Summary
Circadian clocks are encoded by a transcription-translation feedback loop that aligns energetic processes with the solar cycle. Here we show that genetic disruption of the clock activator BMAL1 in skeletal myotubes and fibroblasts increased levels of the hypoxia-inducible factor 1α (HIF1α) under hypoxic conditions. Bmal1−/− myotubes displayed reduced anaerobic glycolysis, mitochondrial respiration with glycolytic fuel, and transcription of HIF1α targets Phd3, Vegfa, Mct4, Pk-m, and Ldha, whereas abrogation of the clock repressors CRY1/2 stabilized HIF1α in response to hypoxia. HIF1α bound directly to core clock gene promoters and, when co-expressed with BMAL1, led to transactivation of PER2-LUC and HRE-LUC reporters. Further, genetic stabilization of HIF1α in Vhl−/− cells altered circadian transcription. Finally, induction of clock- and HIF1α-target genes in response to strenuous exercise varied according to the time-of-day in wild-type mice. Collectively, our results reveal bi-directional interactions between circadian and HIF pathways that influence metabolic adaptation to hypoxia.
Graphical Abstract
Introduction
The circadian system drives internal rhythms of physiology in synchrony with the rotation of the Earth. In mammals, the molecular clock is encoded by a transcription-translation feedback loop composed of activators (CLOCK/BMAL) that induce the transcription of repressors (PER/CRY) that feedback to inhibit the forward limb in a cycle that repeats itself every ~24 hrs, including an additional stabilizing loop comprised of REV-ERB/ROR transcription factors (TFs) (Bass, 2012). Extensive investigation has now shown that peripheral tissue clocks influence metabolism in a tissue-specific manner (Mohawk et al., 2012). For example, abrogation of clock function within pancreas in adult life impairs glucose tolerance, whereas clock disruption within liver results in fasting-induced hypoglycemia and mitochondrial dysfunction (Marcheva et al., 2010; Peek et al., 2013).
While the SCN clock is entrained by light, peripheral clocks can be altered by serum factors, glucocorticoids, and core body temperature (Bass, 2012; Bass and Takahashi, 2010). Environmental signals that impact ATP/AMP levels, redox state, NAD+-dependent class III deacetylases (sirtuins), and nuclear receptor ligands also directly impact clock TFs (Bass and Takahashi, 2010; Peek et al., 2012), raising the possibility that the clock system functions not only to anticipate the light cycle, but also to alter timing in response to metabolic flux, although a gap remains in understanding how the circadian clock regulates adaptation to hypoxia.
Clues to how hypoxia may impact the clock derive from the discovery that both clock and hypoxia-inducible factors belong to the basic helix-loop-helix PER-ARNT-SIM (bHLH-PAS) TF superfamily. HIF proteins are present as heterodimers consisting of HIF1α and HIF1β (ARNT) that bind to E-box-like hypoxia-response elements (HREs) within gene promoters that contain the sequence 5′-[A/G]CGTG-3′ (Kaelin and Ratcliffe, 2008). In normoxic conditions, HIF1α is post-translationally modified by prolyl-hydroxylases and marked for proteasomal degradation by the Von Hippel-Lindau (VHL) E3 ubiquitin ligase. In contrast, during hypoxia when oxygen levels are limiting, or during mitochondrial stress, HIF1α subunits are protected from degradation due to inactivation of the oxygen-dependent prolyl-hydoxylases (Semenza, 2007). The stabilized HIF1α/β heterodimer activates gene transcription pathways involved in angiogenesis, erythropoiesis, and anaerobic glycolysis (Semenza, 2012). Although in silico and in vitro biochemical models suggest that the core clock TF BMAL1 dimerizes with the related bHLH-PAS proteins HIF1α and HIF2α, whether these interactions occur in a physiologic setting is unknown (Bersten et al., 2013; Hogenesch et al., 1998), particularly during exercise when oxygen depletion in skeletal muscle increases energy production through HIF-mediated anaerobic glycolysis.
Here, we apply a combination of genetic, biochemical, and cell-type specific physiological approaches to dissect the interplay between molecular clock and HIF proteins in oxygenic metabolism. Our findings indicate that circadian clock-HIF interactions regulate skeletal muscle anaerobic glycolysis during exercise and that hypoxia reciprocally regulates muscle circadian function. Our studies elucidate tissue-specific differences in the regulation of circadian and HIF TFs important in fuel utilization and daily energy constancy across the sleep-wake/rest-activity cycle.
Results
Tissue-specific circadian control of HIF-mediated anaerobic glycolysis
To determine whether the cell autonomous circadian clock in skeletal muscle contributes to regulation of fuel utilization and glycolytic flux, we assessed oxygen consumption (OCR) and extracellular medium acidification (ECAR) rates, which quantify mitochondrial respiration and glycolysis, respectively, in Bmal1−/− C2C12 mouse myoblasts generated using CRISPR-Cas9-mediated homologous recombination (Fig S1). Similar to our previous findings in isolated mitochondria from Bmal1−/− liver and mouse skeletal muscle (Fig S1C), we observed impaired OCR in Bmal1−/− myotubes compared to WT myotubes under basal conditions and in response to the ATP synthase inhibitor oligomycin and the drug FCCP, which measure uncoupled respiration and maximal flux through the electron transport chain (ETC), respectively (Fig 1A) (Peek et al., 2013). A major difference was observed, however, in comparing anaerobic glycolysis in liver and skeletal muscle. In contrast to Bmal1−/− liver, which exhibited increased anaerobic glycolysis and lactate production (Peek et al., 2013), Bmal1−/− myotubes exhibited reduced extracellular lactate production, as indicated by decreased ECAR in response to added glucose and when the ETC is inhibited by oligomycin (Fig 1B). Importantly, ECAR displayed circadian rhythmicity in synchronized C2C12 myotubes which exhibit an opposing phase to our previously characterized rhythms of mitochondrial fatty acid oxidation (Peek et al., 2013) (Fig S2A), indicating that oxidative versus glycolytic fuel selection is indeed clock-controlled in skeletal muscle. Together, these data suggest key tissue-specific differences in the circadian regulation of fuel selection between liver and muscle.
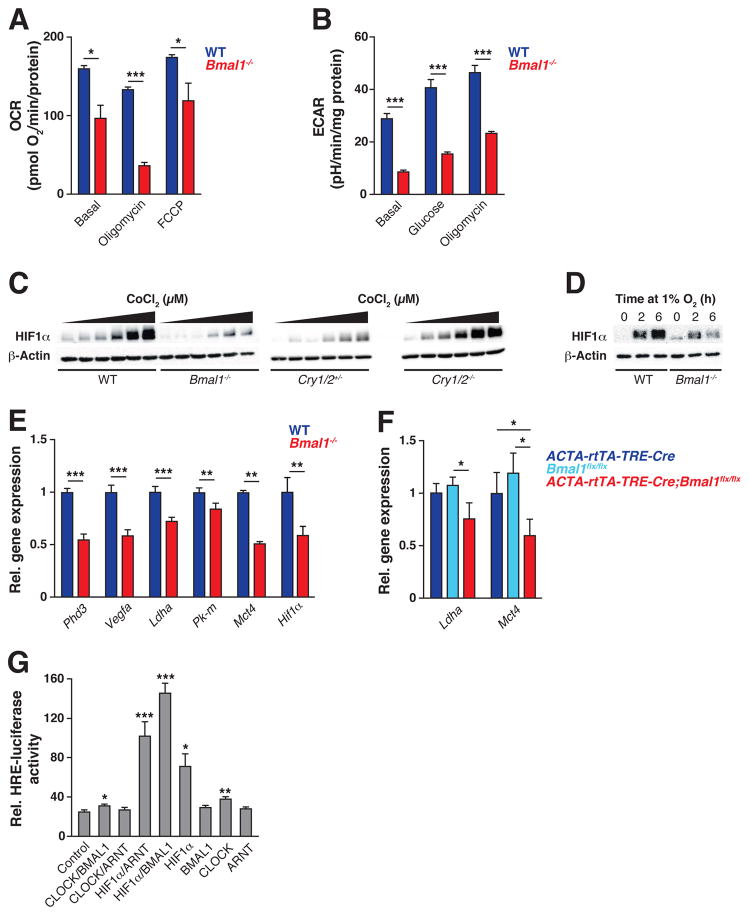
Figure 1. Circadian clock controls oxygen consumption and anaerobic glycolysis through regulation of HIF1α.
(A) OCR from WT and Bmal1−/− C2C12 myotubes treated sequentially with oligomycin and FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone) (n=9–10). (B) ECAR from intact WT and Bmal1−/− C2C12 myotubes treated sequentially with glucose and oligomycin (n=10). (C) Immunoblots of HIF1α and β-actin in WT vs Bmal1−/− (left) and Cry1/2+/− vs Cry1/2−/− (right) MEFs exposed for 14 hours to increasing doses of CoCl2 (0 μM and a dilution curve from 7.8 to 125 μM). (D) Immunoblots of HIF1α and β-actin following exposure to 1% O2 for indicated times in WT vs Bmal1−/− C2C12 myotubes. (E) Expression of HIF target genes in WT vs Bmal1−/− C2C12 myotubes exposed to 1% O2 for 6 hours (n=7–14). (F) Expression of HIF target genes in gastrocnemius muscle from adult life-inducible skeletal muscle Bmal1−/− mice (ACTA-rtTA-TRE-Cre;Bmalfx/fx) and controls (ACTA-rtTA-TRE-Cre and Bmalfx/fx) (n=4–5). (G) Relative luciferase activity of C212 myoblasts transfected with HRE-LUC and plasmids expressing circadian and HIF TFs (n=3). Data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. See also Figures S1–S2.
Since HIF1α is known to be important for glycolytic metabolism in skeletal muscle during both rest and in response to hypoxia (Lindholm and Rundqvist, 2016), we reasoned that reduced HIF1α within Bmal1−/− skeletal muscle may underlie their impaired ECAR, and that the muscle clock may mediate anaerobic glycolytic metabolism through interactions with the HIF pathway. Further, in contrast to tissues such as liver where basal levels of HIF1α are low, skeletal muscle expresses relatively high levels of HIF1α protein even in normoxia (Stroka et al., 2001), suggesting potential tissue-specific roles of HIF1α in normoxic and hypoxic conditions. Thus, we assessed the impact of genetic disruption of both the circadian clock activator versus repressor TFs on the HIF-mediated response to hypoxia. In embryonic fibroblasts (MEFs) isolated from mice lacking BMAL1, we observed reduced HIF1α accumulation (Fig 1C) in response to increasing doses of cobalt chloride (CoCl2), a ‘hypoxia-mimetic’ which stabilizes HIF1α by inactivating the hydroxylases that trigger HIF1α degradation (Wang et al., 1995). Conversely, MEFs lacking the circadian repressors CRY1 and CRY2 displayed increased HIF1α accumulation compared to controls following CoCl2 exposure (Fig 1C), indicating that the core circadian clock feedback loop controls HIF protein levels and the hypoxic response. We also observed a similar reduction in HIF accumulation in Bmal1−/− myotubes exposed to environmental hypoxia (1% O2 for 6 hrs) (Fig 1D), in parallel with reduced expression of known HIF1α target genes, including its own negative regulator Prolyl hydroxylase 3 (Phd3), the pro-angiogenesis factor Vascular endothelial growth factor A (Vegfa), and several genes important for anaerobic glycolysis, including Lactate dehydrogenase A (Ldha), Pyruvate kinase muscle isoform (Pk-m), and the Monocarboxylate transporter 4 (Mct4) (Fig 1E) (Semenza, 2012). Finally, we observed reduced expression of Hif1α mRNA in Bmal1−/− myotubes (Fig 1E), suggesting that reduced HIF function may arise due to impaired Hif1α transcription. In addition, we also observed increased turnover of HIF1α in Bmal1−/− cells following CoCl2 exposure in the presence of the translation inhibitor cycloheximide (Fig S1D), indicating that VHL-dependent HIF1α degradation also contributes to reduced HIF activity in Bmal1−/− cells.
To test whether Bmal1 disruption similarly impacts HIF in vivo, we generated adult-life inducible skeletal muscle-specific Bmal1 knockout mice (ACTA-rtTA-TRE-Cre;Bmalfx/fx). We observed a similar decrease in Ldha and Mct4 expression in gastrocnemius muscle as compared to controls (Fig S2B, 1F) (Rao and Monks, 2009) indicating cell-autonomous regulation of the glycolysis in skeletal muscle. Together, these data indicate that the circadian clock mediates HIF-dependent control of muscle glycolytic lactate production at both the cell autonomous level in myoblasts and in the intact animal.
One explanation for impaired hypoxia-induced HIF1α accumulation and activity in the Bmal1−/− cells could be reduced physical interaction between HIF1α and BMAL1, leading to reduced stability of monomeric HIF1α, as is the case in cells lacking HIF1β (Chilov et al., 1999). In support of a physical interaction between HIF1α and BMAL1, previous biochemical studies indicate dimerization between bHLH-PAS proteins, as a high degree of sequence- and structure-level similarity exists between HIF1β (also termed ARNT) and the core clock activator BMAL1 (also termed ARNT-like), particularly within the protein-protein PASA and PASB domain interaction surfaces (Fig S3) (Hogenesch et al., 1998). To test for functional interactions between HIF1α and BMAL1, we examined their ability to transactivate the hypoxia-response element (HRE), a canonical regulatory motif present within the promoter of HIF1α-target genes, in mammalian skeletal muscle cells (Emerling et al., 2008). We found that co-expression of HIF1α and BMAL1 activated the HRE-luciferase reporter to a similar extent as HIF1α and HIF1β (ARNT), whereas CLOCK/BMAL1 and CLOCK/ARNT did not activate HRE-luciferase, demonstrating transcriptional co-regulation by HIF1α and BMAL1 in mouse skeletal muscle cells (Fig 1G), consistent with circadian clock regulation of the hypoxic response through transactivation of HIF1α.
Hypoxia and HIF1α exert reciprocal control of circadian transcription cycles
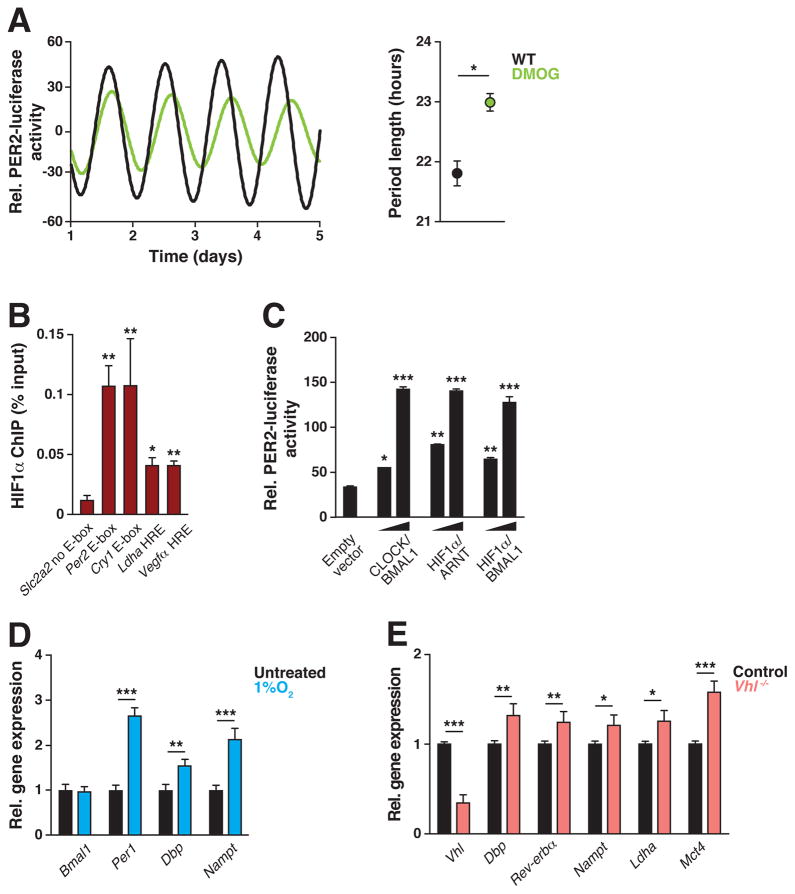
Given in vitro interaction between HIF1α and BMAL1, and similarities in the target transcriptional motifs of the two TFs, we hypothesized that a bidirectional relationship may exist between the circadian and hypoxic pathways. We took several approaches to determine whether the HIF pathway exerts reciprocal effects upon the core clock. First, we treated synchronized C2C12 myotubes that stably express the circadian reporter PERIOD2:LUCIFERASE (PER2:LUC) with the HIF1/2α-stabilizing drug dimethyloxalyl glycine (DMOG), which inhibits VHL-mediated HIF1/2α degradation similarly to CoCl2 but without causing toxicity seen following long-term culture (Cunliffe et al., 1992). Continuous monitoring of luciferase activity revealed significant period lengthening of PER2:LUC oscillations in the presence of DMOG (21.80 ± 0.2 SEM hrs in DMSO-treated controls compared to 22.97 ± 0.15 SEM hrs in DMOG-treated cells, p<0.01) (Fig 2A), demonstrating that HIF directly impacts the core circadian clock within muscle.
Figure 2. HIF regulates circadian clock function.
(A) Normalized photomultiplier detection of PER2:LUC reporter oscillations in synchronized C2C12 myotubes following exposure to 125 μM dimethyloxalylglycine (DMOG), a HIF1/2α stabilizer (green line) or untreated controls (black line) (n=3). (B) ChIP of HIF1α and occupancy of the E-box-containing promoter regions of Per2 and Cry1, the HRE-containing promoter regions of Ldha and Vegfa, and the Slc2a2 gene promoter which has no E-box or HRE (n=3). (C) Relative luciferase activity of PER2:LUC in the presence of indicated BMAL1 and HIF combinations in C212 myoblasts (n=4). (D–E) Relative gene expression of clock target genes in (D) non-synchronized C2C12 myotubes exposed to 1% O2 for 6 hours vs normoxic conditions (n=7–14) and (E) MEFs isolated from Cag-CRE-ER;Vhlfx/fx vs control mice (n=4). Data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001. See also Figure S3.
Second, to determine whether HIF1α localizes to regulatory regions within core clock genes in skeletal muscle, we performed directed chromatin immunoprecipitation (ChIP) of HIF1α at endogenous CLOCK/BMAL1 targets containing canonical E-box binding sites (5′-CACGTG-3′) (Koike et al., 2012). We found significantly enhanced binding of HIF1α to the E-box within the promoter regions of Per2 and Cry1, as well as to canonical HRE targets sites within the promoters of Ldha and Vegfa (Gomes et al., 2013; He et al., 2011), but not within the promoter of Slc2a2 (the solute carrier family 2 gene encoding the glucose transporter GLUT2), which does not contain a canonical CLOCK/BMAL1 E-box motif (Fig 2B). Direct binding of HIF1α to circadian target genes suggests a role for the hypoxic response in regulating circadian gene transcription (Fig 2A). Third, we assessed the ability of HIF1α to transactivate the Per2 gene through co-expression of HIF1α and BMAL1 and found that HIF1α/BMAL1 stimulated the transcription of the PER2:LUC expressed within C2C12 myoblasts to a similar extent as CLOCK/BMAL1 (Fig 2C). Furthermore, we found increased expression of core clock genes in both myotubes (Fig 2D) and MEFs (Fig S4) in response to either 1% O2 or CoCl2. These findings are consistent with the previous observations that increased period length correlates with increased expression of circadian repressors (Bae et al., 2001). Importantly, nearly all of the induced genes are direct targets of CLOCK/BMAL1 containing a canonical E-box regulatory site. Finally, to test the relationship between HIF and CLOCK/BMAL1 activity in response to hypoxia, we examined clock gene expression in MEF cells generated from mice carrying a tamoxifen-inducible CRE-mediated deletion of Vhl (Cag-CRE-ER;Vhlfx/fx) (Haase et al., 2001). Consistent with the effect of hypoxia and HIF stabilization on clock gene expression in wild-type fibroblasts and C2C12 myotubes (Fig 2D, Fig S4), Vhl−/− MEFs displayed increased expression of core clock genes (Fig 2E), indicating that hypoxia induces circadian gene expression through HIF. Together, these data uncover a bidirectional relationship between the circadian and hypoxic pathways.
The circadian clock generates time-of-day-dependent hypoxic response to exercise
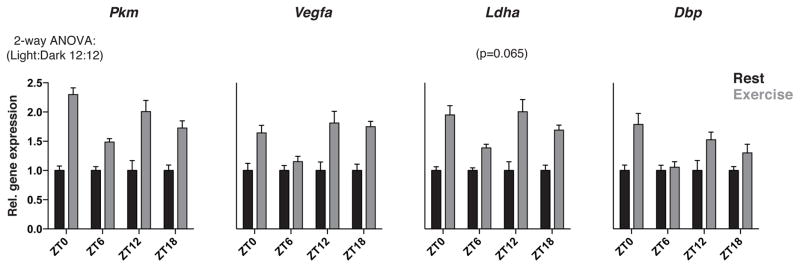
Our findings above highlight a reciprocal functional interaction between circadian and HIF TFs in skeletal muscle. To determine whether the circadian clock and HIF TFs act cooperatively to control gene expression in muscle tissue in vivo in response to a hypoxic challenge, we employed a model of acute strenuous exercise in mice to induce hypoxic stress. Mice lacking HIF1α in skeletal muscle fail to induce the expression of HIF1α-regulated genes important in the production of ATP via glycolysis and lactate and display reduced tolerance to strenuous exercise, indicating that HIF1α is important in acute exercise tolerance (Mason et al., 2004). Thus, using strenuous exercise a paradigm, we aimed to test 1) whether HIF induction exhibits time-of-day variation and 2) whether clock gene expression is altered by hypoxic stress in skeletal muscle tissue in vivo. WT mice were exercised by treadmill running to exhaustion at ZT0, 6, 12 and 18 and whole gastrocnemius muscle (i.e. a primarily type II fiber-containing muscle) was then rapidly excised, snap frozen, and assayed for both HIF1α- and clock-target gene expression. We observed greater induction of both HIF1α and clock targets when mice were exercised at ZT12, 18 and 0 than at ZT6 (Fig 3) (statistical significance between time points determined by 2-way ANOVA), indicating that circadian timing controls the induction of the HIF- and clock-dependent transcriptional response to exercise. These data reinforce the reciprocal nature of the circadian and hypoxic response pathways in response to both time of day and alterations in the oxygenic environment.
Figure 3. Clock and HIF transcriptional response to strenuous exercise varies according to time of day in skeletal muscle.
WT mice were exercised by treadmill running to exhaustion at ZT0 (start of light period), ZT6 (mid-light period), ZT12 (start of dark period), or ZT18 (mid-dark period) prior to immediate extraction of gastrocnemius muscle and subsequent quantification of HIF and clock target mRNAs (n=7). Data are represented as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 using 2-way ANOVA statistical analysis.
Discussion
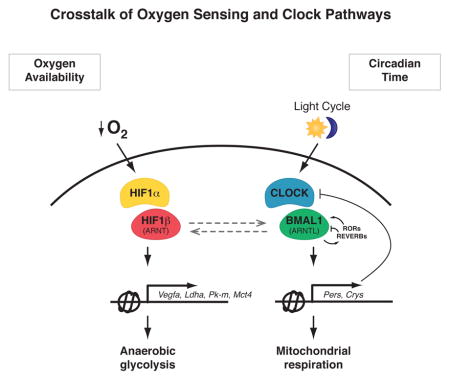
Circadian clocks are unique in that they are capable of not only anticipating daily changes in the solar cycle, but also enable adaptation to flux in the nutrient and oxygen environment. The capacity of circadian clocks to exhibit flexibility can be understood at the molecular level since clock TFs contain PAS domains that are canonical environmental response modules important in sensing xenobiotic, metabolite, and oxygen and transducing such changes into transcriptional programs (McIntosh et al., 2010). Here, we present a previously uncharacterized relationship between the molecular clock and HIF1α in mouse skeletal muscle, revealing a novel mechanism by which peripheral clocks function together with the central oxygen-responsive TF HIF1α to promote rhythmic tissue-specific metabolic fuel selection. While in silico and in vitro biochemical analyses have led to the proposal that HIF TFs may form complexes with circadian clock proteins, a hypothesis supported by studies in the vertebrate zebrafish model (Egg et al., 2013; Hogenesch et al., 1998; Pelster and Egg, 2015), it has remained unclear whether functional interactions between the bHLH-PAS proteins might occur in mammalian tissues. To address the integration of circadian and oxygen-sensing mechanisms under physiologic conditions, we focused on skeletal muscle for several reasons including: (i) muscle tissue displays abundant HIF1α protein levels and transcriptional activity relative to other tissue types (Stroka et al., 2001), suggesting that circadian clock/HIF interactions may participate in basal (i.e. ‘normoxic’) metabolic function; (ii) HIF1α is a determinant of exercise tolerance in glycolytic type IIX muscle fibers (Mason et al., 2004; Slot et al., 2014); and (iii) genetic disruption of the ARNT-like circadian activator, BMAL1, leads to impaired muscle fiber distribution, glycolytic gene expression, and glucose tolerance (Dyar et al., 2014; Hodge et al., 2015). Thus, we hypothesized that the skeletal muscle circadian clock may gate the capacity of oxidative skeletal muscle to augment glycolytic energy production through regulation of HIF signaling.
The ability of cells to respond to acute changes in oxygen availability is an important feature of aerobic organisms. As oxygen levels decrease, the generation of ATP shifts from mitochondrial oxidative phosphorylation to oxygen-independent glycolysis, a HIF1α-dependent process. Our findings show a critical role for the clock in this process, as we reveal circadian clock control of HIF activity may regulate glucose metabolism in a tissue-specific manner. Remarkably, whereas Bmal1−/− liver showed increased anaerobic glycolytic gene expression and lactate production under normoxic conditions (Peek et al., 2013), Bmal1−/− myotubes display reduced extracellular lactate levels and expression of HIF1α target genes. Thus we speculate that clock-HIF interactions play a tissue-specific and pivotal role in determining glycolytic capacity of skeletal muscle.
While future studies will be important to uncover the precise mechanisms by the which circadian clock and HIF transcriptional pathways interact, our data indicate potential functional and physical interactions between HIF1α and BMAL1. Of note, we observed transactivation of both the HRE- and PER2-luciferase reporters when BMAL1 and HIF1α were co-expressed, suggesting that these factors form a transcriptionally competent complex, since overexpression of BMAL1 or HIF1α on their own do not activate HRE-LUC. Future studies will be required to determine how HIF1α and BMAL1 interact during hypoxia in physiological conditions, as well as their impact on transcriptional hypoxic stress response.
The studies presented here reveal endogenous reciprocal interactions between circadian clock and HIF transcriptional pathways in skeletal muscle that may have broader implications for understanding the interplay between circadian and oxygen sensing pathways. We provide evidence for time-of-day ‘gating’ of HIF activation, which appears greater during the active period, corresponding to daytime in humans. These data indicate that HIF is primed by the clock to respond more robustly to a hypoxic stimulus during the time of day when demand for strenuous activity may be greatest, and raise interesting teleological implications for human exercise physiology. This finding raises additional questions as to whether the clock controls response to hypoxic stimuli in other tissues. For example, it would be interesting to investigate whether the circadian clock and HIF interact to mediate time-of-day differences in cardiac tissue recovery following ischemic injury (Durgan et al.). Furthermore, it is possible that circadian regulation of HIF may contribute to pathologies including tumors in which HIF drives a pseudohypoxic state (Dang, 2012). In summary, the clock system functions not only to anticipate changes in the external light cycle, but peripheral clocks also act as a rheostat to regulate oxygen sensing in oxidative tissues under both basal and hypoxic conditions.
Experimental Procedures
Animals
All animal procedures were in accordance with guidelines of the Institutional Animal Care and Use Committee. Bmalfx/fx mice (Johnson et al., 2014) were crossed with ACTA-rtTA-TRE-Cre transgenic mice (provided by Dr. Grant Barish) to generate ACTA-rtTA-TRE-Cre;Bmal1fx/lx mice, as well as Bmal1fx/fx and ACTA-rtTA-TRE-Cre littermate controls. Cre-mediated recombination was achieved with 2 mg/ml doxycycline (Sigma) in drinking water for 21 days, and animals were sacrificed 10–14 days thereafter. Vhlfx/fx mice and CAG-Cre-ER transgenic mice were obtained from Jackson Laboratories. All experiments were performed using male C57BL/6J mice between 3–5 months of age, and mice were maintained on a 12:12 light dark (LD) cycle.
CRISPR-Mediated Gene Deletion in C2C12 Myoblasts
Exon 8 of the Bmal1 gene was deleted in C2C12 myoblasts by CRISPR-Cas9 and homology-directed repair. Intronic DNA flanking Bmal1 exon 8 was cloned into pTOPO2.1 (pBmal1-HR) (Invitrogen). Cells were co-transfected with guide RNA, CAS9 (using pSpCas9(BB)-2A-Puro from Addgene) and pBmal1-HR plasmids. Stably-integrated clones were selected for neomycin resistance (G418, Mediatech) and assayed for loss of Bmal1 mRNA and protein. Data shown are averaged data from two independent Bmal1−/− clones.
Oxygen Consumption Rate (OCR) and Extracellular Medium Acidification Rate (ECAR)
OCR and ECAR were measured in differentiated C2C12 myotubes and in isolated mitochondria as previously described (Peek et al., 2013). Myotubes were plated on Seahorse Biosciences 96-well culture plates and transferred to medium in the presence (for OCR) or absence (for ECAR) of glucose and without sodium bicarbonate. Skeletal muscle mitochondria isolation is described in Supplemental Experimental Procedures.
Statistical Analysis
Statistical analysis was performed by unpaired two-tailed Student’s t-test in most cases except when otherwise noted within figure legends. Where appropriate, data are represented as mean ± SEM. Two-way ANOVA was performed to compare the effect of both exercise and time-of-day on gene expression. Differences were considered statistically significant when p<0.05.
Protein Gel Electrophoresis and Immunoblotting
Immunoblotting performed as described previously (Peek et al., 2013). Details are provided in Supplemental Experimental Procedures.
Quantitative Real-Time PCR
qPCR performed as described previously (Peek et al., 2013). Details are provided in Supplemental Experimental Procedures.
Luciferase Assays
Details are provided in Supplemental Experimental Procedures.
C2C12 Myotube Synchronization
Myotubes synchronization was performed as described previously (Peek et al., 2013). Details are provided in Supplemental Experimental Procedures.
Chromatin Immunoprecipitation (ChIP)
ChIP methods were adapted from previously described experimental procedures (Barish et al., 2010). Details are provided in Supplemental Experimental Procedures.
Mouse Embryonic Fibroblast (MEF) Isolation
MEFs were isolated as previously described (Peek et al., 2013). Details are provided in Supplemental Experimental Procedures.
Mouse Treadmill Exercise Experiments
Details are provided in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank P. Schumacker, N. Chandel, G. Barish and members of the Bass lab for helpful discussion, N. Chandel for the HRE-luciferase plasmid, B. Marcheva for help with figures, and W. Song and C. Omura for technical assistance. This research was supported by: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01DK090625 and grant R01DK100814, National Institute on Aging grant P01AG011412, Chicago Biomedical Consortium S-007, and University of Chicago Diabetes Research and Training Center grant P60DK020595, Lynn Sage Cancer Research Foundation grant (J.B.); NIDDK award K01DK105137-02 (C.B.P.), Swedish Research Council and the Swedish Society for Medical Research (J.C.), Manpei Suzuki Diabetes Foundation Fellowship (A.T.). J.B. has financial interest in Reset Therapeutics.
Footnotes
Author Contributions
C.B.P. performed and analyzed all experiments with help from D.C.L., J.C., S.J.T., M.R.M., N.A.B., and Y.K.. A.T. designed and generated reagents for making CRISPR cell lines. J.B., K.M.R, and C.B.P. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Bass J. Circadian topology of metabolism. Nature. 2012;491:348–356. doi: 10.1038/nature11704. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersten DC, Sullivan AE, Peet DJ, Whitelaw ML. bHLH-PAS proteins in cancer. Nature reviews Cancer. 2013;13:827–841. doi: 10.1038/nrc3621. [DOI] [PubMed] [Google Scholar]
- Chilov D, Camenisch G, Kvietikova I, Ziegler U, Gassmann M, Wenger RH. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1alpha. J Cell Sci. 1999;112(Pt 8):1203–1212. doi: 10.1242/jcs.112.8.1203. [DOI] [PubMed] [Google Scholar]
- Cunliffe CJ, Franklin TJ, Hales NJ, Hill GB. Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J Med Chem. 1992;35:2652–2658. doi: 10.1021/jm00092a016. [DOI] [PubMed] [Google Scholar]
- Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res. 106:546–550. doi: 10.1161/CIRCRESAHA.109.209346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyar KA, Ciciliot S, Wright LE, Bienso RS, Tagliazucchi GM, Patel VR, Forcato M, Paz MI, Gudiksen A, Solagna F, et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Molecular metabolism. 2014;3:29–41. doi: 10.1016/j.molmet.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egg M, Koblitz L, Hirayama J, Schwerte T, Folterbauer C, Kurz A, Fiechtner B, Most M, Salvenmoser W, Sassone-Corsi P, et al. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol Int. 2013;30:510–529. doi: 10.3109/07420528.2012.754447. [DOI] [PubMed] [Google Scholar]
- Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300:E877–885. doi: 10.1152/ajpendo.00626.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge BA, Wen Y, Riley LA, Zhang X, England JH, Harfmann BD, Schroder EA, Esser KA. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skeletal muscle. 2015;5:17. doi: 10.1186/s13395-015-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci U S A. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BP, Walisser JA, Liu Y, Shen AL, McDearmon EL, Moran SM, McIntosh BE, Vollrath AL, Schook AC, Takahashi JS, et al. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc Natl Acad Sci U S A. 2014;111:18757–18762. doi: 10.1073/pnas.1421708111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm ME, Rundqvist H. Skeletal muscle hypoxia-inducible factor-1 and exercise. Experimental physiology. 2016;101:28–32. doi: 10.1113/EP085318. [DOI] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:571–572. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason SD, Howlett RA, Kim MJ, Olfert IM, Hogan MC, McNulty W, Hickey RP, Wagner PD, Kahn CR, Giordano FJ, et al. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh BE, Hogenesch JB, Bradfield CA. Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annual review of physiology. 2010;72:625–645. doi: 10.1146/annurev-physiol-021909-135922. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, Ilkayeva O, Marcheva B, Kobayashi Y, Omura C, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342:1243417. doi: 10.1126/science.1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek CB, Ramsey KM, Marcheva B, Bass J. Nutrient sensing and the circadian clock. Trends Endocrinol Metab. 2012;23:312–318. doi: 10.1016/j.tem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelster B, Egg M. Multiplicity of hypoxia-inducible transcription factors and their connection to the circadian clock in the zebrafish. Physiological and biochemical zoology : PBZ. 2015;88:146–157. doi: 10.1086/679751. [DOI] [PubMed] [Google Scholar]
- Rao P, Monks DA. A tetracycline-inducible and skeletal muscle-specific Cre recombinase transgenic mouse. Developmental neurobiology. 2009;69:401–406. doi: 10.1002/dneu.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Science’s STKE : signal transduction knowledge environment. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot IG, Schols AM, Vosse BA, Kelders MC, Gosker HR. Hypoxia differentially regulates muscle oxidative fiber type and metabolism in a HIF-1alpha-dependent manner. Cellular signalling. 2014;26:1837–1845. doi: 10.1016/j.cellsig.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.