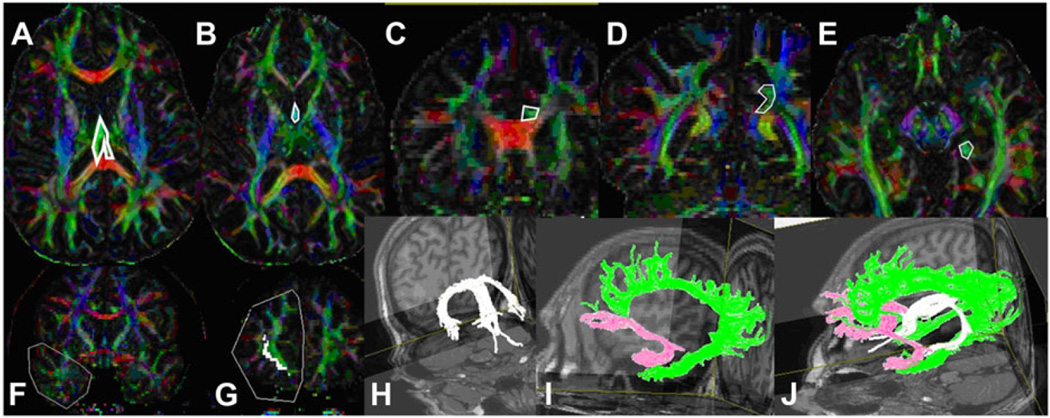
We used a brute force and multiple region-of-interest (ROI) tracking method and the fiber assignment with continuous tractography (FACT) algorithm13,23 (DTI Studio, Johns Hopkins University, Baltimore, MD) to reconstruct fornix, uncinate, and cingulum with a FA threshold of .15, and an angle threshold of 70°. Reproducibility of the fiber construction in both hemispheres was tested on all subjects by two experienced raters (ZK, KMH). We used color-coded principal eigenvector red-green-blue (RGB) map derived from DTI to seed ROIs. Multiple ROI-based deterministic tractography was used.23 For fornix (see Figs 2H and J), we seeded first ROI in subcallosal green fibers seen in axial slice where body and crus of fornix can be observed (see Fig 2A) and second ROI was seeded in the anterior part of body of fornix (see Fig 2B).24 For cingulum (see Figs 2I and J), we seeded first ROI and second ROI in supracallosal green projection fibers anteriorly (see Fig 2C) and posteriorly (see Fig 2D). Third ROI was seeded in the green hippocampal cingulum fibers seen in the medial temporal lobe (see Fig 2E).23 For UF (see Figs 2I and J), we seeded first ROI in the coronal section of temporal lobe at the anterior commissure level (see Fig 2F) and second ROI in the coronal section of frontal lobe (see Fig 2G).25 Once a fiber tract was reconstructed, its entire trajectory was verified on a slice-by-slice basis to compare with established anatomical landmarks described in the human brain neuroanatomy atlases.2,3
Fig 2.
Illustration of the regions-of-interest used for fornix (A and B), cingulum (C, D, and E), and uncinated fasciculus (F and G) in color-coded map of diffusion tensor imaging (DTI) along with 3-dimensional representation of these tracts (H, I, and J) in the fused T1-weighted map.
Statistical Analyses
Kolmogorov-Smirnov test could not assure the normal distribution for all the DTI metrics. Comparisons between group means and medians were performed using a nonparametric Mann-Whitney U-test and significance defined at P-value < .05. In the whole cohort (n = 46), age, total brain lesion volume, and years of education-adjusted correlations between CI score and all other DTI-derived variables were computed using the Spearman coefficient. Generalized linear models were used for adjustments. After excluding borderline cognitively impaired subjects with MS, MSNI (n = 10) and MSCI (n = 29) formed the two groups for the analysis. Multiple comparisons were not performed. All analyses and generation of the Figures 1 and 3 were performed in SPSS Statistics 24.0-IBM software.
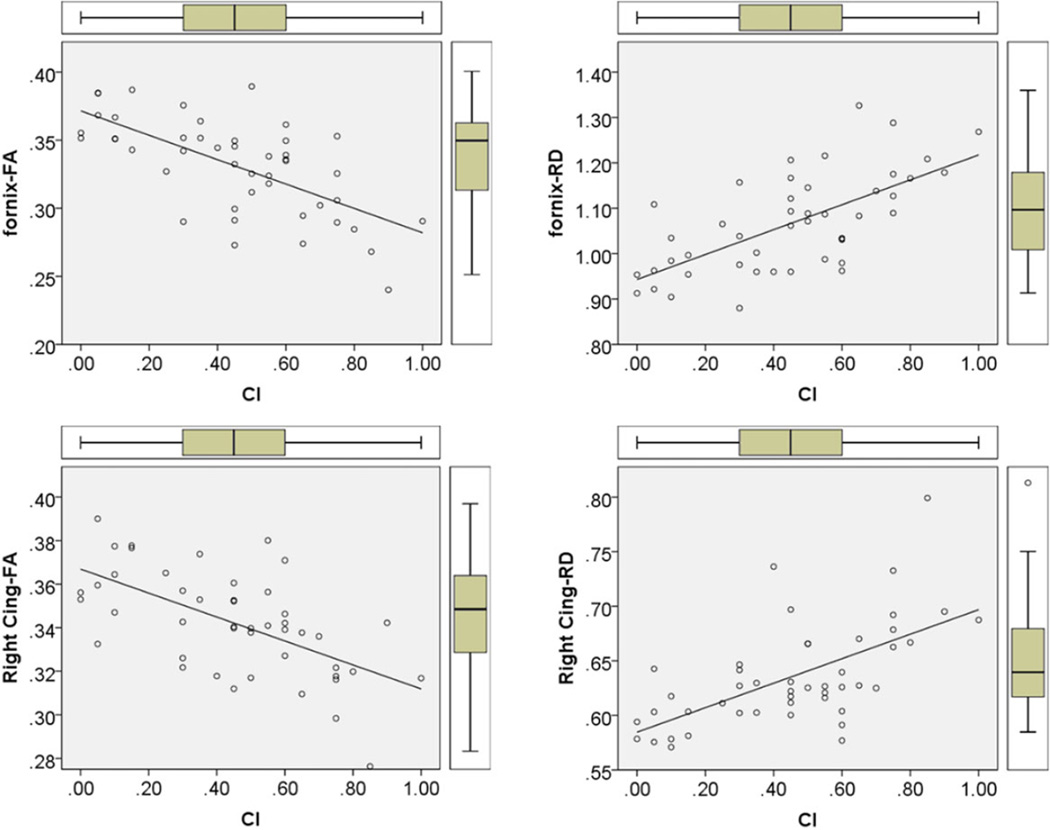
Fig 3.
Illustration of the scatter plot, distribution, box plots, and best fit linear regression line of fractional anisotropy (FA) and radial diffusivity (RD) of fornix and right cingulum with cognitive impairment (CI) index.
Results
Table 1 provides the results from whole cohort and group analysis. In the entire cohort total lesion volume (T1 and T2 LV in mL) was found to be 19.85 ± 19.17 (7.57 ± 7.10 in MSNI, 27.01 ± 20.55 in MSCI). Note that fornix FA (.36 ± .02 in MSNI; .31 ± .03 in MSCI) and RD (.97 ± .06 in MSNI; 1.11 ± .10 in MSCI); left cingulum FA (.37 ± .02 in MSNI; .35 ± .02 in MSCI), MD (.76 ± .02 in MSNI, .82 ± .02 in MSCI), and RD (.59 ± .02 in MSNI, .65 ± .05 in MSCI); right cingulum FA (.36 ± .02 in MSNI, .33 ± .02 in MSCI), MD (.76 ± .02 in MSNI, .81 ± .05 in MSCI), and RD (.59 ± .02 in MSNI, .65 ± .05 in MSCI); and left UF FA (.39 ± .02 in MSNI; .34 ± .07 in MSCI) were statistically different between MSNI and MSCI (P < .001). Spearman’s rank order analysis showed that fornix FA (r = −.61, P = .00002), fornix RD (r = .52, P = .0003), right cingulum FA (r = −.54, P = .0002), and right cingulum RD (r = .5, P = .0007) were strongest correlates of CI index (see Fig 3). Table 2 illustrates the other correlations of DTI values with CI index.
Table 2.
Correlations (r values) of cognitive impairment (CI) index with normalized volume (Nvol), fractional anisotropy (FA), mean (MD), axial (AD), and radial (RD) diffusivities of fornix, cingulum (Cing), uncinated fasciculus (UF).
| CI Index | |
|---|---|
| Fornix-Nvol* | −.33 |
| Fornix-FA*** | −.61 |
| Fornix-MD* | .38 |
| Fornix-AD | .12 |
| Fornix-RD*** | .52 |
| Left Cing-Nvol | −.10 |
| Left Cing-FA** | −.42 |
| Left Cing-MD* | .38 |
| Left Cing-AD | .26 |
| Left Cing-RD** | .42 |
| Right Cing-Nvol | −.01 |
| Right Cing-FA*** | −.54 |
| Right Cing-MD** | .45 |
| Right Cing-AD | .29 |
| Right Cing-RD*** | .50 |
| LeftUFNvol | −.08 |
| Left UF-FA | −.22 |
| Left UF-MD | −.06 |
| Left- UF AD | −.09 |
| Left Uf-RD | −.04 |
| Right UF-Nvol** | −.42 |
| Right UF-FA* | −.33 |
| Right UF-MD | .31 |
| Right UF-AD | .19 |
| Right UF-RD* | .36 |
For the analysis Spearman’s rank-order analysis was used and for significance level, *represents p < 0.05,
p < 0.01, and
p < 0.001.
Discussion
In this study, we provided systematically in vivo delineation and 3-Dimensional reconstruction of major limbic pathways including fornix, cingulum, and uncinate fasciculi using DTI-based deterministic tractography in our MS cohort. We found significant differences in DTI measures of fornices, cingulum, and UF between cognitively normal (MSNI) and impaired MS patients (MSCI), which may reflect the effects of the extent of damage to these limbic white matter pathways on their cognitive performance. We also found that FA and RD of fornices and FA of right cingulum were significantly correlated with the MACFIMS scoring. MACFIMS scoring measures processing speed and working memory, two sensitive domains in MS-related CI.26
These findings suggest that FA and RD values of fornix and FA values of cingulum may be potential neuroimaging markers of CI of the type common to MS. Previous studies attempted to relate fornix, cingulum, and UF to various realms of cognition such as visuospatial memory,27 episodic memory,9 and speed of processing.28,29 As these tracts are found to be predictors of different cognitive skills in various studies, we attempted to provide an MS-tailored cognitive impairment approach through the use of comprehensive MACFIMS scoring.
Consistent with previous studies,10 we found very strong difference in DTI values of fornix between MSNI and MSNI. Fornix was shown to be a predictor of visuospatial memory,27 verbal memory,29 and spatial working memory.30 In our cohort, FA and RD of fornix were found highly correlated with the extent of CI in MS.
Cingulum was associated with attention/executive function, auditory information processing speed and flexibility, visual perception and memory, and verbal learning skills.7,28,29 FA and RD of right cingulum were found to be strong correlates of CI in MS in our cohort. It has been previously demonstrated that there is right-left asymmetry in the cingulum, with the cingulum bundle FA being higher in the left hemisphere.23 Laterality of the cingulum has not been addressed in the previous MS studies. We are not aware of any apparent factor besides the characteristics of our cohort, making right cingulum more strongly correlated with MS CI than the left cingulum.
UF showed moderate difference between MSCI and MSNI groups. Kern and colleagues found that FA of UF correlated with spatial memory and processing speed in MS.29 However, in the correlation analysis, we could not define strong correlations between DTI values of UF and CI index. This finding is somewhat consistent with previous studies28,31 and suggests that UF is not a strong indicator of the CI in MS. The UF is the major infero-anterior pathway connection between temporal and frontal lobes25,32 and cognitive impairment in our MS cohort may be attributed to more involvement of superior circuits.
We have constructed limbic pathways with their entire trajectory being verified on a slice-by-slice basis while being guided by basic neuroanatomy atlases using in vivo DTI-based quantification instead of using fully automated voxel-based methods such as tract-based spatial statistics (TBSS) that would require spatial normalization. We have demonstrated that the microstructural integrity of these pathways is correlated with the presence and extent of CI seen in this MS cohort using a comprehensive MS-tailored cognitive evaluation tool, the MAC-FIMS CI index.
Our study has several limitations that include a lack of age-and gender-matched healthy controls, the limitation of a cross-sectional study to associations rather than predictive value of the metrics, the heterogeneity of MS subgroups studied, and our limited sample size. Adequately powered, properly stratified and controlled studies with serial design are now required to reach definite conclusions in the role of microcellular tissue integrity of main limbic tracts in contributing to and predicting the presence and development of CI in MS.
Acknowledgments
We wish to thank Vipul Kumar Patel for helping in data acquisition. This study was funded by a K-23 training award to Dr. Flavia M. Nelson.
Footnotes
Disclosure: The authors have no competing interest related to the study.
References
- 1.Mesulam M. Principles of Behavioral and Cognitive Neurology. 2nd. New York: Oxford University Press; 2000. pp. 64–66. [Google Scholar]
- 2.Mendoza J, Foundas A. Clinical Neuroanatomy: A Neurobehavioral Approach. New Orleans, LA: Springer; 2007. pp. 213–271. [Google Scholar]
- 3.Catani M, de Schotten T. Atlas of Human Brain Connections. 1st. New York: Oxford University Press; 2012. pp. 49–76. [Google Scholar]
- 4.Ron MA, Callanan MM, Warrington EK. Cognitive abnormalities in multiple sclerosis: a psychometric and MRI study. Psychol Med. 1991;21:59–68. doi: 10.1017/s0033291700014653. [DOI] [PubMed] [Google Scholar]
- 5.Rocca MA, Pagani E, Absinta M, et al. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136–2145. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- 6.Mesaros S, Rocca MA, Kacar K, et al. Diffusion tensor MRI tractography and cognitive impairment in multiple sclerosis. Neurology. 2012;78:969–975. doi: 10.1212/WNL.0b013e31824d5859. [DOI] [PubMed] [Google Scholar]
- 7.Dineen RA, Vilisaar J, Hlinka J, et al. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132:239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- 8.Audoin B, Zaaraoui W, Reuter RF, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry. 2010;81:690–695. doi: 10.1136/jnnp.2009.188748. [DOI] [PubMed] [Google Scholar]
- 9.Koenig KA, Sakaie KE, Lowe MJ, et al. The relationship between cognitive function and high-resolution diffusion tensor MRI of the cingulum bundle in multiple sclerosis. Mult Scler. 2015;21:1794–1801. doi: 10.1177/1352458515576983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer KA, Muhlert N, Cercignani M, et al. White matter tract abnormalities are associated with cognitive dysfunction in secondary progressive multiple sclerosis. Mult Scler. 2016 doi: 10.1177/1352458515622694. pii: 1352458515622694. [DOI] [PubMed] [Google Scholar]
- 11.Oishi K, Mielke MM, Albert M, et al. The fornix sign: a potential sign for Alzheimer’s disease based on diffusion tensor imaging. J Neuroimaging. 2012;22:365–374. doi: 10.1111/j.1552-6569.2011.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosgrove J, Alty JE, Jamieson S. Cognitive impairment in Parkinson’s disease. Postgrad Med J. 2015;91:212–220. doi: 10.1136/postgradmedj-2015-133247. [DOI] [PubMed] [Google Scholar]
- 13.Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 15.Hasan KM, Walimuni IS, Abid H, et al. A review of diffusion tensor magnetic resonance imaging computational methods and software tools. Comput Biol Med. 2011;41:1062–1072. doi: 10.1016/j.compbiomed.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80:201–206. doi: 10.1136/jnnp.2008.148403. [DOI] [PubMed] [Google Scholar]
- 17.Amato MP, Ponziani G, Pracucci G, et al. Cognitive impairment in early-onset multiple sclerosis. Arch Neurol. 1995;52:168–172. doi: 10.1001/archneur.1995.00540260072019. [DOI] [PubMed] [Google Scholar]
- 18.Reitan RM, Wolfson D. In: Theoretical, methodological, and validational bases of the Halstead-Reitan neuropsychological test battery, in comprehensive handbook of psychological assessment: intellectual and neuropsycological assessment. Goldstein G, Beers GS, Hersen M, editors. Hoboken, NJ: John Wiley & Sons; 2003. pp. 105–133. [Google Scholar]
- 19.Nelson F, Datta S, Garcia N, et al. Intracortical lesions by 3T magnetic resonance imaging and correlation with cognitive impairment in multiple sclerosis. Mult Scler. 2011;17:1122–1129. doi: 10.1177/1352458511405561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan KM, Narayana PA. Computation of the fractional anisotropy and mean diffusivity maps without tensor decoding and diagonalization: theoretical analysis and validation. Magn Reson Med. 2003;50:589–598. doi: 10.1002/mrm.10552. [DOI] [PubMed] [Google Scholar]
- 21.Hasan KM, Halphen C, Sankar A, et al. Diffusion tensor imaging-based tissue segmentation: validation and application to the developing child and adolescent brain. Neuroimage. 2007;34:1497–1505. doi: 10.1016/j.neuroimage.2006.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasan KM, Walimuni IS, Abid H, et al. Multi-modal quantitative MRI investigation of brain tissue neurodegeneration in multiple sclerosis. J Magn Reson Imaging. 2012;35:1300–1311. doi: 10.1002/jmri.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamali A, Yousem DM, Lin DD, et al. Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neurosci Lett. 2015;608:45–50. doi: 10.1016/j.neulet.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Hasan KM, Iftikhar A, Kamali A, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SM, Leo GJ, Bernardin L, et al. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 27.Koenig KA, Sakaie KE, Lowe MJ, et al. Hippocampal volume is related to cognitive decline and fornicial diffusion measures in multiple sclerosis. Magn Reson Imaging. 2014;32:354–358. doi: 10.1016/j.mri.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu HJ, Christodoulou C, Bhise V, et al. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage. 2012;59:3713–3722. doi: 10.1016/j.neuroimage.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 29.Kern KC, Gold SM, Lee B, et al. Thalamic-hippocampal-prefrontal disruption in relapsing-remitting multiple sclerosis. Neuroimage Clin. 2015;8:440–447. doi: 10.1016/j.nicl.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberlin LE, Verstynen TD, Burzynska AZ, et al. White matter microstructure mediates the relationship between cardiorespiratory fitness and spatial working memory in older adults. Neuroimage. 2016;131:91–101. doi: 10.1016/j.neuroimage.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahin N, Selouan R, Markowitz CE, et al. Limbic pathway lesions in patients with multiple sclerosis. Acta Radiol. 2016;57:341–347. doi: 10.1177/0284185115578689. [DOI] [PubMed] [Google Scholar]
- 32.Hulst HE, Steenwijk MD, Versteeg A, et al. Cognitive impairment in MS: impact of white matter integrity, gray matter volume, and lesions. Neurology. 2013;80:1025–1032. doi: 10.1212/WNL.0b013e31828726cc. [DOI] [PubMed] [Google Scholar]