Abstract
Macrophages are a heterogeneous population of phagocytic cells present in all tissues. Recently, several drugs that target the epigenetic machinery have emerged as attractive molecules for treating infection and inflammation by modulating macrophages. Treatment of lipopolysaccharide (LPS)-challenged macrophages with epigenetic modifiers leads to phenotype switching. This could provide stimulatory/destructive (M1) or suppressive/protective (M2) therapeutic strategies, which are crucial in the cytokine milieu in which the macrophages reside. In this review, we provide an overview of macrophage functional diversity during various diseases, including infection, as well as the current status in the development and clinical utility of epigenetic modifiers.
Keywords: epigenetic modifiers, macrophages, polarization, inflammation
Introduction
Every year, sepsis-induced acute lung injury (ALI) and cardiovascular diseases (CVDs) affect millions of people worldwide [1,2]. Several risk factors, such as smoking, dyslipoproteinemia, hypertension, diabetes, and obesity, increase a person's chances of acquiring CVDs and related inflammation, but the major underlying culprit remains to be identified [3]. Specifically, in the CVD atherosclerosis, persistent inflammation occurs in the arterial wall. Similarly, in sepsis, overinflammation occurs in the lungs when this organ is attacked by a pathogen. Overall, CVDs and sepsis remain a prime public health concern and serve as a financial burden to many healthcare systems worldwide [4]. For years, doctors have used anti-inflammatory medications and antibiotics to treat many diseases. In a similar fashion, antibiotics and volume replacement are used to treat sepsis, but even these therapies fail to suppress the overinflammation that is the root cause of sepsis [5]. Thus, therapies based on a new understanding of epigenetic changes during disease pathogenesis are needed.
In recent years, macrophages have been in the spotlight because of their significant role in inflammation. In general, when a pathogen enters the body, macrophages detect and engulf it and can present the fragmented peptide to T-helper (Th) cells. Simultaneously, macrophages release proinflammatory cytokines and chemokines to destroy the pathogen and secrete anti-inflammatory cytokines and chemokines to protect the body. During this time, the macrophage undergoes specific differentiation depending on the local environment [6]. Two distinct polarized states of macrophages have been identified. The macrophages that release proinflammatory cytokines are known as M1 classically activated macrophages (CAM), and M2 alternatively activated macrophages (AAM) are those that release anti-inflammatory cytokines [7,8]. More specifically, when a macrophage is attacked by pathogenic inflammatory stimuli, the cell alters its gene expression to conceive an activated macrophage, which is more armed to combat the inflammatory stimuli [9].
In sepsis, there is a high expression of M1 macrophages, which release many proinflammatory cytokines and promote cell apoptosis as a consequence. Coupled with this, there is decreased expression of M2 macrophages, which minutely discharge anti-inflammatory cytokines and chemokines. The obvious solution to counteract the unchecked inflammation is to increase anti-inflammatory cytokines while simultaneously reducing proinflammatory cytokines. Studies have observed that the administration of intravenous immunoglobulin led macrophages to secrete increasing amounts of anti-inflammatory interleukin 10 (IL10). However, this works only if the intravenous immunoglobulin is administered 1 h after the inflammatory attack, and the effect only lasts for 24 h [10]. Thus, there is a dire need to find a more permanent solution to this uncontrolled inflammation. Given that the pathogen-induced response causes a change in the gene expression of the macrophages, many researchers are currently seeking ways to revert this affect. Specifically, they aim to manipulate macrophage gene expression through epigenetic modulations to generate more M2 macrophages than M1 macrophages. More specific is the desire to manipulate post-translational modifications of histones and noncoding RNA [3] by using epigenetic modifiers. Recently, studies have shown that using agents that modulate chromatin systems in macrophages are of potential therapeutic value in controlling proinflammatory responses [11–13]. Thus, in an attempt to seek a treatment for inflammation, researchers have directed their attention to identify drugs that epigenetically target macrophages. In this review, we provide an overview of different types of macrophage with important roles in disease pathogenesis and how the epigenetic-modifying agents target these macrophages to modulate disease prognosis during infection and inflammation.
Macrophages
Origin
For many years, it was believed that macrophages solely arose from the local differentiation of circulating monocytes [14]. However, recent studies revealed that macrophages originate during embryonic development [15,16]. Studies have shown that each organ has its own unique combination of embryonically and adult-derived macrophages and governs the level to which circulating monocytes replace resident macrophages [17,18]. Additionally, it was noted that embryonic macrophages are involved in tissue remodeling, whereas adult macrophages primarily assist in host defense [19]. The major difference between the embryonic- and adult-derived macrophages and stage-specific functions has been reviewed recently [20]. Aside these differences, it was observed that embryonically derived macrophages and adult-derived macrophages coexist in many organs. To prove the independent existence of these two types of macrophage, 6 h after whole-body irradiation, recipient mice received 5×107 bone marrow cells from GFP-expressing donor mice [21]. The resultant chimeric mice had macrophages from both bone marrow and peripheral blood of donor GFP origin 28 days after transplantation. Importantly, the alveolar macrophages were GFP negative, indicating that bone marrow transplantation had no effect on alveolar macrophages up to 8 months. This study suggests a system in which the bone marrow and circulation of recipient mice could be fully replaced without disturbing the residential population of macrophages in the lungs [21]. Together, these two populations are flawlessly synchronized, specialized, and performing organ-specific tasks.
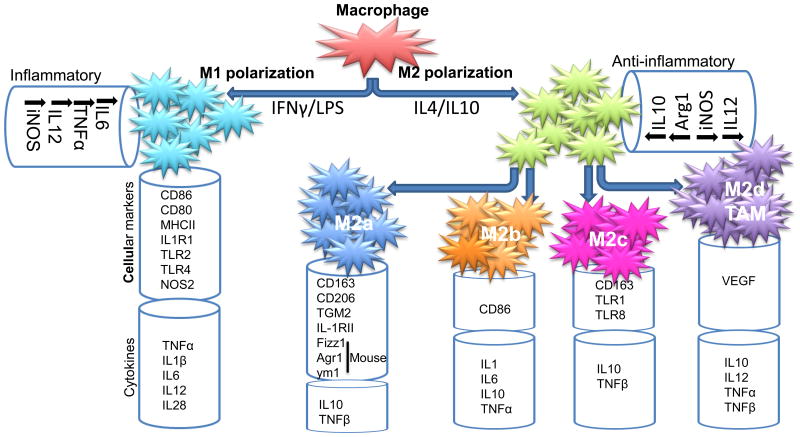
These macrophages, under different pathophysiological conditions and surrounding microenvironments, can acquire distinct functional phenotypes via different phenotypic polarization. The activated state of macrophages presents as classical activated M1 and alternatively activated M2 forms. M2 macrophages are further divided into M2a (induced by IL4 and IL13), M2b (activated by immune complexes or TLR agonist), and M2c (induced by IL10) based on their induction and function [22]. A fourth type of macrophage, M2d, has been reported and is characterized by an IL10high IL12low M2 profile with the features of tumor-associated macrophages (TAMs) [23]. The M1 and M2 macrophages, the different subclasses of M2, and the secretion of chemokines and cytokines are summarized in Figure 1.
Figure 1.
Different types of macrophage and their secretory products. M1 and M2 macrophages and the different subclasses of M2 and their relevant chemokines and cytokines are summarized. For definitions of abbreviations, please see the main text.
Macrophages in different organs
In general, macrophages are found in all parts of the body, residing in various tissue types and adapted to their local microenvironment [17]. They are found in the spleen, lung, peritoneal cavity, the central nervous system (CNS), liver, skin, bone, blood, and gastrointestinal (GI) tract. Alveolar and interstitial macrophages are found only in the lungs, where the former maintain surfactant homeostasis and the latter avert allergic reactions to airborne antigens by governing dendritic cell function [24]. There are two macrophage subtypes in the peritoneal cavity of adult mice. Large peritoneal cavity macrophages represent approximately 90% of the peritoneal cavity macrophages in unstimulated mice, but disappear rapidly after stimulation with LPS or thioglycolate. Moreover, small peritoneal cavity macrophages predominate in the peritoneal cavity after stimulation with LPS or thioglycolate. These macrophages function phagocytically to clear apoptotic cells [25]. Microglias are the resident phagocytic macrophages predominantly present in the CNS and are thought to be involved in inflammatory responses. There are perivascular, meningeal, and choroid plexus macrophages also present in the CNS and these have been referred to as ‘microglia’ [26]. M1 microglia are proinflammatory and secrete inflammatory cytokines and chemokines, including reactive oxygen species (ROS). M2 microglia suppress proinflammatory cascades and promote repair [27].
Kupffer cells or liver macrophages primarily reside in the liver. These cells represent approximately 10-15% of all liver cells. Kupffer cells attack blood-borne particles, whereas other macrophages serve in immune surveillance [28]. Dermal macrophages and Langerhans cells take up residence in the skin. Bone marrow macrophages support erythropoiesis, whereas multinucleated osteoclasts cause bone resorption. In blood, Ly6Clow patrolling monocytes help sustain vascular integrity. In the GI track, macrophages of the intestinal lamina propria help maintain stable conditions in the intestine and generate an immune response to bacteria [19]. Activation of M1 macrophages has also been found in atopic dermatitis, where a large number of M1 macrophages accumulate in acutely or chronically inflamed skin and release inflammatory cytokines [29]. In the daunorubicin (DNR)-induced model of nephrotoxicity, expression of an M1 phenotype was increased with the release of inflammatory cytokines, which were attenuated by modulating the macrophage polarization to an M2 state [30]. Thus, in various tissue types, macrophages perform specific functions. In general terms, macrophages maintain homeostasis by modulating proinflammatory and anti-inflammatory cytokines [31].
The concept of macrophage polarization and its functions
Macrophage polarization is a process whereby macrophages phenotypically mount a specific functional response to the microenvironment [32]. In the host immune system, macrophages have an important role in both normal and disease conditions. The concept of macrophage plasticity during tissue repair and regeneration was recently reviewed elsewhere [33]. Macrophages mainly sustain homeostasis by mediating the release of proinflammatory and anti-inflammatory cytokines. Lymphocytes activate macrophages by releasing either interferon-γ (IFN γ) or IL4. The release of IFNγ compels macrophages to secrete proinflammatory cytokines, leading them to generate toxic mediators in great quantities [34]. Specifically, these macrophages release tumor necrosis factor α (TNFα), IL1, IL6, and reactive oxygen intermediates, and are recognized as M1 macrophages [35]. By contrast, the macrophages that release IL4 trigger the expression of anti-inflammatory cytokines, such as Arginase-1 (Arg1) and IL10, as well as increasing the expression of cell surface receptor CD206 [36]. These macrophages are found to inhibit activated macrophages (M1) and are known as M2 macrophages [37]. This phenomenon of the two contrasting M1/M2 phenotypes is referred to as ‘macrophage polarization’. The proinflammatory M1 macrophages have robust microbicidal and tumoricidal activity, whereas anti-inflammatory M2 macrophages orchestrate the remodeling of the tissue and tumor formation [38,39]. Recently, a study analyzing transcriptional mRNA profiling data identified novel markers for M1 (CD38, Gpr18, and Fpr2) and M2 (Myc and Egr2) macrophages [40]. In different microenvironments, the regulation of transcription factors directs the M1/M2 phenotype of the macrophage (Figure 1). It is particularly interesting that inactivation of signal transducer and activator of transcription 3 (STAT3) polarizes macrophages to a proinflammatory phenotype associated with a higher expression of M1 markers [41]. Studies have shown that, apart from environmental factors, the cellular signaling components that have an important role in macrophage polarization are STATs, IFN-regulatory factor (IRF) [42], peroxisome proliferator-activated receptor (PPAR)-γ [43], nuclear factor (NF)-κB [32], and activator protein (AP). Macrophage polarization is closely governed by the balance between the cellular signaling event of STAT1 and STAT3/STAT6. The M1 and M2 switch in macrophages, present at the same location, has also been observed in vivo [44]. Thus, researchers are aiming to manipulate the signaling events to regulate the expression of M1/M2 macrophages to control the prevalence of a disease.
The role of macrophages in different diseases
The involvement of macrophage phenotypes during disease condition indicates that the detection or modulation of macrophage responses could be a useful approach for diagnostics or therapies, respectively, in different diseases. However, this field is hampered by the lack of potential biomarkers for M1 and M2 macrophages.
CVDs
Macrophages have an essential role in the progression of CVDs. Several studies have revealed the role of M1/M2 macrophages, and their availability in a balanced ratio decides the resolution of the atherosclerotic lesion [45,46]. Atherosclerotic lesions are formed by an accumulation of lipids in the intima of medium and large arteries, followed by penetration by T cells, mast cells, and monocytes. Eventually, this accumulation destabilizes the lesions, causing them to split and start thrombosis [47]. Thrombosis eventually leads to acute cardiac injury, or myocardial infarction (MI), which is a major source of CVD [48]. The M1 and M2 polarization in atherosclerosis was comprehensively reviewed recently [49].
During myocardial infarction, transcription-1, IRF-5, and NF-κB are activated [50]. They signal a huge amount of M1 macrophages to rush to the site of cardiac injury to initiate cardiac remodeling [51]. Once this initial period passes, a second set comprising STAT-6, IRF-4, and PPAR-α reaches the injury site. The migrated macrophages now begin to exhibit a pro-resolution M2 profile, leading toward M2 macrophage polarization. This new macrophage phenotype boosts cardiac repair by governing profibrotic responses [52]. These macrophages resolve the damage caused by inflammation and return the system to homeostasis.
A study showed that there were an increased number of CD68-positive macrophages at the border zone of the mouse myocardium 3 days post-MI. This increased infiltration of macrophages was associated with an increase in mRNA expression of proinflammatory cytokines and chemokines [53]. Moreover, exogenous administration of IL10 promoted remodeling through the inhibition of various proinflammatory responses. This study strongly suggests that small molecules, which activate macrophages to secrete IL10, would be a potential therapeutic approach for treatment post-MI.
Inflammatory processes have a major role in ischemia/reperfusion (IR) injury, where elevated levels of MCP-1-CCR2 chemokine/monocyte receptor interactions and increased expression of endothelial adhesion molecules increase the population of M1 proinflammatory macrophage [54]. Inflammation promotes activation of NF-κB and IRF-1, which are subsequently downregulated with the release of IRF-4 as well as the anti-inflammatory cytokines IL4, IL13, IL10, and transforming growth factor β1 (TGF-β1) [55]. This shifts tissue inflammation toward tissue repair with the release of CCL13, CCL8, or CCL26 [55]. In vitro studies showed that the STAT6 signaling molecule is increased by IL4 and IL13, which promotes alternatively activated macrophage phenotypes that principally release extracellular matrix molecules [56] and stops the inflammatory signaling cascade. M2 macrophages express increased level of arginase, which is a substrate for the synthesis of collagen and thereby directly promotes cell division and tissue repair [57]. Macrophages produce profibrotic mediators that activate fibroblasts and regulate different processes associated with the remodeling of tissue IR injury by secreting chemokines and releasing anti-inflammatory molecules [58,59].
Macrophages also have a crucial role in human ischemic and idiopathic dilated cardiomyopathy hearts and release inflammatory cytokines, such as IFNγ, IL6, IL1β, and TNFα. A recent study showed that aging SAMP8 mice hearts induced proinflammatory signals that were associated with the accumulation of M1-type macrophages. These were CD68 positive cells that upregulated the release of proinflammatory factors and cytokines, such as IFNγ, IL6, IL1β, and TNFα, and also TNF receptor 1 (TNFR1) and cyclooxygenase (COX)-2 expression [60]. Overall, these studies suggest that macrophages have an important role in cardiac remodeling during disease conditions.
Infection
Human monocyte-derived macrophages initiate particular patterns of gene expression upon their exposure to bacteria and viruses. After bacterial exposure, macrophages utilize their pattern recognition receptors/sensors (PRRs) to identify pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). PAMPs are the lipids, proteins, lipoproteins, and glycan obtained from bacteria, viruses, and other parasites, whereas DAMPs are endogenous danger signal molecules that act as potent activators of innate immunity [61]. The specific receptors of PAMP/DAMP interact with the invading agent and activate a pathway for defense [62]. Thus, with the help of Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain-like (NOD)-like receptors, macrophages sense the presence of bacteria [63]. Upon recognition, macrophages trigger the production of M1 proinflammatory cytokines, such as TNFα, IL6, IL12, and IL1β [64]. Once the infection is controlled, the immune system aims to neutralize the effects of M1 macrophages by producing more M2 macrophages [65].
Macrophages also commonly combat viruses. With the help of PRRs, the macrophage recognizes a virus [66]. Then, the active endocytic processes allow the macrophages to not only consume the invading pathogens, but also display the remnants of these pathogens to T lymphocytes via major histocompatibility complex (MHC) molecules. In turn, these T cells release IFNγ, a soluble mediator, which strengthens the attack by the macrophages on the pathogens. Macrophages generate two major mechanisms: innate defense and acquired defense. As soon as a pathogen is recognized, an innate defense mechanism effectively ingests and destroys the pathogen. Then, the antigens initiate an adaptive immune response. Through the systematic release of cytokines and chemokines and the recruitment of other immune cells, an inflammatory response is generated to protect the cells from further infection [67]. Thus, viral or bacterial infection and the related responses in modulating macrophage polarization signify the important role of macrophages during infection and inflammatory responses.
Wounds
Given that macrophages are the first line of defense, they have a distinct role in protecting cells from injury. When an injury occurs, wound macrophages, which are derived from circulating monocytes, assist in protecting the host. In particular, inflammatory monocytes respond to injury by marching to the sites of the wound, where they mature into M1 macrophages and generate an inflammatory response by releasing ROS and reactive nitrogen species (RNS), proteases, proinflammatory lipids, and cytokines [68,69]. They also continue to recruit neighboring monocytes and form lesions [70]. During this time, neutrophils also enter the site to clear the debris and necrotic tissue. Once this short-lived process of debridement terminates, neutrophils undergo apoptotic cell death [71]. As more neutrophils begin to die, a second population of monocytes arrives, mostly from tissue-resident macrophages [70,71]. These monocytes evolve into M2 macrophages and govern the remodeling phase. The M2 macrophages continue to debride the site by phagocytosis and also clear the remains of neutrophils. In addition, they not only downregulate inflammation, but also discharge growth factors to promote fibroblast proliferation and angiogenesis. They further endorse tissue regeneration and, thus, lead the cells back to homeostasis to maintain tissue morphology and function [55,68,71,72]. Recently, a study showed that naringenin, a flavonoid compound, activated anti-inflammatory gene expression by switching from the M1 to M2 phenotype, which led to increased CD36 and IL10 in atopic dermatitis [73]. These above findings strongly suggest that macrophages have an important role during wound-mediated tissue remodeling.
Macrophage polarization in ALI
Sepsis-induced ALI is an inflammatory disorder characterized by bilateral alveolar infiltrates, lung edema, and respiratory failure, with limited treatment options. During lung inflammation, the M1 and M2 macrophages are two distinct phenotypes with important roles in producing inflammatory and anti-inflammatory factors, respectively, to combat the injury [74,75]. Many studies have clearly demonstrated that, after sensing the presence of a pathogen, macrophages first display an M1 phenotype. Then, to return to homeostasis, they embrace the M2 phenotype [76]. When macrophages first sense the presence of a pathogen, they become activated and produce proinflammatory cytokines, such as IFNγ, TNFα, and IL1β. These cytokines signal other tissue-resident macrophages to secrete chemokines for neutrophil recruitment and also to direct macrophages and lymphocytes to the origin of infection. This action initiates lung inflammation [77].
Later, when the pathogen is suppressed, all the steps that initiated inflammation must be reversed to stop the inflammation. The release of IL1β is blocked at the receptor IL1R1. This action lowers the release of the neutrophil chemokine macrophage inflammatory protein 2 (MIP-2) and blocks neutrophil recruitment as a consequence. Additionally, the IL1β antagonism and the high concentration of TNFα initiate neutrophil apoptosis. The ingestion of apoptotic neutrophils triggers macrophages to take the anti-inflammatory route. Specifically, they secrete immunosuppressive cytokines, such as TGFβ and IL10. The anti-inflammatory M2 macrophages further promote angiogenesis, tissue remodeling, and wound healing [77,78]. Hence, because of their distinct phenotypes, macrophages have a significant role in not only the initiation of the inflammation, but also the termination of inflammation [79].
Epigenetic modifiers and macrophage polarization
Epigenetic modifications are associated with altered gene expression and, therefore, regulate different cellular functions, including macrophage polarization. Epigenetic modifications include DNA methylation, histone modifications by acetylation/deacetylation and methylation/demethylation, ubiquitination, and sumoylation. All these modifications are involved in the regulation of macrophage activation and functional differentiation. Aberrant histone modifications are associated with multiple human diseases and are potential diagnostic biomarkers or therapeutic targets for some inflammatory or autoimmune diseases [80]. Studies have shown that histone methylation at lysine residues has an important role in M2 macrophage activation. Ash1I is a H3K4 methyltransferase that regulates macrophage activation by modulating IL6 and TNFγ production, whereas Jmjd3 has a significant role in M2 macrophage activation. Sumoylation and miRNA-mediated regulation of gene expression and functions of macrophages, [QA2]PPARγ has been found to undergo sumoylation, which attenuates LPS-mediated inactivation of NF-κB and, therefore, reduces the production of inflammatory cytokines, such as IL12, TNFα, and nitric oxide (NO) molecules [81].
Thus, epigenetic mechanisms regulate chromatin marks in the DNA and histones that control gene expression pattern. These epigenetic modifications have recently been studied in response to environmental cues, which regulate the expression level of different transcription factors, such as NF-κB and/or STAT, and transcription activators/inhibitors that are activated by inflammatory signals [82].
Inhibition of histone deacetylases (HDACs) regulates the acetylation status of different genes associated with inflammation [5,83] or other diseases, such as cancer [84], CVDs [85], and metabolic homeostasis [86]. In recent years, HDAC inhibitors have demonstrated great potential in serving as a therapeutic treatment for the suppression of inflammation in macrophages. Many studies have shown that HDAC inhibitors tend to reduce the expression of M1 macrophages. By reducing the expression of M1 cytokines, the macrophages are compelled to take the anti-inflammatory M2 route. There are two important epigenetic events that direct the fate of a cell and its expression: DNA methylation or demethylation and histone deacetylation or acetylation [5,87,88]. Currently, researchers aim to utilize HDAC inhibitors (iHDACs) and DNA methyltransferases inhibitors (iDNMTs) to achieve this task. Variable results have been shown with the use of iHDACs that attenuated the generation of inflammatory cytokines and inflammatory chemokines, and inflammatory injury in the airway, digestive tract, and joints in animal models [89-92]. This variability in earlier studies might be because of the focus on the effect of higher concentrations and pretreatment protocols with the drugs rather than on therapeutic potential. Given that histone deacetylation regulates gene expression, iHDAC is expected to increase the gene expression of M2 macrophages.
One study demonstrated that the alteration of HDAC expression triggered proinflammatory gene expression in macrophages. In this study, LPS-induced macrophages expressed the genes Ccl2, Ccl7, and Edn1. When Trichostatin A (TSA), an HDACi, was introduced into the cells, it suppressed the expression of these genes, consequently reducing the inflammation [93]. In a similar fashion, the use of Scriptaid, another HDACi, has been shown to lead microglia in the M2 direction by increasing the expression of glycogen synthase kinase 3β (GSK3β). This increase in expression triggers a chain of reactions that enhance PI3K/Akt signaling, leading microglia in the M2 direction [94]. Similarly, TSA was shown to lead M2 polarization in macrophages. Specifically, by chelating the central zinc finger motif of HDACs, TSA is able to inhibit enzyme activity. It reduces the expression of proinflammatory cytokines, such as TNFα and nitric oxide, through the phosphorylation of p38MAPK. Additionally, it also suppresses the expression of cytokines and chemokines of ROS. In addition, macrophages exhibit an overexpression of IL6 and the IL6 receptor (IL6R) when stimulated by LPS [95]. However, the presence of the HDACi ITF2357 has been shown to reduce the expression of IL6 and IL6R [96]. Another HDACi, valproic acid (VPA), reduces M1 macrophage expression by downregulating the expression of proinflammatory cytokines, and the cellular markers CD40 and CD80. Simultaneously, it increases the expression of M2 macrophages by downregulating the expression of CD86, an anti-inflammatory cytokine [96]. Recently, it was shown that inhibition of HDAC6 protects mice from LPS-mediated toxicity by downregulating proinflammatory responses and upregulating expression of IL10 [97]. Therefore, from all these observations, it can be concluded that iHDACs are a new class of small molecules that can modulate the transcriptional state of different diseases and gradually gain importance in targeted therapies of a variety of diseases, from cancer to inflammation.
In epigenetic regulation, DNA methylation has been shown to regulate the gene expression of macrophages in response to the pathogenesis of several diseases, including inflammation. Macrophages are primed in response to different stimuli, which dictates the expression and function of a particular phenotypic state, that is, M1/M2. TLR signaling induces trimethylation of H3K4 on cytokine gene promoters that activates proinflammatory gene expression and M1 macrophage activation. 5-azacytidine is a common DNMTi that induces DNA hypomethylation by inhibiting DNMTs. Studies have shown that DNMT3b regulates macrophage polarization and inflammation. Obese mice express increased levels of DNMT3b associated with the expression of proinflammatory M1 macrophages, and knockdown of DNMT3b switches the polarity of macrophages to the alternative M2 state [98]. Another DNMT, DNMT1 is also associated with the release of proinflammatory cytokines in macrophages by hypermethylating Suppressor of cytokine signaling 1 (SOCS1). Inhibition of DNMT1 downregulates the activation of the Janus kinase (JAK)-2/STAT3 pathway in RAW264.7 cells after LPS induction and thereby prevents the inflammatory phenotype [99]. These examples provide clear evidence that inhibition of demethylases modulates the transcriptional activation of genes for M2 macrophages and drugs that target DNMTs could be used to promote anti-inflammatory responses to reduce inflammation.
Combination of epigenetic modifiers
Studies have shown that DNA methylation and histone deacetylation dictate the fate of a cell and its gene expression [88,100]. Recently, it was shown that 5-Aza 2-deoxycytidine (Aza), a DNMTi and TSA, a total inhibitor for HDACs, have the potential to treat inflammation. The study demonstrated that the combination of Aza+TSA not only decreased the expression of M1 macrophages, but also increased M2 macrophage expression in LPS-induced primary mouse bone marrow-derived macrophages (BMDMs) [13].
Recently, the role of Aza+TSA in LPS-induced BMDMs was examined using qRT-PCR. The data revealed 23 inflammatory mRNA transcripts to be significantly expressed in BMDMs stimulated with LPS when compared with controls. However, treatment with Aza+TSA significantly reduced the LPS-induced mRNA expression of chemokines and cytokines [83]. Furthermore, the immunofluorescence analysis of lung tissues isolated from LPS-induced mice either untreated or treated with either Aza+TSA or Aza alone or TSA alone for 24 h showed a reduced expression of NO synthase (NOS)-2 in LPS-challenged macrophages treated with Aza+TSA compared with untreated LPS-induced macrophages. Likewise, the expression of CD206 showed increased protein expression in Aza+TSA-treated BMDMs compared with untreated control cells [83]. These data indicated that LPS-challenged macrophages treated with Aza+TSA have fewer M1 and more M2 macrophages to favor the secretion of anti-inflammatory factors to combat the inflammatory responses. These data support earlier findings that M1 and M2 macrophages coexist and the dominance of either type has a major role in disease progression [38]. Hence, the above study clearly demonstrates that Aza+TSA have the potential to suppress LPS-mediated inflammation through the manipulation of epigenetic factors.
Future perspectives and challenges
It is now known that macrophage polarization governs the fate of an organ. When an organ is attacked by infection or injury, macrophages first exhibit the proinflammatory M1 phenotype to release proinflammatory cytokines against the stimulus. However, if this M1 phase continues, it can cause tissue damage. Thus, after the initial response, macrophages with the anti-inflammatory M2 phenotype not only suppress the inflammation, but also promote tissue remodeling and retain homeostasis. Thus, to treat inflammatory diseases, sepsis in particular, researchers aim to reverse this macrophage polarization. Recently, it was shown that the combination of two epigenetic modifiers (Aza+TSA) decreased the expression of the M1 phenotype while augmenting the expression of the M2 phenotype in LPS-induced macrophages [13]. Even though these epigenetic modifiers have only recently received attention from researchers, they have a promising future. Using these previous studies as an example, more researchers can aim to utilize epigenetic modifiers to manipulate the pathogenesis of other diseases. Given that these epigenetic modifiers relieve the cause of the inflammatory disease, they could also serve as a more effective therapeutic treatment.
Concluding remarks
Macrophages are heterogeneous cells, and the surrounding environment regulates their function and phenotype. M1/M2 macrophages have different functions and different transcriptional profiles, but these are all required to maintain homeostasis. These macrophages have unique abilities to destroy pathogens or repair the inflammatory injury. Epigenetic modifiers have the ability to determine the fate of a macrophage at the site of tissue injury. Thus, such molecules could have substantial therapeutic value during the treatment of infection and inflammation.
Highlights (for review).
This review is focusing on polarization of macrophages by epigenetic modifications.
M1/M2 macrophages are flawlessly synchronized, specialized, and perform organ-specific tasks.
Drugs targeting epigenetic machineries are becoming an important field to study.
Combinations of epigenetic modifying agents activate M2 macrophages to reduce inflammation.
Acknowledgments
This work was supported, in part, by American Heart Association Grant-in-Aid 16GRNT30950010 and National Institutes of Health COBRE grant P20GM104936 (to J.R.).
Footnotes
Teaser: Epigenetics is useful way to study physiological and pathophysiological conditions. Inflammation triggers the activation of immune system, antimicrobial defense, tissue repair, and remodeling. Here, we summarize the functional role of epigenetically modified M2 macrophages and their role in immunomodulatory activities.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nemeth K, et al. Modulation of bone marrow stromal cell functions in infectious diseases by toll-like receptor ligands. J Mol Med. 2010;88:5–10. doi: 10.1007/s00109-009-0523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, et al. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Bekkering S, et al. The epigenetic memory of monocytes and macrophages as a novel drug target in atherosclerosis. Clin Ther. 2015;37:914–923. doi: 10.1016/j.clinthera.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Mulchandani N, et al. Stimulation of brain AMP-activated protein kinase attenuates inflammation and acute lung injury in sepsis. Mol Med. 2015;XX:YYY–ZZZ. doi: 10.2119/molmed.2015.00179. [QA3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thangavel J, et al. Combinatorial therapy with acetylation and methylation modifiers attenuates lung vascular hyperpermeability in endotoxemia-induced mouse inflammatory lung injury. Am J Pathol. 2014;184:2237–2249. doi: 10.1016/j.ajpath.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 8.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hume DA. The many alternative faces of macrophage activation. Front Immunol. 2015;6:370. doi: 10.3389/fimmu.2015.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozicky LK, et al. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL10–producing activation state. J Leukoc Biol. 2015;XX:YYY–ZZZ. doi: 10.1189/jlb.3VMA0315-078R. [QA4] [DOI] [PubMed] [Google Scholar]
- 11.De Santa F, et al. Jmjd3 contributes to the control of gene expression in LPS-activated macrophages. EMBO J. 2009;28:3341–3352. doi: 10.1038/emboj.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruidenier L, et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature. 2012;488:404–408. doi: 10.1038/nature11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thangavel J, et al. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J Cell Sci. 2015;XX:YYY–ZZZ. doi: 10.1242/jcs.170258. [QA5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Italiani P, Boraschi D. New insights into tissue macrophages: from their origin to the development of memory. Immune Netw. 2015;15:167–176. doi: 10.4110/in.2015.15.4.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 16.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epelman S, et al. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolattukudy P. MCPIP: a key player in macrophage polarization. Oncotarget. 2015;XX:YYY–ZZZ. doi: 10.18632/oncotarget.5451. [QA6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldar M, Murphy KM. Origin, development, and homeostasis of tissue-resident macrophages. Immunol Rev. 2014;262:25–35. doi: 10.1111/imr.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 21.Janssen WJ, et al. Development and characterization of a lung-protective method of bone marrow transplantation in the mouse. J Immunol Methods. 2010;357:1–9. doi: 10.1016/j.jim.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 22.Brown MB, et al. M1/M2 macrophage polarity in normal and complicated pregnancy. Front Immunol. 2014;5:606. doi: 10.3389/fimmu.2014.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duluc D, et al. Tumor-associated leukemia inhibitory factor and IL6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- 24.Rubins JB. Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med. 2003;167:103–104. doi: 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- 25.Ghosn EE, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc Natl Acad Sci U S A. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsumoto A, et al. Ontogeny and functions of central nervous system macrophages. J Immunol. 2014;193:2615–2621. doi: 10.4049/jimmunol.1400716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitani H, et al. Characterization of the liver-macrophages isolated from a mixed primary culture of neonatal swine hepatocytes. Results Immunol. 2014;4:1–7. doi: 10.1016/j.rinim.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karuppagounder V, et al. Resveratrol attenuates HMGB1 signaling and inflammation in house dust mite-induced atopic dermatitis in mice. Int Immunopharmacol. 2014;23:617–623. doi: 10.1016/j.intimp.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Karuppagounder V, et al. Curcumin alleviates renal dysfunction and suppresses inflammation by shifting from M1 to M2 macrophage polarization in daunorubicin induced nephrotoxicity in rats. Cytokine. 2016;84:1–9. doi: 10.1016/j.cyto.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Vasconcelos DP, et al. Macrophage polarization following chitosan implantation. Biomaterials. 2013;34:9952–9959. doi: 10.1016/j.biomaterials.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Wang N, et al. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das A, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–2606. doi: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasegawa Y, et al. Impaired cytokine production by peripheral blood mononuclear cells and monocytes/macrophages in Parkinson's disease. Acta Neurol Scand. 2000;101:159–164. doi: 10.1034/j.1600-0404.2000.101003159.x. [DOI] [PubMed] [Google Scholar]
- 35.Yin K, et al. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arterioscler Thromb Vasc Biol. 2015;XX:YYY–ZZZ. doi: 10.1161/ATVBAHA.115.306132. [QA7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makita N, et al. IL10 enhances the phenotype of M2 macrophages induced by IL4 and confers the ability to increase eosinophil migration. Int Immunol. 2015;27:131–141. doi: 10.1093/intimm/dxu090. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, et al. IL4 modulates macrophage polarization in ankylosing spondylitis. Cell Physiol Biochem. 2015;35:2213–2222. doi: 10.1159/000374026. [DOI] [PubMed] [Google Scholar]
- 38.Tian S, Chen SY. Macrophage polarization in kidney diseases. Macrophage. 2015;2:YYY–ZZZ. doi: 10.14800/macrophage.679. QA8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagalkot V, et al. Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J Control Release. 2015;XX:YYY–ZZZ. doi: 10.1016/j.jconrel.2015.09.027. QA9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jablonski KA, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE. 2015;10:e0145342. doi: 10.1371/journal.pone.0145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura R, et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunthner R, Anders HJ. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chawla A. Control of macrophage activation and function by PPARs. Circ Res. 2010;106:1559–1569. doi: 10.1161/CIRCRESAHA.110.216523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khallou-Laschet J, et al. Macrophage plasticity in experimental atherosclerosis. PLoS ONE. 2010;5:e8852. doi: 10.1371/journal.pone.0008852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mantovani A, et al. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol. 2009;29:1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 46.Boyle JJ, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kassiteridi C, Monaco C. Macrophages and dendritic cells: the usual suspects in atherogenesis. Curr Drug Targets. 2015;16:373–382. doi: 10.2174/1389450116666150330115809. [DOI] [PubMed] [Google Scholar]
- 48.Neele AE, et al. Epigenetic pathways in macrophages emerge as novel targets in atherosclerosis. Eur J Pharmacol. 2015;763:79–89. doi: 10.1016/j.ejphar.2015.03.101. [DOI] [PubMed] [Google Scholar]
- 49.Aluganti Narasimhulu C, et al. Atherosclerosis - do we know enough already to prevent it? Curr Opin Pharmacol. 2016;27:92–102. doi: 10.1016/j.coph.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Bolego C, et al. Macrophage function and polarization in cardiovascular disease: a role of estrogen signaling? Arterioscler Thromb Vasc Biol. 2013;33:1127–1134. doi: 10.1161/ATVBAHA.113.301328. [DOI] [PubMed] [Google Scholar]
- 51.Gliem M, et al. Hyperglycemia and PPARgamma antagonistically influence macrophage polarization and infarct healing after ischemic Stroke. Stroke. 2015;XX:YYY–ZZZ. doi: 10.1161/STROKEAHA.115.010557. QA10. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Velasco M, et al. Involvement of monocytes/macrophages as key factors in the development and progression of cardiovascular diseases. Biochem J. 2014;458:187–193. doi: 10.1042/BJ20131501. [DOI] [PubMed] [Google Scholar]
- 53.Krishnamurthy P, et al. IL10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res. 2009;104:e9–e18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Couto G, et al. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125:3147–3162. doi: 10.1172/JCI81321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832:989–997. doi: 10.1016/j.bbadis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Wang N, et al. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [QA11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dzik JM. Evolutionary roots of arginase expression and regulation. Front Immunol. 2014;5:544. doi: 10.3389/fimmu.2014.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karuppagounder V, et al. Modulation of macrophage polarization and HMGB1-TLR2/TLR4 cascade plays a crucial role for cardiac remodeling in senescence-accelerated prone mice. PLoS ONE. 2016;11:e0152922. doi: 10.1371/journal.pone.0152922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 62.Vural A, Kehrl JH. Autophagy in macrophages: impacting inflammation and bacterial infection. Scientifica. 2014;2014:825463. doi: 10.1155/2014/825463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Benoit M, et al. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 65.Gong L, et al. Autophagy as a macrophage response to bacterial infection. IUBMB Life. 2012;64:740–747. doi: 10.1002/iub.1070. [DOI] [PubMed] [Google Scholar]
- 66.Varela M, et al. Cellular visualization of macrophage pyroptosis and interleukin-1beta release in a viral hemorrhagic infection in zebrafish larvae. J Virol. 2014;88:12026–12040. doi: 10.1128/JVI.02056-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercer J, Greber UF. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013;21:380–388. doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Laskin DL, et al. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahdavian Delavary B, et al. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adamson R. Role of macrophages in normal wound healing: an overview. J Wound Care. 2009;18:349–351. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 72.Inoue T, Okusa MD. Neuroimmune control of acute kidney injury and inflammation. Nephron. 2015;XX:YYY–ZZZ. doi: 10.1159/000438496. QA12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karuppagounder V, et al. Naringenin ameliorates skin inflammation and accelerates phenotypic reprogramming from M1 to M2 macrophage polarization in atopic dermatitis NC/Nga mouse model. Ex p Dermatol. 2016;XX:YYY–ZZZ. doi: 10.1111/exd.12962. QA13. [DOI] [PubMed] [Google Scholar]
- 74.Chavez-Galan L, et al. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR(+) macrophages. Front Immunol. 2015;6:263. doi: 10.3389/fimmu.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vereyken EJ, et al. Classically and alternatively activated bone marrow derived macrophages differ in cytoskeletal functions and migration towards specific CNS cell types. J Neuroinflammation. 2011;8:58. doi: 10.1186/1742-2094-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aggarwal NR, et al. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–725. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Herold S, et al. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hristodorov D, et al. Targeting CD64 mediates elimination of M1 but not M2 macrophages in vitro and in cutaneous inflammation in mice and patient biopsies. MAbs. 2015;7:853–862. doi: 10.1080/19420862.2015.1066950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alber A, et al. The role of macrophages in healing the wounded lung. Int J Exp Pathol. 2012;93:243–251. doi: 10.1111/j.1365-2613.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koch MW, et al. Epigenetic changes in patients with multiple sclerosis. Nat Rev Neurol. 2013;9:35–43. doi: 10.1038/nrneurol.2012.226. [DOI] [PubMed] [Google Scholar]
- 81.Jennewein C, et al. Sumoylation of peroxisome proliferator-activated receptor gamma by apoptotic cells prevents lipopolysaccharide-induced NCoR removal from kappaB binding sites mediating transrepression of proinflammatory cytokines. J Immunol. 2008;181:5646–5652. doi: 10.4049/jimmunol.181.8.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ivashkiv LB. Epigenetic regulation of macrophage polarization and function. Trends Immunol. 2013;34:216–223. doi: 10.1016/j.it.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thangavel J, et al. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J Cell Sci. 2015;128:3094–3105. doi: 10.1242/jcs.170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nilubol N, et al. A Phase II trial of valproic acid in patients with advanced, radioiodine-resistant thyroid cancers of follicular cell origin. Clin Endocrinol. 2016;XX:YYY–ZZZ. doi: 10.1111/cen.13154. QA14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morales CR, et al. Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression. Sci Signal. 2016;9:ra34. doi: 10.1126/scisignal.aad5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Crunkhorn S. Metabolic disease: new role for HDACs in glucose homeostasis. Nat Rev Drug Discov. 2011;10:492. doi: 10.1038/nrd3483. [DOI] [PubMed] [Google Scholar]
- 87.Hashimshony T, et al. The role of DNA methylation in setting up chromatin structure during development. Nat Genet. 2003;34:187–192. doi: 10.1038/ng1158. [DOI] [PubMed] [Google Scholar]
- 88.Rajasingh J, et al. Improvement of cardiac function in mouse myocardial infarction after transplantation of epigenetically-modified bone marrow progenitor cells. PLoS ONE. 2011;6:e22550. doi: 10.1371/journal.pone.0022550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi Y, et al. Histone deacetylase inhibitor KBH-A42 inhibits cytokine production in RAW 264.7 macrophage cells and in vivo endotoxemia model. Exp Mol Med. 2008;40:574–581. doi: 10.3858/emm.2008.40.5.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, et al. Histone deacetylase inhibitors attenuate acute lung injury during cecal ligation and puncture-induced polymicrobial sepsis. World J Surg. 2010;34:1676–1683. doi: 10.1007/s00268-010-0493-5. [DOI] [PubMed] [Google Scholar]
- 91.Iwata K, et al. Trichostatin A, a histone deacetylase inhibitor, down-regulates interleukin-12 transcription in SV-40-transformed lung epithelial cells. Cell Immunol. 2002;218:26–33. doi: 10.1016/s0008-8749(02)00523-3. [DOI] [PubMed] [Google Scholar]
- 92.Rahman I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem Pharmacol. 2002;64:935–942. doi: 10.1016/s0006-2952(02)01153-x. [DOI] [PubMed] [Google Scholar]
- 93.Aung HT, et al. LPS regulates proinflammatory gene expression in macrophages by altering histone deacetylase expression. FASEB J. 2006;20:1315–1327. doi: 10.1096/fj.05-5360com. [DOI] [PubMed] [Google Scholar]
- 94.Wang G, et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci U S A. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabanel M, et al. Epigenetic control of macrophage shape transition towards an atypical elongated phenotype by histone deacetylase activity. PLoS ONE. 2015;10:e0132984. doi: 10.1371/journal.pone.0132984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Glauben R, et al. Histone deacetylase inhibitors modulate interleukin 6-dependent CD4+ T cell polarization in vitro and in vivo. J Biol Chem. 2014;289:6142–6151. doi: 10.1074/jbc.M113.517599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang B, et al. Microtubule acetylation amplifies p38 kinase signalling and anti-inflammatory IL-10 production. Nat Commun. 2014;5:3479. doi: 10.1038/ncomms4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang X, et al. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol. 2014;28:565–574. doi: 10.1210/me.2013-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng C, et al. SOCS1 hypermethylation mediated by DNMT1 is associated with lipopolysaccharide-induced inflammatory cytokines in macrophages. Toxicol Lett. 2014;225:488–497. doi: 10.1016/j.toxlet.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 100.Araki H, et al. Chromatin-modifying agents permit human hematopoietic stem cells to undergo multiple cell divisions while retaining their repopulating potential. Blood. 2007;109:3570–3578. doi: 10.1182/blood-2006-07-035287. [DOI] [PubMed] [Google Scholar]