Abstract
Evolution of metazoans resulted in the specialization of cellular and tissue function. This was accomplished by division of labor, which allowed tissue parenchymal cells to prioritize their core functions while ancillary functions were delegated to tissue accessory cells, such as immune, stromal, and endothelial cells. In metabolic organs, the accessory cells communicate with their clients, the tissue parenchymal cells, to optimize cellular processes, allowing organisms to adapt to changes in their environment. Here, we discuss tissue immunometabolism from this vantage point, and use examples from adipose tissues (white, beige, and brown) and liver to outline the general principles by which accessory cells support metabolic homeostasis in parenchymal cells. A corollary of this model is that disruption of communication between client and accessory cells might predispose metabolic organs to the development of disease.
Over the last 15 years, we have witnessed a renaissance in the fields of immunology and metabolism, which has given rise to the field of immunometabolism. This interdisciplinary field, which employs experimental approaches and paradigms from classical immunology and metabolism, can be divided into two subdisciplines: cellular immunometabolism and tissue immunometabolism. The former focuses on how changes in cellular metabolism support the cell fate decisions made by immune cells, including activation, proliferation, differentiation, and polarization, whereas the latter investigates how immune cells instruct tissue and systemic metabolism to support organismal adaptation to environmental challenges. While this review primarily focuses on tissue immunometabolism, we also discuss concepts from cellular immunometabolism to broaden the scope of presented findings and to highlight the interdependent progress made in these two disciplines.
Although evidence for crosstalk between immunity and metabolism has sporadically existed in the literature for quite some time (Ardawi and Newsholme, 1983; Hausberger, 1966; Williamson, 1901), the contemporary fields of cellular and tissue immunometabolism can trace their origins to a handful of pivotal papers. For cellular immunometabolism, the classic paper by Thompson and colleagues in 2002 demonstrated that the costimulation of T cells with anti-CD28 increased glucose uptake and glycolysis to support anaplerotic metabolism of proliferating lymphocytes (Frauwirth et al., 2002). Nearly all subsequent studies built upon the general principle established in this paper; i.e. extracellular signals control the uptake and metabolism of nutrients in immune cells. For example, it is now appreciated that the recognition of pathogen associated molecular patterns (PAMPs), the ligation of co-stimulatory or co-inhibitory molecules, or the activation of immune cells by cytokines results in changes in cellular metabolism that fuel the effector functions of immune cells (DeBerardinis and Thompson, 2012; Fox et al., 2005; O’Neill and Pearce, 2016; Pearce and Pearce, 2013). In some cases, these changes in cellular metabolism are simply permissive, whereas in others, they represent a metabolic checkpoint that needs to be cleared for full enactment of immune functions.
In a similar manner, the discovery by Hotamisligil and Spiegelman in 1993 that obesity results in increased expression of tumor necrosis factor-α (TNF) in adipose tissue to promote insulin resistance formed the cornerstone for the foundation of tissue immunometabolism (Hotamisligil et al., 1993). The explosive growth of this field, however, awaited the discovery by Ferrante and Chen in 2003 that adipose tissue of obese mice is infiltrated with macrophages that contribute to adipose tissue inflammation and insulin resistance (Weisberg et al., 2003; Xu et al., 2003). Since these initial discoveries in tissue immunometabolism, a large number of immune cells and pathways have been implicated in the maintenance of metabolic homeostasis in obese animals (Hotamisligil, 2006; Mathis, 2013; Odegaard and Chawla, 2013; Osborn and Olefsky, 2012). Rather than summarizing this extensive literature, we review tissue immunometabolism here from the vantage point of the development of metabolic organs and the licensing of their metabolic functions. Using examples from adipose tissues (white, beige, and brown) and liver, we propose that the seeding of metabolic organs by immune and stromal cells is temporally and spatially coordinated to support their physiologic functions during development and in the adult organism. A corollary of this hypothesis is that disruption of communication between the immune cells and their client cells, the tissue parenchymal cells, might contribute to the pathogenesis of metabolic diseases.
Tissue specialization in complex metazoans
One aspect of metazoan evolution is the progressive specialization of tissue function, both at the level of parenchymal cells that make up each individual tissue, and at level of tissue-resident accessory cells, such as immune cells and stromal cells (Okabe and Medzhitov, 2016). For example, in mammals, skeletal muscle (myocytes) generate force and movement, adipose tissue (adipocytes) store excess nutrients, liver (hepatocytes) provide nutrients during fasting and participate in xenobiotic detoxification, gastrointestinal cells (epithelial cells) support nutrient absorption, and immune cells (innate and adaptive) perform host surveillance functions. This specialization, also termed ‘division of labor,’ optimizes parenchymal cell function, but reduces their autonomy. This decrease in autonomy is evident by the supportive functions of accessory cells, such as stromal and immune cells, which have indispensable roles for maintaining physiologic tissue function and homeostasis. It is important to note that while these accessory cells cannot perform the bread-and-butter functions of parenchymal cells, such as generation of contractile force and movement (skeletal muscle), storage of excess nutrients (white adipocytes), generation of heat from uncoupled respiration (beige and brown adipocytes), and detoxification of xenobiotics and toxins (hepatocytes), they can optimize the functions of parenchymal cells to facilitate adaptations to environmental challenges. Although less well appreciated, immune cells in metabolic organs likely also perform their classical functions in host defense by carrying out surveillance of metabolic tissues, which are discussed later in this review.
An examination of simple metazoans, Caenorhabditis elegans and Drosophila melanogaster, nicely highlights the principle of tissue specialization during vertebrate evolution. Although C. elegans and D. melanogaster lack the mammalian equivalents of adipocytes, hepatocytes, and professional immune cells, they retain the conserved nutrient and pathogen sensing pathways found in mammals and other vertebrates. The epithelial cells that form the external and internal barrier surfaces of C. elegans are thus multi-functional (Shivers et al., 2008) (Tan and Shapira, 2011). For example, in C. elegans, which feed on bacterial blooms found on decaying organic matter, the intestinal tract is in immediate contact with commensal and pathogenic microbial species, and performs multiple functions, including nutrient absorption, storage, toxin detoxification, and immune surveillance (Sifri et al., 2005). To perform these varied functions, the intestinal epithelium expresses lipid synthases and lipases to store and mobilize lipids, antimicrobial and cytoprotective proteins for host defense, and various enzymes for detoxification of ingested toxins and xenobiotics (Jones and Ashrafi, 2009; Melo and Ruvkun, 2012; Pellegrino et al., 2014). In a similar manner, the fat body of D. melanogaster, which is distributed throughout the insect body, performs multiple metabolic and immune functions. In addition to being the primary site of nutrient storage, the fat body plays an important role in regulating systemic metabolism through the secretion of hormones and lipases, produces antimicrobial peptides, and participates in the detoxification of xenobiotics and nitrogen waste (Arrese and Soulages, 2010; Ferrandon et al., 2007; Palanker et al., 2009). However, unlike C. elegans, D. melanogaster has innate immune cells termed hemocytes, which provide cell-mediated immunity against pathogens via phagocytosis (Charroux and Royet, 2009). Thus, functions that are partitioned into multiple cell types and tissues (adipocytes, hepatocytes, intestinal epithelial cells and, innate and adaptive immune cells) in mammals are performed by a single cell type/tissue in C. elegans or two cell types/tissue in D. melanogaster, suggesting that tissue specialization occurred progressively over the course of evolution, as the size, lifespans, and complexity of organisms increased.
Cellular architecture of metabolic organs
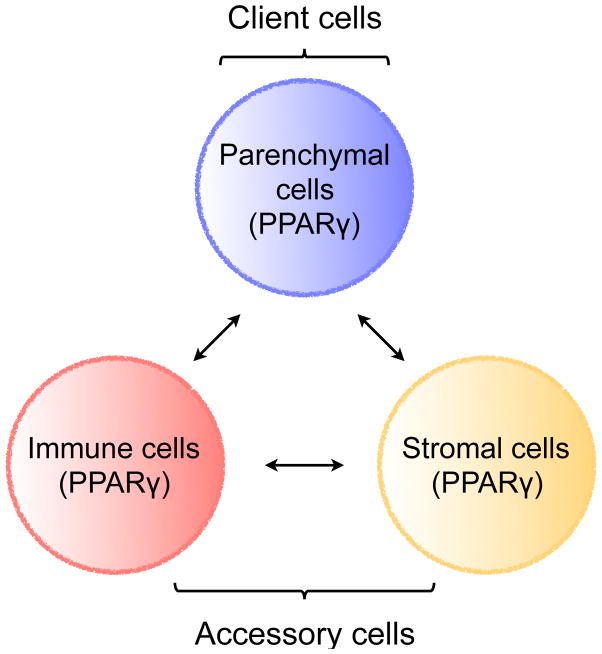
As a consequence of division of labor, the various cell types that make up metabolic tissues need to collaborate and communicate with each other in order to perform their physiologic functions. The trio of tissue parenchymal, stromal, and immune cells forms the basic functional unit of metabolic organs (Figure 1). While the parenchymal cells (white, beige or brown adipocytes, hepatocytes, skeletal myocytes, and enterocytes) perform the core metabolic functions, tissue resident stromal and immune cells carry out accessory functions to support their clients, the parenchymal cells. A number of factors, including chemokines, cytokines, growth factors, hormones, and small molecules, mediate communication between parenchymal, immune, and stromal cells. In addition, expression of common sensors of the metabolic state allows for coordinated sensing of metabolites across the three cell types. For example, in adipose tissue, this is mediated by PPARγ, which senses fatty acids and fatty acid metabolites to coordinate programs across parenchymal, immune, and stromal cells (Figure 1)
Figure 1. The core functional unit of metabolic organs.
Metabolic organs, such as WAT, BAT, beige fat, and liver, are seeded by immune and stromal cells during development. These accessory cells support their clients, the tissue parenchymal cells. Communication between client and accessory cells is mediated by chemokines, cytokines, growth factors, hormones, small molecules, and direct contact. In addition, expression of common sensors of the metabolic state allows for coordinated sensing of metabolites across the three cell types. For example, in adipose tissue (designated by parentheses), communication between parenchymal, immune, and stromal cells is mediated by PPARγ, which coordinates cellular responses by sensing changes in fatty acids and fatty acid metabolites.
The tissue stromal cells consist of mesenchyme-derived mural cells, pericytes, and fibroblasts, whereas macrophages, innate lymphoid cells (ILCs), and regulatory T cells (Tregs) primarily comprise the group of tissue resident immune cells. While certain basal functions are performed by accessory cells in all metabolic organs, such as production of extracellular matrix (ECM), host surveillance, and the clearance of apoptotic cells, other functions of accessory cells are deployed in a tissue-specific manner. Although our understanding of how stromal and immune cells support the tissue-specific functions of parenchymal cells is incomplete, some available examples from adipose tissue growth and remodeling nicely highlight this general principle. For example, stromal cells are recruited to give rise to white or beige adipocytes when mice are challenged with a higher intake of nutrients or environmental cold, respectively (Berry et al., 2014; Berry and Rodeheffer, 2013; Lee et al., 2012). In a similar manner, exposure to environmental cold stimulates the recruitment and alternative activation of macrophages to enhance the adrenergic tone of inguinal WAT (Nguyen et al., 2011), which supports the biogenesis of thermogenic beige fat (Qiu et al., 2014).
Communication between parenchymal, stromal, and immune cells is critical for the maintenance of metabolic homeostasis. A central question then arises: how is this communication established and maintained between these three cell types in various metabolic tissues? Potential insights into this question can be obtained from examining the ontogeny and development of tissue resident macrophages. In mammals, nearly all organs are populated with tissue-resident macrophages, which are seeded during development by precursor cells derived from the yolk-sac, fetal liver, or the bone marrow (Geissmann et al., 2010; Ginhoux and Guilliams, 2016; Lavin et al., 2015). While the commitment to the macrophage lineage is regulated by the ets transcription factor PU.1, environmental signals impart tissue-specific functional characteristics on these resident cells (Ghisletti et al., 2010; Heinz et al., 2010). Local environmental signals (retinoic acid in the peritoneum, fatty acids in adipose tissue, surfactants in lung, and oxysterols in liver and spleen) induce and/or activate regulatory transcription factors (GATA6 in peritoneal macrophages, PPARγ in the adipose tissue and lung macrophages, and LXRα in Kupffer cells and splenic marginal zone macrophages) that cooperate with the master regulator PU.1 to activate and maintain tissue-specific macrophage enhancers, which confer tissue-specific characteristics onto these macrophages (Gosselin et al., 2014; Lavin et al., 2014). In support of this, genetic deletion of these regulatory factors impairs the development of tissue-resident macrophages at their respective sites (Gautier et al., 2014; N et al., 2013; Odegaard et al., 2007; Okabe and Medzhitov, 2014; Rosas et al., 2014; Schneider et al., 2014). Thus, the activation of tissue-specific enhancers by transcription factors not only provides a mechanism for establishing heterogeneity in tissue macrophages, but also serves as a means by which these cells can functionally support their client cells, the parenchymal cells. For example, Kupffer cells and adipose tissue macrophages (ATMs) might use LXRα and PPARγ to sense flux of oxysterols and fatty acids, respectively, allowing for coordinated sensing of lipids across client and accessory cells in liver and adipose tissue, respectively.
Tissue resident stromal cells are derived from the mesenchyme and widely dispersed in mammalian organs. Similar to tissue resident immune cells, stromal cells are heterogeneous, express tissue-specific markers, and populate organs during embryogenesis. However, unlike tissue resident immune cells, stromal cells are not terminally differentiated and display a great deal of plasticity in their capacity to proliferate and differentiate. In their basal state, these mesenchyme-derived cells can be found wrapped around endothelial cells of capillaries and venules, where they are referred to as pericytes, mural cells or stellate cells (in the liver), or they can be dispersed amongst the parenchyma of organs. Upon injury or tissue damage, stromal cells rapidly proliferate, and in response to environmental cues, can give rise to fibroblasts and/or adipocytes. Although some studies have suggested that stromal cells can differentiate into other cell lineages, these data are less conclusive and have not been confirmed by in vivo fate-mapping studies (Nombela-Arrieta et al., 2011).
While little is known about the signaling and transcriptional pathways that confer tissue specificity to stromal cells, these cells support the functions of parenchymal, immune, and tissue stem cells. In their basal state, stromal cells produce ECM, growth factors (IGF-1, Wnts, BMP antagonists), and cytokines (M-CSF), which provides structural support to the parenchyma and trophic factors for survival and proliferation of parenchymal, immune, and tissue stem cells. Upon injury or tissue damage, stromal cells rapidly proliferate, giving rise to a population of fibroblasts that secrete ECM components, chemokines, cytokines, and matrix metalloproteinases (MMPs), which facilitate the recruitment, retention and activation of immune cells, and provide a scaffold for the regeneration of the damaged tissue. For example, fibroadipogenic progenitors (FAPs) are stromal cells in the skeletal muscle, which are found next to the skeletal muscle stem cells, the satellite cells (Joe et al., 2010; Uezumi et al., 2010). After injury, FAPs rapidly proliferate and release chemokines and cytokines to enhance recruitment of immune cells to remodel damaged tissue and restore its functionality. FAPs also are the major source of IL-6 and IGF-1, which support the proliferation of satellite cells and their differentiation into myocytes, respectively.
Physiologic control of client cell metabolism by accessory cells
In the sections below, we will use the developmental model of functional organization to discuss how physiologic homeostasis is maintained in adipose depots (white, beige, and brown) and liver. Although similar principles likely apply to skeletal muscle myocytes and small intestine enterocytes, our knowledge on how immune and stromal cells support the metabolic functions of these organs is currently limited.
White Adipose Tissue
The primary function of white adipose tissue (WAT) is in nutrient storage and energy balance (Rosen and Spiegelman, 2014). The parenchymal cells of this tissue, white adipocytes, take up lipids from the circulation and store them within a large cytoplasmic lipid droplet. These cells increase storage of lipids when nutrient intake by the organism exceeds its energetic requirements. However, when nutrient intake is insufficient to meet energetic demands, adipocytes engage in lipolysis to mobilize the stored lipid into the circulation, allowing other tissues to use it as a fuel (Young and Zechner, 2013). In addition to serving as the professional lipid storing cells, white adipocytes secrete a variety of adipokines (leptin, adiponectin, and resistin), lipokines (palmitoleic acid), and chemokines (Ccl2), which coordinate local and systemic metabolic homeostasis (Schwartz and Lazar, 2011; Stern et al., 2016; Yamauchi and Kadowaki, 2013).
Although WAT depots are present in number of discrete locations (perigonadal, inguinal, perirenal, and mesenteric), their developmental origin can be traced back to the mesenchyme (Rosen and Spiegelman, 2014). In rodents, WAT generally develops after birth with the inguinal depots emerging before the perigonadal depots (Han et al., 2011; Hong et al., 2015; Jiang et al., 2014; Wang et al., 2013b). In nearly all of these depots, the terminal differentiation to adipocytes is regulated by the nuclear receptor PPARγ, which cooperates with C/EBPα to control nearly every facet of adipocyte metabolism (Cristancho and Lazar, 2011; Rosen and Spiegelman, 2014; Tontonoz et al., 1994; Umek et al., 1991). Since the transcriptional activity of PPARγ is gated by ligands, in particular by dietary and endogenous fatty acids, it functions as a nutrient sensor to coordinate cellular programs of energy storage and utilization (Chawla et al., 2001; Evans et al., 2004). Consistent with this, genetic deletion or loss of function mutations in PPARγ result in lipoatrophy and lipodystrophy in mice and humans, respectively (Semple et al., 2006; Wang et al., 2013a).
The housekeeping functions of adipocytes in nutrient storage and mobilization are supported by tissue resident stromal and immune cells. The stromal cells present in white adipose depots have been identified as PDGFRβ+ mural cells, PDGFRβ+ pericytes, and PDGFRα+ mesenchymal cells (Berry and Rodeheffer, 2013; Joe et al., 2009; Lee et al., 2012; Tang et al., 2008). Although there is considerable heterogeneity in expression of PDGFRα or β amongst the stromal cell types in the various WAT depots (Berry et al., 2014; Jiang et al., 2014), these cells support parenchymal cell functions in multiple ways. First, they form a pool of precursor cells, which can give rise to new adipocytes when excess energy storage capacity is needed or for the homeostatic replacement of dying adipocytes. This differentiation of stromal cells into adipocytes is regulated by the fatty acid sensor PPARγ (Rosen and Spiegelman, 2014) (Figure 1). Second, these cells secrete ECM that provides structural integrity to adipose tissue and forms a scaffold for the differentiation of adipocytes. Third, stromal cells are an important source of growth factors (IGF-1, M-CSF), chemokines and cytokines, which modulate the recruitment, survival and activation of immune cells. These basal and homeostatic functions of WAT stromal cells have been mostly discerned from studies in which mice were challenged with high fat diet or environmental cold. However, it is likely that stromal cells also participate in maintaining WAT homeostasis during other environmental conditions, such as infection, fasting, and tissue injury. To address some of these additional supportive functions, it will be important to understand the molecular basis for heterogeneity of WAT stromal cells, and identify signaling pathways that control their proliferation and differentiation into adipocytes and ECM-producing fibroblasts.
The resident immune cell population of WAT includes a variety of innate and adaptive immune cells, including macrophages, eosinophils, group 2 innate lymphoid cells (ILC2s), and conventional and regulatory T (Tregs) cells (Mathis, 2013; Odegaard and Chawla, 2013). Research over the last decade has shed light on the important roles of these cells in adipose tissue homeostasis. Resident macrophages support the structural integrity of the tissue by remodeling extracellular matrix, phagocytosing dying adipocytes, and elaborating anti-inflammatory cytokines (Odegaard and Chawla, 2013; Wernstedt Asterholm et al., 2014). In lean animals, the network of ILC2s, eosinophils, and to a lesser extent, Th2 cells secrete type 2 cytokines, such as IL-4, IL-5, and IL-13, which perform three important functions (Odegaard and Chawla, 2013). First, they regulate the type 2 program of alternative macrophage activation (M2) and support the survival of eosinophils in perigonadal WAT (Molofsky et al., 2013; Odegaard et al., 2007). Second, IL-4/13 signaling is critical for postnatal expansion of PDGFRα+ stromal cells, illustrating the dynamic crosstalk between immune and stromal cells in WAT (Lee et al., 2015a). Third, this cytokine milieu supports insulin responsiveness and other metabolic characteristics of healthy WAT, as evidenced by the reduction in these parameters upon genetic deletion of these type 2 cytokines or disruption of cells that secrete them (Bapat et al., 2015; Cipolletta et al., 2012; Molofsky et al., 2013; Odegaard et al., 2007).
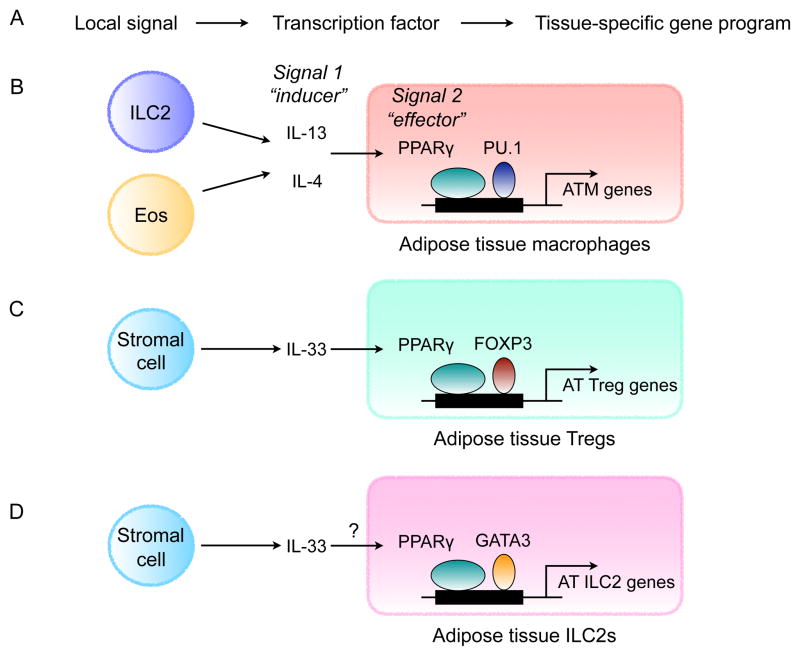
How do tissue resident immune cells support the metabolic functions of the parenchymal adipocytes in WAT? We propose a model in which two signals cooperate to establish the tissue- specific programs of immune cells that fine tune nutrient storage and release by adipocytes. In this model, signal 1 (the “inducer”) is a cytokine or growth factor that instructs the survival, proliferation and/or activation of immune and stromal cells (Figure 2A). For example, IL-5 from ILC2s regulates survival and proliferation of eosinophils, IL-4 from eosinophils and IL-13 from ILC2s support alternative activation of macrophages and proliferation of PDGFRα+ stromal cells, and IL-33 from stromal cells regulates the expansion of Tregs and ILC2s in WAT (Kolodin et al., 2015; Lee et al., 2015a; Molofsky et al., 2013; Nussbaum et al., 2013; Vasanthakumar et al., 2015; Wu et al., 2011). In addition to regulating their survival and expansion, signal 1 induces the expression of a tissue-specific transcription factor (signal 2, the “effector”) that cooperates with a lineage-specific factor to induce expression of tissue-specific programs in immune cells (Figure 2A). For immune cells in WAT, the tissue-specific factor is PPARγ, which is induced by IL-4 in adipose tissue macrophages (Figure 2B) and by IL-33 in adipose tissue Tregs (Figure 2C) (Huang et al., 1999; Vasanthakumar et al., 2015). Although ILC2s are known to express PPARγ and ST2 (the receptor for IL-33) (Molofsky et al., 2015; Robinette et al., 2015), it is not known whether IL-33 directly induces the expression of PPARγ in the WAT ILC2s (Figure 2D). Since the transcriptional activity of PPARγ is gated by fatty acids and fatty acid metabolites (Chawla et al., 2001), immune cells can sense the changes in the flux of fatty acids in adipose tissue, thereby optimizing their ability to work together with their client cells, the adipocytes, in maintenance of fatty acid homeostasis. For example, PPARγ transcriptionally regulates programs of lipogenesis and oxidative metabolism in adipocytes (Way et al., 2001; Wilson-Fritch et al., 2003; Wilson-Fritch et al., 2004), metabolic programs that it also controls in ATMs (Odegaard et al., 2007). Thus, by using PPARγ as a nutrient sensor in immune cells, the division of labor does not compromise tissue functionality but rather enhances it, as it allows for coordination amongst the different cell types by sensing of metabolites. In support of this idea, deletion of inducing signals (IL-4/13 or IL-33) or effector (PPARγ) impairs the survival and maturation of adipose tissue immune cells, including macrophages, Tregs, and ILC2s, and the maintenance of metabolic homeostasis in WAT (Bapat et al., 2015; Cipolletta et al., 2012; Molofsky et al., 2013; Odegaard et al., 2007).
Figure 2. Tissue-specific programming of immune cells in WAT.
(A) Local signals (the “inducers”) induce expression of transcription factors (the “effectors”) that confer tissue-specific identity to immune cells.
(B) Maturation of adipose tissue macrophages (ATMs) is dependent of type 2 cytokines, IL-4 and IL-13, and PPARγ. ILC2-derived IL-13 and eosinophil-derived IL-4 signal via the IL-4Rα in ATMs to induce PPARγ, which cooperates with lineage-specific transcription factor PU.1 to direct expression of adipose tissue-specific gene expression in ATMs.
(C) Maturation of adipose tissue (AT) Tregs is dependent on IL-33 and PPARγ. Stromal cell-derived IL-33 signals via ST2, the receptor for IL-33, to induced expression of PPARγ in Tregs, which cooperates with lineage-specific transcription factor FOXP3 to direct transcriptional programs in adipose tissue Tregs.
(D) Maturation of ILC2s in adipose tissue. ILC2s are known to express PPARγ, however, it is not known whether its expression is regulated by IL-33. IL-33 and ST2 are required for the maintenance of adipose tissue ILC2s. PPARγ might cooperate with GATA3, which is necessary for the development of ILC2s, to regulate adipose tissue-specific gene expression in ILC2s.
Beige Adipose Tissue
Upon exposure to environmental cold or adrenergic stimulation, certain white adipose tissues in mice, such as inguinal or subcutaneous WAT, gain competence for thermogenesis by inducing the expression of uncoupling protein-1 (UCP-1) (Harms and Seale, 2013; Rosen and Spiegelman, 2014). Although this phenomenon was known for decades, it was presumed that the clusters of UCP-1+cells present in WAT were ectopically recruited brown adipocytes. However, fate-mapping and genome-wide profiling studies have revealed that the recruited UCP-1+ cells in WAT are developmentally and transcriptionally distinct from brown adipocytes (Seale et al., 2011; Wu et al., 2012). These cells have now been named “beige” or “brite” adipocytes (Harms and Seale, 2013; Wu et al., 2012), and their recruitment upon cold exposure can contribute up to 10–15% of total body energy expenditure (Qiu et al., 2014; Shabalina et al., 2013). Since beige adipocytes can prevent and ameliorate established obesity in thermoneutral mice (Cohen et al., 2014; Qiu et al., 2014), there is great interest in understanding their developmental origins, functions, and mechanisms of recruitment in mice and humans.
Developmental origins of beige adipocytes are complex, involving contributions from PDGFRα+ stromal cells, PDGFRβ+ stromal cells, and smooth muscle cells (Berry et al., 2016; Lee et al., 2015a; Lee et al., 2012; Long et al., 2014; Vishvanath et al., 2016). These findings suggest that either there is great deal of plasticity in expression of these fate mapping markers (PDGFRα, PDGFRβ and Myh11) or the recruited beige fat in the adult mice is mosaic in its origins. An additional variable that has been largely overlooked is the time at which beige adipocytes appear in inguinal WAT. While most studies have focused on recruitment of beige adipocytes in adult animals after exposure to environmental cold, the inguinal WAT undergoes physiologic browning at the time of weaning (postnatal day (P) 21), suggesting that the commitment of precursor cells to the beige fat lineage likely precedes expression of UCP1 in the inguinal WAT on P21 (Lee et al., 2015a; Odegaard et al., 2016). Thus, it will be important to repeat the fate-mapping studies in younger animals to ascertain the precise contribution of PDGFRα+ stromal cells, PDGFRβ+ stromal cells, and smooth muscle cells to beige adipocytes that are recruited in the adult.
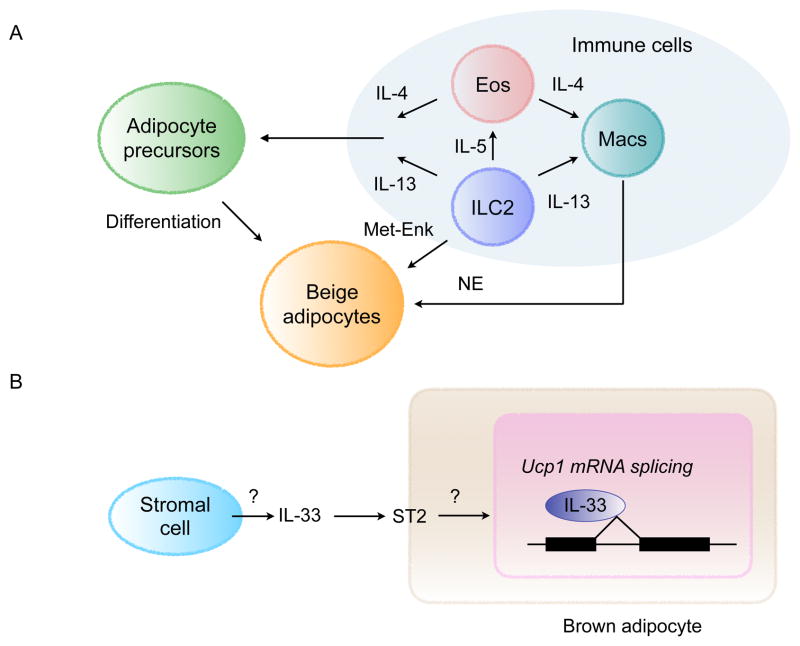
The physiologic and cold-induced recruitment of beige adipose tissue nicely illustrates how intercellular crosstalk orchestrates the development and activation of beige adipocytes (Figure 3A). While commitment to the beige fat lineage requires communication between immune and stromal cells, crosstalk between immune and parenchymal cells regulates the thermogenic activity of beige adipocytes. For example, gain- and loss-of-function studies have revealed that ILC2-derived IL-13 and eosinophil-derived IL-4 stimulate the proliferation and commitment of PDGFRα+ stromal cells to the beige fat lineage (Lee et al., 2015a). In addition, these type 2 cytokines are required for the alternative activation (M2) of ATMs to support the adrenergic tone of this tissue and the terminal differentiation of stromal cells to beige adipocytes (Nguyen et al., 2011; Qiu et al., 2014). These supportive functions of ATMs are dependent on their capacity to produce norepinephrine via tyrosine hydroxylase, which promotes the differentiation and thermogenic activation of beige adipocytes (Qiu et al., 2014). While myeloid cell-derived catecholamines are beneficial in the adaptation to environmental cold, they can also be harmful, as was observed in the setting of autoimmune encephalomyelitis, a mouse model of multiple sclerosis (Shaked et al., 2015). In addition to norepinephrine production by ATMs, activation of ILC2s by IL-33 stimulates production of methionine-enkephalin peptides, which induce beige fat biogenesis by an unclear mechanism (Brestoff et al., 2015).
Figure 3. Interactions between immune and stromal cells regulate thermogenesis.
(A) A model of how interactions between immune and stromal cells regulate biogenesis of beige fat. Type 2 immune cells, including ILC2s, eosinophils (Eos), and alternatively activated macrophages (Macs), communicate with stromal cells and beige adipocytes to regulate remodeling of inguinal WAT into thermogenic beige fat. Signaling via the IL-4Rα in macrophages regulates catecholamine biosynthesis and secretion. IL-4/13 signaling in PDGFRα+ cells regulates the numbers and fate of adipocyte precursors in WAT. Differentiation and activation of beige adipocytes is stimulated by myeloid-cell derived norepinephrine (NE) and ILC2-derived methionine-enkephalin (Met-Enk).
(B) IL-33 and ST2 regulate licensing of beige and brown adipocytes for uncoupled respiration. IL-33 is an atypical cytokine that is normally located in the nucleus. Its release from cells results in extracellular cleavage by proteases derived from mast cells and neutrophils. Cleaved IL-33 can bind to cell surface receptor ST2 to initiate downstream responses via the adaptor protein MyD88. In BAT, IL-33 and ST2 are required for perinatal licensing of uncoupled respiration by controlling the splicing of Ucp1 mRNA. Although it is not known how IL-33 and ST2 regulate the splicing of Ucp1 mRNA, it is likely to be a non-canonical function of IL-33 because it does not require the adaptor protein MyD88. While it is likely that stromal cells are the cellular source of IL-33, this remains to be determined.
This cellular circuit of immune and stromal cells is likely to be a core mechanism by which mice recruit additional thermogenic capacity upon cold exposure, because a number of other browning pathways converge upon it. First, the browning effects of the myokine meterorin-like (Metrnl) require an intact type 2 cytokine signaling pathway, including eosinophils and alternatively activated (M2) macrophages, to increase adrenergic and thermogenic activity of the tissue (Rao et al., 2014). Second, a similar requirement for alternatively activated (M2) macrophages has been observed for adiponectin-mediated beige fat recruitment during prolonged cold exposure (Hui et al., 2015). Third, microbiota depletion has been shown to promote browning of WAT by inducing type 2 cytokine production (IL-4, IL-5, and IL-13), eosinophil recruitment, and alternative macrophage activation (Suarez-Zamorano et al., 2015). However, there is one caveat that one needs to keep in mind when interpreting the results from the depletion of host microbiota. Since cellular metabolism in prokaryotes and eukaryotes generates heat as a by-product, depletion of the microbiome might decrease obligatory heat production in mice, necessitating an increase in beige fat thermogenesis to maintain thermal balance.
Brown Adipose Tissue
While WAT is the primary site for energy storage in mammals, brown adipose tissue (BAT) participates in energy expenditure to generate heat, allowing mammals to adapt to colder environments (Cannon and Nedergaard, 2004; Gordon, 1993; Lowell and Spiegelman, 2000). This unique capacity of BAT is dependent on UCP-1 (initially called thermogenin), which dissipates the mitochondrial H+ gradient to allow for fast substrate oxidation without the need for ATP generation. As a result of this increase in metabolic rate, heat is generated as a by-product, which is dissipated throughout the animal to maintain the core temperature. The presence of thermogenic BAT in mammals has generally been suggested to be the basis for homeothermy, i.e. the maintenance of stable core temperature regardless of the ambient temperature. However, neonatal mice are unable to defend their body temperature and are essentially poikilothermic: their body temperature closely tracks with the ambient environmental temperature (Lagerspetz, 1962). While the defense of body temperature in cooler environments improves considerably by 2 weeks of age, homeothermy is achieved around 3 weeks of age in mice (Lagerspetz, 1962). Since neonatal mice primarily use behavioral thermoeffectors (huddling, nesting, and consuming warm milk) for thermoregulation (Gordon, 1993), it suggests that energy-consuming BAT is less active and these animals primarily use the available energy to accelerate their growth to maturity.
Although it was initially thought that white and brown adipocytes have similar developmental origins, fate-mapping studies and genomic analysis have revealed that brown adipocytes have distinct developmental origins, which they share with skeletal muscle. For example, fate-mapping with Myf5- or Pax7-reporter mice shows that brown but not beige adipocytes arise from a Myf5+/Pax7+ myogenic precursors (Lepper and Fan, 2010; Seale et al., 2008; Seale et al., 2011). At a genome-wide level, transcriptional and proteomic signatures of brown adipocytes are also more similar to those of skeletal muscle than white adipocytes (Forner et al., 2009; Timmons et al., 2007), findings that are consistent with the increased reliance of brown adipocytes on oxidative metabolism. While the differentiation of brown adipocytes is regulated by transcriptional cascades involving PPARγ and C/EBPα (Rosen and Spiegelman, 2014), PR-domain containing protein-16 (Prdm16) and early B cell factor-2 (Ebf2) are necessary for the maintenance of brown adipocytes in adult animals (Harms et al., 2014; Rajakumari et al., 2013). In line with this, deletion of Prdm16 or Ebf2 results in suppression of brown fat gene expression and function, but not its specification during embryogenesis, indicating that these two transcriptional regulators are primarily required for the maintenance of brown adipose tissue.
At present, our knowledge of how brown adipocytes interact with parenchymal and immune cells is limited. Although BAT contains a resident population of innate (macrophages and eosinophils) and adaptive (Tregs) immune cells (Medrikova et al., 2015; Nguyen et al., 2011; Tian et al., 2016), their supportive roles in the maintenance of brown fat functions are poorly understood. For example, BAT contains a unique population of Tregs, whose depletion results in macrophage infiltration and BAT inflammation without significantly altering its thermogenic functions (Medrikova et al., 2015). In contrast, two studies have identified PDGFRα+ and Sca-1+ stromal cells in adult BAT, which can differentiate into brown adipocytes (Lee et al., 2015b; Schulz et al., 2011). Since the cell surface marker profile of PDGFRα+ cells in BAT is similar to that of stromal cells in inguinal WAT, it suggests that homeostatic replacement of brown adipocytes in adult animals might occur through a different mechanism than its development during embryogenesis, which depends on Myf5+ myogenic precursor cells.
A recent study demonstrated a unique requirement for an immune signal, which likely comes from stromal cells, to license BAT for its postnatal functions in thermogenesis (Figure 3B). In placental mammals, although BAT develops during embryogenesis, its thermogenic functions are only needed for the maintenance of core temperature during postnatal life. This one-way transition from intrauterine to extrauterine life requires that uncoupling thermogenesis be licensed in BAT. This function is uniquely performed by IL-33 and its receptor ST2 during the perinatal period in mice (Odegaard et al., 2016). Unlike other cytokines, IL-33 is a nuclear cytokine that is chiefly expressed in stromal cells and thought to be released into the extracellular environment by necroptosis or mechanical stretch (Cayrol and Girard, 2014; Molofsky et al., 2015). In immune cells, binding of IL-33 to its receptor ST2 initiates canonical downstream responses via the adaptor protein MyD88. In contrast, during the perinatal period, IL-33 and ST2, but not MyD88, are required for normal splicing of Ucp1 mRNA, which is necessary for expression of a functional UCP1 protein in BAT (Odegaard et al., 2016). In line with this, genetic deletion of IL-33 or ST2 results in complete loss of UCP1 protein in BAT, rendering mice susceptible to cold-induced hypothermia. Although the precise mechanisms by which IL-33 and ST2 regulate splicing of Ucp1 mRNA are not known, these findings suggest a non-canonical model in which extracellular IL-33 might signal via ST2 in brown adipocytes to induce expression of nuclear IL-33 to control splicing of Ucp1 mRNA (Figure 3B). Since a similar requirement of IL-33 and ST2 in splicing and expression of UCP1 is observed in beige adipocytes, it suggests that licensing of thermogenesis is also a critical checkpoint that needs to be cleared for postnatal development of beige fat.
Liver
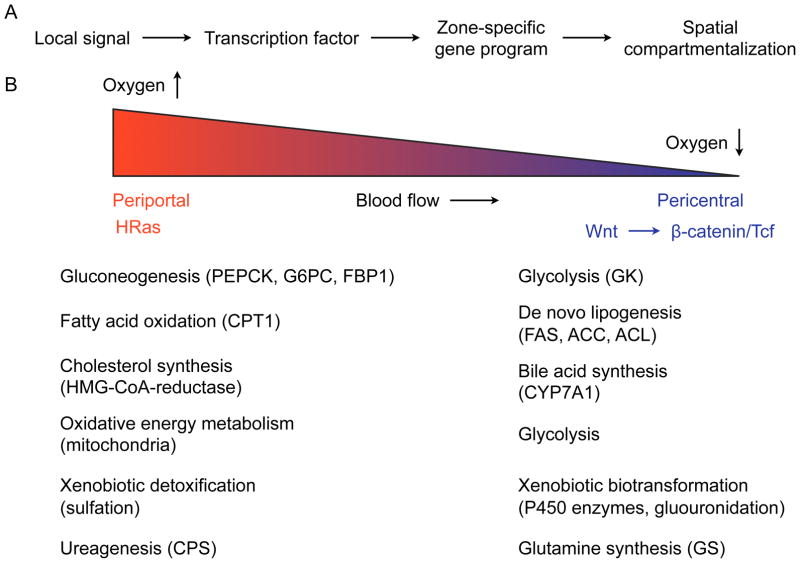
The liver is a major metabolic organ that participates in the maintenance of carbohydrate, protein, and lipid metabolism, and iron homeostasis. In addition, the liver secretes hormones (IGF-1, hepcidin), synthesizes proteins (coagulation factors, albumin), and is a key site for the detoxification of xenobiotics and toxins (Si-Tayeb et al., 2010). These synthetic and degradative functions of liver are compartmentalized into zones often referred to as ‘metabolic zonation’ (Gebhardt, 1992; Jungermann, 1988). The two major metabolic zones of the liver lie along the porto-central axis of the hepatic lobules, which form the functional unit of the liver. The periportal zone is the region near the hepatic triad of portal vein, hepatic artery and bile duct, and receives nutrients from the portal vein and oxygen from the hepatic artery. At the other end of the lobule is the pericenteral region, which is adjacent to the central vein and receives the least oxygenated blood (Figure 4A, B). Studies dating back almost half a century have noted considerable spatial and functional heterogeneity in hepatocytes along the porto-central axis (Katz et al., 1976). The periportal zone has higher expression of enzymes necessary for gluconeogenesis, fatty acid oxidation and urea synthesis, whereas the hepatocytes in the pericentral zone predominantly participate in glutamine, fatty acid, ketone and bile acid synthesis, and in glycolysis (Gebhardt and Matz-Soja, 2014; Jungermann, 1988) (Figure 4B). This spatial heterogeneity in hepatocyte metabolism is maintained by factors that control zonation of the liver, including Wnt/β-catenin and Ha-RAS pathway (Benhamouche et al., 2006; Gebhardt et al., 2007). While basal metabolic functions are highly zonated, metabolic stress results in recruitment of additional hepatocytes in adjacent zones to maintain homeostasis. This is analogous to the recruitment of additional myofibers for the generation of larger contractile force by skeletal muscles.
Figure 4. Metabolic and immune zonation of liver.
(A) Model of how metabolic and immune functions might be zonated in the liver. (B) Metabolic zonation of liver. In the periportal region, portal vein and hepatic artery provide nutrients and oxygen, respectively, whereas the pericentral region is oxygen-poor. In general, energy consuming catabolic functions are localized to the periportal region, whereas anabolic functions are performed by hepatocytes in the pericentral region. Pericentral zonation is regulated by the Wnt-β-catenin/Tcf pathway, whereas the HRas pathway controls the zonation program around the periportal region. Enzymes or enzymatic activities that show preferential localization to the periportal or pericentral zones are listed next to the metabolic programs they participate in. PEPCK- posphoenolpyruvate carboxy- kinase, G6PC- glucose-6- phosphatase, FBP1- fructosel,6-bisphosphatase, GK-glucokinase, CPT1- carnitine palmitoyltransferase I, FAS-fatty acid synthase, ACC- acetyl-CoA carboxylase, ACL- ATP-dependent citrate lyase, CPS- Carbamoylphosphate synthetase, GS- Glutamine synthetase.
In addition to this spatial zonation of metabolic functions, the circadian clock imposes a temporal rhythm on hepatocyte gene expression that synchronizes hepatocyte metabolism with the daily rhythms of feeding-and-fasting (Bass and Takahashi, 2010). These two mechanisms allow biochemically incompatible reactions or futile metabolic cycles to be separated in space and time. For example, gluconeogenesis is higher during fasting and is localized to the periportal region, whereas glycolysis predominates during the fed state and occurs in the pericentral region. A similar separation between catabolic (periportal) and anabolic (pericentral) reactions is observed for cholesterol, fatty acid, and glutamine metabolism.
If the metabolic functions of hepatocytes (the client cells) are gated by zonation, then it follows that the supportive functions of accessory cells, Kupffer and stellate cells, might also exhibit a similar pattern of zonation to form a functional unit. Although evidence in support of this is currently lacking, there are suggestions in the literature that Kupffer cells might exhibit a similar zonal distribution, which functionally aligns them with their client cells. First, Kupffer cells are more dominant in the periportal region, which is high in nutrients and oxygen (Gebhardt, 1992). Second, the metabolic dichotomy of macrophages, i.e. classically activated (M1-type) are glycolytic and alternatively activated (M2-type) are oxidative (O’Neill and Pearce, 2016; Vats et al., 2006), nicely follows the oxygen gradient along the porto-central axis; oxygen tension is higher in the periportal area to support oxidative phosphorylation and lower in the pericentral zone where glycolysis predominates. This observation suggests that the metabolic alignment of accessory cells (Kupffer cells) with their client cells (hepatocytes) might result in their zonal distribution with M2-type Kupffer cells localized to the periportal regions and M1-type near the pericentral zones. In support of this idea, PPARδ has been shown to regulate the alternative (M2) activation of Kupffer cells, which in turn can support oxidative metabolism in hepatocytes (Kang et al., 2008; Odegaard et al., 2008). Third, genome-wide profiling of tissue macrophages has revealed enrichment for transcription factors in Kupffer cells that are involved in parenchymal cell metabolism. For example, LXRα and RXRα, which are sensors of oxysterols and retinoids (derivatives of Vitamin A), respectively (Chawla et al., 2001), are highly enriched in Kupffer cells (Kierdorf et al., 2015), indicating they might regulate liver-specific programs in these cells. Since LXRα- and RXRα-mediated degradation of cholesterol into bile acids via CYP7A occurs in pericentral hepatocytes (Peet et al., 1998; Wan et al., 2000), these findings suggest that the supportive functions of LXRα+ Kupffer cells in cholesterol homeostasis might be localized to the pericentral region.
Although the parenchymal or stromal cell signals (the inducers) that control tissue-specific adaptations of Kupffer cells are not known, one can build a general model based on transcription factors that regulate liver metabolic programs, which exhibit high degree of zonation. For example, gluconeogenesis and fatty acid oxidation are enriched in the periportal region, functions that are transcriptionally coordinated by FoxO1, CREB, and PPARδ in hepatocytes (Lin and Accili, 2011). These transcription factors also control the M2 program of alternative macrophage activation (Chung et al., 2016; Kang et al., 2008; Luan et al., 2015; Odegaard et al., 2008), raising the possibility that M2 macrophages in the periportal region might support stimuli driven gluconeogenesis and fatty acid oxidation in hepatocytes. In a similar manner, fatty acid and bile acid synthesis are performed by hepatocytes in the pericentral zone, anabolic programs that are transcriptionally coordinated by Srebp-1c and LXRα, respectively (Brown and Goldstein, 1999; Kalaany and Mangelsdorf, 2006). Based on the proposed model, enrichment of Srebp-1c and LXRα in pericentral Kupffer cells, the former being the transcriptional target of the latter (Repa et al., 2000), will provide a mechanism by which these cells might support the synthetic functions of hepatocytes. Although the zonation pattern for iron metabolism genes is not known, the liver is a major site of iron storage and mobilization (Andrews, 2008). In this context, the enrichment of Spi-C in Kupffer cells, a transcription factor that is induced by heme and regulates the recycling of iron, provides another example of how coordination between tissue resident immune and parenchymal cells might be orchestrated by metabolites (Haldar et al., 2014; Kohyama et al., 2009; Lavin et al., 2014). Thus, in the future, it will be important to determine whether Kupffer cells exhibit heterogeneity in their gene expression programs and functions that parallel the metabolic zonation of hepatocytes.
Licensing of metabolic functions during physiologic transitions
Although licensing of postnatal functions in metabolic organs was initially demonstrated in BAT (Odegaard et al., 2016), it likely occurs in other organs when placental mammals transition from intrauterine to extrauterine life. As used here, the term “licensing” refers to a switch from a previous default state to a new permissive state. For example, in BAT, this represents a switch from coupled respiration in the embryo to uncoupled respiration in the neonate, a switch that occurs once and then is maintained for the life of the animal. In general, the licensing of perinatal functionalities is thought to be signal dependent, and independent of developmental regulators that control programs of patterning and cell fate specification. For instance, while IL-33 and ST2 license BAT for uncoupled respiration, the development of BAT is unaltered in IL-33 and ST2 deficient mice. However, it is currently not known whether BAT represents a special example of this paradigm or whether signal dependent licensing of postnatal functions occurs in other metabolic organs.
We suggest that the liver is another organ where licensing of postnatal functions occurs during the perinatal period. During embryogenesis, fetal liver only participates in nutrient storage via glycogenesis and lipogenesis, because the developing embryo has unfettered access to maternal nutrients. This is supported by the observation that fetal liver does not express PEPCK or G6Pase, two enzymes necessary for gluconeogenesis (Burch et al., 1963). During the transition from intrauterine to extrauterine life, the liver becomes licensed to carry out gluconeogenesis, as evidenced by massive induction of PEPCK and G6Pase (unpublished observations). One prediction of this model is that impaired licensing of gluconeogenesis will result in postnatal hypoglycemia and lethality, a phenotype that has been observed in mice that are unable to induce gluconeogenesis after birth. For example, deletion of the placental miR-379/miR-410 cluster, which is expressed from the maternal allele, results in impaired expression of gluconeogenic genes in the liver of neonatal mice, resulting in hypoglycemia and perinatal lethality (Labialle et al., 2014). Although the targets of this miRNA cluster or the signals that license gluconeogenesis are not known, these findings suggest that signal-dependent licensing of postnatal functions in placentals might be a general paradigm.
Another critical transition that mammals encounter is when the neonates weans from mother’s milk to adult food. This transition from suckling to normal chow in mice requires a major change in the expression of enzymes in the intestinal epithelium to support nutrient intake. For example, the neonatal intestinal brush border is optimized to extract nutrients from mother’s milk, as it expresses lactase and fatty acid metabolizing enzymes. This transition in the intestinal epithelium is regulated by the transcriptional repressor B lymphocyte-induced maturation protein 1 (Blimp1), which is expressed in the neonatal epithelium and functions to repress characteristics of adult intestinal epithelium. In line with this, deletion of Blimp1 in the intestines causes ectopic expression of adult intestinal epithelium genes in the neonates, resulting in poor nutrient absorption, growth retardation, and excessive mortality (Harper et al., 2011; Muncan et al., 2011). A similar transition occurs in the inguinal WAT of mice at the time of weaning. During the suckling period, this tissue is mainly a site of energy storage, as it is packed with unilocular white adipocytes. At the time of weaning, the inguinal white adipose tissue remodels from energy storing to energy burning beige fat, which participates in the maintenance of core temperature (Lee et al., 2015a; Odegaard et al., 2016). As animals age, this tissue reverts back to white fat but retains its capacity to undergo browning after cold exposure. While IL-33 and ST2 are required for licensing of uncoupled respiration in beige adipocytes of juvenile and adult mice (Odegaard et al., 2016), they are dispensable for the commitment to and differentiation of beige adipocytes, indicating that other signals regulate the physiologic transition from white to beige at weaning.
Classical functions of immunity in defense of metabolic organs
The homeostatic functions of the immune system in metabolic organs were likely shaped by the organism’s need to adapt to changes in nutrient availability, environmental temperature, and infectious challenges. The latter represents the canonical functions of the immune system in the defense and surveillance of metabolic organs. While it has long been recognized that resident immune cells in the liver provide a defense against dietary pathogens (Jenne and Kubes, 2013), the majority of the studies on adipose tissue have focused on how obesity alters the activation of innate and adaptive immunity during infectious challenges. These studies in mice and humans have collectively revealed that obesity increases susceptibility to many viral and bacterial infections, and the comorbidities, such as tissue damage, associated with them (Gardner et al., 2011; Huttunen and Syrjanen, 2013; Karlsson and Beck, 2010). Both direct and indirect effects of obesity likely contribute to these changes in host susceptibility to infection and tissue damage. For instance, leptin signaling in immune cells provides a direct mechanism by which genetic and dietary obesity might alter the activation of innate and adaptive immune cells (Naylor and Petri, 2016), whereas changes in neural, hormonal or vascular function represent indirect mechanisms by which obesity alters the susceptibility of the host to infections and tissue damage (Karlsson and Beck, 2010).
New insights into the tissue surveillance functions of immune cells in WAT can be derived from studies that identified adipocytes and adipose tissue as important reservoirs for chronic infections by viruses, bacteria, and parasites. For example, human immunodeficiency virus (HIV)-infected CD4+ T cells evade host immunity and antiviral therapy by hiding in adipose tissue, thereby providing a reservoir for viral persistence (Damouche et al., 2015). In a similar manner, human cytomegalovirus (HCMV) not only infects and replicates in human adipose stromal/stem cells, but can also alter their adipogenic potential and immunomodulatory properties (Zwezdaryk et al., 2016). Mycobacterium tuberculosis, which co-evolved with its human host and infects up to one third of world’s population, can persist in a non-replicative form in adipocytes that evades host immunity and anti-microbial therapy (Agarwal et al., 2014; Neyrolles et al., 2006). A similar picture has emerged for persistence of parasites in adipose tissue, including Plasmodium falciparum, Trypanosoma brucei, and Trypanosoma cruzi. While P. falciparum and T. brucei are sequestered in adipose tissue in the extracellular space (Franke-Fayard et al., 2005; Trindade et al., 2016), T. cruzi directly infects adipocytes, and like M. tuberculosis, localizes to the lipid droplet (Combs et al., 2005; Ferreira et al., 2011). These observations collectively suggest that many pathogens exhibit a tropism for adipose tissue, which might allow for their persistence in the host.
If one considers that adipose tissue is a site for pathogen persistence, then one potential function of adipose tissue resident immune cells might be the immune surveillance of this metabolic organ. These surveillance functions of tissue resident immune cells, in particular the ATMs, might be performed by two distinct mechanisms. First, ATMs might directly sense PAMPs to coordinate local and systemic immune responses. Although direct evidence in support of this idea is currently lacking, signals that regulate physiologic trafficking of ATMs into adipose tissues provide a clue. For example, previous studies have revealed that lipolytic stimuli, such as fasting and adrenergic activation, promote the recruitment of macrophages into adipose tissue, which form crown-like structures around adipocytes, accumulate cytoplasmic lipid droplets, and induce programs of lysosomal catabolism (Kosteli et al., 2010; Mottillo et al., 2007; Xu et al., 2013). While these findings have been interpreted as macrophages buffering the release of fatty acids from adipocytes (Xu et al., 2013), an alternative interpretation might be that lipolysis releases potential PAMPs that are then sampled by macrophages to detect evasive intracellular pathogens.
Sequestration by ATMs of micro or macronutrients that are essential for pathogen growth might be a second mechanism by which they protect adipose tissue from infections. In support of this, Trindale et. al. recently reported that the replicative and infective forms of T. brucei found in adipose tissues are morphologically, transcriptionally, and metabolically distinct from those found in the blood (Trindade et al., 2016). These adipose tissue forms of T. brucei exhibit high reliance on β-oxidation of fatty acids, which they presumably acquire from their lipid rich environment. In this context, the sequestration of fatty acids by ATMs might function to limit the growth of T. brucei and other microbes, which preferentially oxidize fatty acids to meet their metabolic needs. This is analogous to the antimicrobial functions of hepcidin, which targets the iron exporter ferroportin for degradation, during bacterial infections (De Domenico et al., 2011). As a consequence, iron is sequestered within macrophages and hepatocytes, resulting in reduction of circulating iron, which is essential for the growth of nearly all microbial pathogens. Thus, the sequestration of micronutrients (iron) or macronutrients (fatty acids) by macrophages might be a conserved mechanism for slowing the growth of pathogens in tissues. These potential functions of ATMs in host defense will be important to consider in future studies that investigate the physiologic role of these cells in tissue homeostasis.
Pathobiology: immune contributions to metabolic disease
Over the last two decades, the interest in tissue immunometabolism has been driven by the correlation between obesity and the onset of inflammation in WAT (Hotamisligil et al., 1993; Weisberg et al., 2003; Xu et al., 2003). In general, these studies have revealed that, compared to lean animals, WAT of obese mice has low-grade inflammation that is associated with accumulation of pro-inflammatory macrophages, neutrophils, CD8+ T cells, Th1 and Th17 cells, and B cells (Mathis, 2013; Osborn and Olefsky, 2012). At the same time, there is a decrease in the number of anti-inflammatory immune cells, including eosinophils, M2-type macrophages, Tregs, and ILC2s (Lumeng et al., 2007; Odegaard and Chawla, 2013). This switch in the immune cell repertoire between lean and obese mice is thought to amplify the inflammatory timbre of WAT, which is initiated as adipocytes expand to accommodate the increased intake of nutrients. The increased influx of carbohydrates, fatty acids, and amino acids into adipocytes causes cellular dysfunction, which manifests as lipid dysregulation (accumulation of intracellular diacylglycerols, saturated fatty acids, and ceramides), abnormal intracellular protein modification (glycosylation and nitration), mitochondrial dysfunction/oxidative stress, and endoplasmic reticulum/membrane stress. This metabolic stress in adipocytes culminates in the activation of stress pathways (Jun N-terminal kinases (JNK), inhibitor of nuclear factor κB (IκB) kinase β (IKKβ), endoplasmic reticulum-to-nucleus signaling 1(IRE-1), and RNA-activated protein kinase (PKR)), which converge to reduce nutrient intake by decreasing insulin signaling and augment the inflammatory response by activating JNK and IKKβ (Hotamisligil, 2010). These findings suggest that both cell intrinsic and extrinsic pathways contribute to obesity-associated inflammation and insulin resistance in obese animals.
The association between inflammation, insulin resistance, and nutrient storage is evolutionarily ancient and conserved across metazoans. For example, activation of Toll signaling pathway in the fat body of D. melanogaster, which is the mammalian equivalent of liver, white adipose tissue, and innate immune system, is sufficient to reduce insulin signaling cell autonomously in the fat body and non-autonomously throughout the organism (DiAngelo et al., 2009). This decrease in insulin action reduces nutrient storage and growth in the fly, suggesting that inflammation-induced insulin resistance reallocates nutrients from storage to host defense. A similar shift from storage to mobilization is observed in mice and humans during infection or innate immune activation, indicating conservation of this response across metazoans (Gallin et al., 1969; Okin and Medzhitov, 2016). These findings also have important implications for modeling and studying obesity-associated inflammation in mice. First, the evolutionary conservation of inflammation-induced insulin resistance from flies to humans, suggests the existence of genetic pathways that can modulate insulin action upon detection of pathogens. Second, these inflammatory signaling pathways might also modulate insulin signaling and prioritize nutrient utilization during other stress states, such as tissue injury and pregnancy. Third, although the crosstalk between inflammation and insulin resistance exists and is conserved across metazoans, its selection during evolution was unlikely to be driven by the need to limit nutrient storage, because our predecessors never encountered prolonged states of nutrient excess that are observed today. These observations lead us to suggest that while acute inflammation during infection promotes insulin resistance to fuel host immunity in flies, mice, and humans, it is less likely to be the culprit that potentiates insulin resistance in an unstressed type 2 diabetic who walks into a physician’s office.
The overwhelming number of studies in mice point to a strong link between the onset of obesity-induced inflammation in WAT and insulin resistance. For example, genetic perturbations that limit the activation of stress signaling pathways in metabolic tissues or impair the activation of pro-inflammatory immune cells improve insulin action and glucose disposal in obese mice. The converse has also been reported; i.e. genetic disruption of anti-inflammatory pathways worsens insulin sensitivity and glucose tolerance in obese animals. However, nearly all of these studies have been performed in thermally stressed mice (20–22°C), a housing temperature that is well below their thermoneutral zone (30°C) (Cannon and Nedergaard, 2011; Gordon, 1993). For example, mice housed at 20–22°C spend twice as much as energy as those housed at 30°C to maintain thermal balance, which is associated with a near doubling in their heart rate (Swoap et al., 2008). This increase in energy expenditure and heart rate is maintained by a proportional increase in the sympathetic tone, raising the question of what might be the similar condition in humans. Two human conditions that acutely mimic the physiology of the mouse at 20–22°C are exercise and the use of stimulants, such as methamphetamines (speed). However, neither is applicable to the obese type 2 diabetic patient who is seen in outpatient clinics. In line with this, when mice are housed at thermoneutrality, obesity-induced metabolic inflammation in WAT is not associated with development of insulin resistance (Tian et al., 2016). While these findings suggest that thermoneutral housing can uncouple metabolic inflammation from insulin resistance, it will be important to understand the effects of housing temperature on other physiologic parameters, such as the gut microbiome, intestinal permeability, and hepatic steatosis.
Metabolic disease: loss of communication between client and accessory cells?
If tissue resident immune and stromal cells facilitate the functions of parenchymal cells, then one prediction of this model might be that loss of accessory cell functions decreases the adaptive capacity of tissues, rendering them susceptible to development of disease. This idea can be experimentally tested in three ways. First, local signals (the inducers in Figure 2) that regulate the survival of tissue resident immune cells can be targeted to determine how they affect parenchymal cell homeostasis. Second, genetic manipulation of the effectors (Figure 2), transcription factors that regulate the tissue-specific programs of immune cells, might be useful to understand how loss of a particular immune cell in a tissue alters the composition of the circuit and the metabolism of the functional unit. For example, this has been partially explored by deletion of PPARγ in macrophages and Tregs (Bapat et al., 2015; Cipolletta et al., 2012; Odegaard et al., 2007). Third, circadian synchrony between parenchymal, immune, and stromal cells is necessary for optimal tissue function (Bass and Takahashi, 2010). If so, then desynchronization amongst these cell types might predispose to the development of disease. This can be experimentally tested by disrupting the cellular clock in immune or stromal cells, and asking how it alters the functional output of this core cellular circuit, as has been performed for myeloid cells in the setting of dietary obesity (Nguyen et al., 2013).
While the previous studies in tissue immunometabolism have primarily focused on indices of organismal metabolism (such as glucose and insulin tolerance tests, and hyperinsulinemic-euglycemic clamps), investigations of homeostatic functions of immune and stromal cells will require focusing on cellular metabolism of parenchymal cells. A number of different techniques, such as metabolic flux and substrate utilization analysis, cellular metabolomics, genome-wide RNA profiling, and proteomics, might be useful in uncovering the contribution of accessory cells to parenchymal cell metabolism. In addition, heterogeneity in tissue architecture and function will also need to be considered. For example, liver’s metabolic functions are highly zonated, necessitating that paracrine effects of Kupffer and stromal cells should be studied in this context. This is likely important because hepatic steatosis and fibrosis is known to initiate in the pericentral region, where lipogenic genes are highly expressed in hepatocytes and fibrotic activity of stellate cells is most active (Hijmans et al., 2014; Rombouts and Marra, 2010).
Additional insights into the physiologic and supportive functions of immune and stromal cells in metabolic tissues can be gained from experimental systems of tissue regeneration. Across vertebrate species, tissue injury results in the activation of immunity, which communicates with tissue stromal, parenchymal, and stem cells to orchestrate programs of tissue repair. In these scenarios, there are three possible regenerative outcomes: restoration of tissue function by stem (skeletal muscle and intestines) or parenchymal (liver) cells, replacement of damaged tissue by scar (fibrosis), or the fatty degeneration of tissue by infiltrating adipocytes. These three outcomes are controlled by the dialogue between stem/parenchymal, immune, and stromal cells. For example, during skeletal muscle regeneration, the immune cells and signals regulate the expansion and functions of FAPs (muscle stromal cells) (Heredia et al., 2013), which provide paracrine support for the differentiating satellite cells (skeletal muscle stem cells) (Heredia et al., 2013; Joe et al., 2010). However, if regeneration is impaired secondary to satellite cell dysfunction or loss of anti-inflammatory Tregs (Burzyn et al., 2013; Kuswanto et al., 2016), local signals direct FAPs to differentiate into fibroblasts or adipocytes for the deposition of scar tissue or fatty degeneration of muscle, respectively (Joe et al., 2010; Uezumi et al., 2010). Thus, given the mesenchymal origin of FAPs and their similarities with adipocyte precursors (Joe et al., 2009), immune modulation of FAPs in skeletal muscle might also provide insights into the pathways that control expansion and fibrosis of WAT during obesity.
Concluding remarks and outstanding questions
Here, we have provided a framework for studying tissue immunometabolism, which focuses on the homeostatic functions of tissue resident immune cells. This perspective builds on the idea that evolution of metazoans is associated with cellular specialization, which resulted in auxiliary functions being relegated to accessory cells (Okabe and Medzhitov, 2016). These tissue accessory cells, which comprise immune and stromal cells, are seeded during development and support the core functions of parenchymal cells in metabolic organs. Thus, the basic functional unit of metabolic organs is composed of parenchymal, immune, and stromal cells, which continuously communicate with each other and their environment. In adipose tissue, this coordinated sensing of metabolites is mediated by the fatty acid sensor PPARγ, whereas in liver it might be orchestrated by the oxysterol receptor LXRα. Overlaid on top of this core functional unit are neuronal, hormonal, and circadian inputs, which establish coordination amongst the metabolic organs necessary for adaptation to environmental challenges. For example, adaptation to environmental cold requires mobilization of lipid stores from WAT for their oxidation in BAT, functions that are facilitated by immune and stromal cells.
Although the field of tissue immunometabolism has made tremendous progress over the last two decades, there still are a number of outstanding questions. First, our understanding of local signals that regulate the tissue-specific programs of immune and stromal cells in metabolic organs is incomplete. Second, the ontogeny and fate of stromal cells that populate WAT, BAT, and liver is not known. In this regard, it will be important to identify the signals and transcriptional programs that regulate the development, maintenance, and differentiation of stromal cells in metabolic tissues. Third, while some signals have been identified that mediate communication between parenchymal, stromal, and immune cells, our understanding in this area is rather patchy and incomplete. Fourth, while we generally view WAT and BAT as homogenous organs, studies in liver have uncovered heterogeneity and zonation of hepatic metabolism. However, it is not known whether anabolic and catabolic programs are similarly segregated in WAT. For example, are energy storage and release zonally compartmentalized in WAT or do all adipocytes participate in lipogenesis and lipolysis? Fifth, does spatial organization of immune and stromal cells impact parenchymal cell metabolism or its communication with the nerves? Sixth, how is labor partitioned between client (parenchymal) and accessory (immune and stromal) cells in metabolic tissues, and what are the tissue-specific mechanisms by which immune cells support parenchymal cell metabolism? And lastly, how does disruption of communication between client and accessory cells contribute to pathogenesis of metabolic diseases? Addressing these and other related questions will likely shed new light on the field of tissue immunometabolism.
Acknowledgments
We thank members of the Chawla laboratory for discussions and A. Loh for comments on the manuscript. The authors’ work was supported by grants from NIH (DK094641, DK101064), and an NIH Director’s Pioneer Award (DP1AR064158) to A.C. V.I.K. was supported by a Predoctoral Fellowship from the Western State Affiliate of American Heart Association. A.C. was also supported by UCSF NORC grant P30DK098722. The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal P, Khan SR, Verma SC, Beg M, Singh K, Mitra K, Gaikwad AN, Akhtar MS, Krishnan MY. Mycobacterium tuberculosis persistence in various adipose depots of infected mice and the effect of anti-tubercular therapy. Microbes Infect. 2014;16:571–580. doi: 10.1016/j.micinf.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983;212:835–842. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature. 2015;528:137–141. doi: 10.1038/nature16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–770. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Berry DC, Jiang Y, Graff JM. Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nature communications. 2016;7:10184. doi: 10.1038/ncomms10184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19:8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci U S A. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch HB, Lowry OH, Kuhlman AM, Skerjance J, Diamant EJ, Lowry SR, Von Dippe P. Changes in patterns of enzymes of carbohydrate metabolism in the developing rat liver. J Biol Chem. 1963;238:2267–2273. [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155:1282–1295. doi: 10.1016/j.cell.2013.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. The Journal of experimental biology. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Current opinion in immunology. 2014;31C:31–37. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc Natl Acad Sci U S A. 2009;106:9797–9802. doi: 10.1073/pnas.0903971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Chung S, Lee TJ, Reader BF, Kim JY, Lee YG, Park GY, Karpurapu M, Ballinger MN, Qian F, Rusu L, et al. FoxO1 regulates allergic asthmatic inflammation through regulating polarization of the macrophage inflammatory phenotype. Oncotarget. 2016 doi: 10.18632/oncotarget.8162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, Mathis D. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews. Molecular cell biology. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damouche A, Lazure T, Avettand-Fenoel V, Huot N, Dejucq-Rainsford N, Satie AP, Melard A, David L, Gommet C, Ghosn J, et al. Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection. PLoS Pathog. 2015;11:e1005153. doi: 10.1371/journal.ppat.1005153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Domenico I, Ward DM, Kaplan J. Hepcidin and ferroportin: the new players in iron metabolism. Semin Liver Dis. 2011;31:272–279. doi: 10.1055/s-0031-1286058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAngelo JR, Bland ML, Bambina S, Cherry S, Birnbaum MJ. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc Natl Acad Sci U S A. 2009;106:20853–20858. doi: 10.1073/pnas.0906749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–874. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- Ferreira AV, Segatto M, Menezes Z, Macedo AM, Gelape C, de Oliveira Andrade L, Nagajyothi F, Scherer PE, Teixeira MM, Tanowitz HB. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forner F, Kumar C, Luber CA, Fromme T, Klingenspor M, Mann M. Proteome differences between brown and white fat mitochondria reveal specialized metabolic functions. Cell Metab. 2009;10:324–335. doi: 10.1016/j.cmet.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, Ramesar J, Buscher P, Que I, Lowik C, Voshol PJ, den Boer MA, van Duinen SG, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- Gallin JI, Kaye D, O’Leary WM. Serum lipids in infection. The New England journal of medicine. 1969;281:1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gardner EM, Beli E, Clinthorne JF, Duriancik DM. Energy intake and response to infection with influenza. Annual review of nutrition. 2011;31:353–367. doi: 10.1146/annurev-nutr-081810-160812. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Ivanov S, Williams JW, Huang SC, Marcelin G, Fairfax K, Wang PL, Francis JS, Leone P, Wilson DB, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. The Journal of experimental medicine. 2014;211:1525–1531. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt R. Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther. 1992;53:275–354. doi: 10.1016/0163-7258(92)90055-5. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Baldysiak-Figiel A, Krugel V, Ueberham E, Gaunitz F. Hepatocellular expression of glutamine synthetase: an indicator of morphogen actions as master regulators of zonation in adult liver. Prog Histochem Cytochem. 2007;41:201–266. doi: 10.1016/j.proghi.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Matz-Soja M. Liver zonation: Novel aspects of its regulation and its impact on homeostasis. World journal of gastroenterology: WJG. 2014;20:8491–8504. doi: 10.3748/wjg.v20.i26.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Barozzi I, Mietton F, Polletti S, De Santa F, Venturini E, Gregory L, Lonie L, Chew A, Wei CL, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44:439–449. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Gordon CJ. Temperature regulation in laboratory rodents. Cambridge England; New York, NY, USA: Cambridge University Press; 1993. [Google Scholar]
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar M, Kohyama M, So AY, Kc W, Wu X, Briseno CG, Satpathy AT, Kretzer NM, Arase H, Rajasekaran NS, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- Harms MJ, Ishibashi J, Wang W, Lim HW, Goyama S, Sato T, Kurokawa M, Won KJ, Seale P. Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metab. 2014;19:593–604. doi: 10.1016/j.cmet.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci U S A. 2011;108:10585–10590. doi: 10.1073/pnas.1105852108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausberger FX. Pathological changes in adipose tissue of obese mice. The Anatomical record. 1966;154:651–660. doi: 10.1002/ar.1091540311. [DOI] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Molecular cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans BS, Grefhorst A, Oosterveer MH, Groen AK. Zonation of glucose and fatty acid metabolism in the liver: mechanism and metabolic consequences. Biochimie. 2014;96:121–129. doi: 10.1016/j.biochi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Hong KY, Bae H, Park I, Park DY, Kim KH, Kubota Y, Cho ES, Kim H, Adams RH, Yoo OJ, et al. Perilipin+ embryonic preadipocytes actively proliferate along growing vasculatures for adipose expansion. Development. 2015;142:2623–2632. doi: 10.1242/dev.125336. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Huang JT, Welch JS, Ricote M, Binder CJ, Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D, Glass CK. Interleukin-4-dependent production of PPAR-gamma ligands in macrophages by 12/15-lipoxygenase. Nature. 1999;400:378–382. doi: 10.1038/22572. [DOI] [PubMed] [Google Scholar]
- Hui X, Gu P, Zhang J, Nie T, Pan Y, Wu D, Feng T, Zhong C, Wang Y, Lam KS, et al. Adiponectin Enhances Cold-Induced Browning of Subcutaneous Adipose Tissue via Promoting M2 Macrophage Proliferation. Cell Metab. 2015;22:279–290. doi: 10.1016/j.cmet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Huttunen R, Syrjanen J. Obesity and the risk and outcome of infection. Int J Obes (Lond) 2013;37:333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell reports. 2014;9:1007–1022. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem cells. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Ashrafi K. Caenorhabditis elegans as an emerging model for studying the basic biology of obesity. Dis Model Mech. 2009;2:224–229. doi: 10.1242/dmm.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungermann K. Metabolic zonation of liver parenchyma. Semin Liver Dis. 1988;8:329–341. doi: 10.1055/s-2008-1040554. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol. 2006;68:159–191. doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EA, Beck MA. The burden of obesity on infectious disease. Exp Biol Med (Maywood) 2010;235:1412–1424. doi: 10.1258/ebm.2010.010227. [DOI] [PubMed] [Google Scholar]
- Katz N, Teutsch HF, Jungermann K, Sasse D. Perinatal development of the metabolic zonation of hamster liver parenchyma. FEBS Lett. 1976;69:23–26. doi: 10.1016/0014-5793(76)80645-x. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Prinz M, Geissmann F, Gomez Perdiguero E. Development and function of tissue resident macrophages in mice. Seminars in immunology. 2015;27:369–378. doi: 10.1016/j.smim.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama M, Ise W, Edelson BT, Wilker PR, Hildner K, Mejia C, Frazier WA, Murphy TL, Murphy KM. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, Mathis D. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity. 2016;44:355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labialle S, Marty V, Bortolin-Cavaille ML, Hoareau-Osman M, Pradere JP, Valet P, Martin PG, Cavaille J. The miR-379/miR-410 cluster at the imprinted Dlk1-Dio3 domain controls neonatal metabolic adaptation. The EMBO journal. 2014;33:2216–2230. doi: 10.15252/embj.201387038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerspetz K. The postnatal development of homoiothermy and cold resistance in mice. Experientia. 1962;18:282–284. doi: 10.1007/BF02148237. [DOI] [PubMed] [Google Scholar]
- Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, Odegaard JI, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015a;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2015b;29:286–299. doi: 10.1096/fj.14-263038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. doi: 10.1016/j.cmet.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- Luan B, Yoon YS, Le Lay J, Kaestner KH, Hedrick S, Montminy M. CREB pathway links PGE2 signaling with macrophage polarization. Proc Natl Acad Sci U S A. 2015;112:15642–15647. doi: 10.1073/pnas.1519644112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrikova D, Sijmonsma TP, Sowodniok K, Richards DM, Delacher M, Sticht C, Gretz N, Schafmeier T, Feuerer M, Herzig S. Brown adipose tissue harbors a distinct sub-population of regulatory T cells. PLoS One. 2015;10:e0118534. doi: 10.1371/journal.pone.0118534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149:452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. The Journal of experimental medicine. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury, and Inflammation. Immunity. 2015;42:1005–1019. doi: 10.1016/j.immuni.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. American journal of physiology. Endocrinology and metabolism. 2007;293:E1188–1197. doi: 10.1152/ajpendo.00051.2007. [DOI] [PubMed] [Google Scholar]
- Muncan V, Heijmans J, Krasinski SD, Buller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, et al. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nature communications. 2011;2:452. doi: 10.1038/ncomms1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAG, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, Casanova-Acebes M, Lopez F, Tabraue C, Beceiro S, et al. The nuclear receptor LXRalpha controls the functional specialization of splenic macrophages. Nat Immunol. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor C, Petri WA., Jr Leptin Regulation of Immune Responses. Trends Mol Med. 2016;22:88–98. doi: 10.1016/j.molmed.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Neyrolles O, Hernandez-Pando R, Pietri-Rouxel F, Fornes P, Tailleux L, Barrios Payan JA, Pivert E, Bordat Y, Aguilar D, Prevost MC, et al. Is adipose tissue a place for Mycobacterium tuberculosis persistence? PLoS One. 2006;1:e43. doi: 10.1371/journal.pone.0000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, Silberstein LE. The elusive nature and function of mesenchymal stem cells. Nature reviews. Molecular cell biology. 2011;12:126–131. doi: 10.1038/nrm3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. The Journal of experimental medicine. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339:172–177. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Lee MW, Sogawa Y, Bertholet AM, Locksley RM, Weinberg DE, Kirichok Y, Deo RC, Chawla A. Perinatal Licensing of Thermogenesis by IL-33 and ST2. Cell. 2016;166:841–854. doi: 10.1016/j.cell.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y, Medzhitov R. Tissue biology perspective on macrophages. Nat Immunol. 2016;17:9–17. doi: 10.1038/ni.3320. [DOI] [PubMed] [Google Scholar]
- Okin D, Medzhitov R. The Effect of Sustained Inflammation on Hepatic Mevalonate Pathway Results in Hyperglycemia. Cell. 2016;165:343–356. doi: 10.1016/j.cell.2016.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363–374. doi: 10.1038/nm.2627. [DOI] [PubMed] [Google Scholar]
- Palanker L, Tennessen JM, Lam G, Thummel CS. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature. 2014;516:414–417. doi: 10.1038/nature13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metab. 2013;17:562–574. doi: 10.1016/j.cmet.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al. Meteorin-like Is a Hormone that Regulates Immune-Adipose Interactions to Increase Beige Fat Thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes & development. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M Immunological Genome C. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts K, Marra F. Molecular mechanisms of hepatic fibrosis in non-alcoholic steatohepatitis. Dig Dis. 2010;28:229–235. doi: 10.1159/000282094. [DOI] [PubMed] [Google Scholar]
- Rosas M, Davies LC, Giles PJ, Liao CT, Kharfan B, Stone TC, O’Donnell VB, Fraser DJ, Jones SA, Taylor PR. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR-gamma by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22:259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O’Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell reports. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Shaked I, Hanna RN, Shaked H, Chodaczek G, Nowyhed HN, Tweet G, Tacke R, Basat AB, Mikulski Z, Togher S, et al. Transcription factor Nr4a1 couples sympathetic and inflammatory cues in CNS-recruited macrophages to limit neuroinflammation. Nat Immunol. 2015;16:1228–1234. doi: 10.1038/ni.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Youngman MJ, Kim DH. Transcriptional responses to pathogens in Caenorhabditis elegans. Curr Opin Microbiol. 2008;11:251–256. doi: 10.1016/j.mib.2008.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Sifri CD, Begun J, Ausubel FM. The worm has turned--microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13:119–127. doi: 10.1016/j.tim.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Zamorano N, Fabbiano S, Chevalier C, Stojanovic O, Colin DJ, Stevanovic A, Veyrat-Durebex C, Tarallo V, Rigo D, Germain S, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. 2015;21:1497–1501. doi: 10.1038/nm.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoap SJ, Li C, Wess J, Parsons AD, Williams TD, Overton JM. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. American journal of physiology. Heart and circulatory physiology. 2008;294:H1581–1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- Tan MW, Shapira M. Genetic and molecular analysis of nematode-microbe interactions. Cell Microbiol. 2011;13:497–507. doi: 10.1111/j.1462-5822.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tonotonoz P, Chawla A. Thermoneutral Housing Accelerates Metabolic Inflammation to Potentiate Atherosclerosis but Not Insulin Resistance. Cell Metab. 2016;23:165–178. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid- activated transcription factor [published erratum appears in Cell 1995 Mar 24;80(6):following 957] Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Trindade S, Rijo-Ferreira F, Carvalho T, Pinto-Neves D, Guegan F, Aresta-Branco F, Bento F, Young SA, Pinto A, Van Den Abbeele J, et al. Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe. 2016;19:837–848. doi: 10.1016/j.chom.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- Umek RM, Friedman AD, McKnight SL. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A, Moro K, Xin A, Liao Y, Gloury R, Kawamoto S, Fagarasan S, Mielke LA, Afshar-Sterle S, Masters SL, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, Wang MY, Kusminski CM, Morley TS, Gupta RK. Pdgfrbeta+ Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 2016;23:350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, et al. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol. 2000;20:4436–4444. doi: 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A. 2013a;110:18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013b;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way JM, Harrington WW, Brown KK, Gottschalk WK, Sundseth SS, Mansfield TA, Ramachandran RK, Willson TM, Kliewer SA. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor gamma activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology. 2001;142:1269–1277. doi: 10.1210/endo.142.3.8037. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RT. On the Treatment of Glycosuria and Diabetes Mellitus with Sodium Salicylate. British medical journal. 1901;1:760–762. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Burkart A, Bell G, Mendelson K, Leszyk J, Nicoloro S, Czech M, Corvera S. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Young SG, Zechner R. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes & development. 2013;27:459–484. doi: 10.1101/gad.209296.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwezdaryk KJ, Ferris MB, Strong AL, Morris CA, Bunnell BA, Dhurandhar NV, Gimble JM, Sullivan DE. Human cytomegalovirus infection of human adipose-derived stromal/stem cells restricts differentiation along the adipogenic lineage. Adipocyte. 2016;5:53–64. doi: 10.1080/21623945.2015.1119957. [DOI] [PMC free article] [PubMed] [Google Scholar]