To the Editor
Hemophilia A is an X-linked bleeding disorder resulting from deficiency of factor VIII (FVIII) and characterized by spontaneous and traumatic bleeding into joints and muscles. Because recurrence of spontaneous bleeds into joints leads to synovial inflammation and degenerative arthritis [1], a major goal of comprehensive care for patients with severe hemophilia A is to prevent bleeds by frequent preventive or “prophylactic” dosing of FVIII [2]. Three times-weekly prophylaxis has been shown to prevent joint bleeds and joint damage in children with severe hemophilia A [2] and is now considered standard of care [3]. However, whether prophylaxis should be continued at the same dose and frequency in adults whose joints and supporting tissues are more mature and potentially less vulnerable to bleeds, is not known. Further, although prophylaxis is effective in reducing bleeds, hospitalizations, and improving days lost at work and quality of life [4], significant barriers exist: it is invasive, inconvenient, and costly. For a 70 kg male with severe hemophilia A using 40–50 IU/kg recombinant factor VIIII (rFVIII) three times weekly, at an estimated $0.80 per unit, the cost approaches up to $500,000 annually, or higher if there is trauma or surgery. According to the CDC Universal Data collection database, prophylaxis is practiced by only 57% overall [5], and among adults, only 33% continue on prophylaxis as it interferes with lifestyle and is costly [6]. Yet, of those reducing prophylaxis frequency, only 20% require a subsequent more intense regimen [7]. These data suggest that, while a significant proportion of young adults reduce or discontinue prophylaxis, once-weekly or twice-weekly regimens may be sufficient to prevent or reduce joint bleeding and joint damage, the latter of which was subsequently studied [8]
We, therefore, designed a pilot NHLBI R34 study to determine the feasibility of conducting a larger R01 clinical trial to compare the safety and efficacy of 40 IU/kg rFVIII prophylaxis given once-weekly vs. three times-weekly in adults ≥ 18 years of age with severe hemophilia A, FVIII ≤ 0.01 U/ml. This was an outpatient 52-week prospective, cross-over, multi-center phase III trial, registered at ClinicalTrials.gov (NCT 01405742). The study was approved by the Institutional Review Board at the University of Pittsburgh (IRB PRO110905888), and by institutional boards at each participating institution. The trial was overseen by a Data and Safety Monitoring Board (DSMB) appointed by NHLBI which monitored trial progress and patient safety. Eligible subjects were adult males with severe hemophilia A, FVIII <0.01 IU/ml, ≥18 years of age, ≥ 150 prior exposures, no history of a detectable inhibitor (<0.6 Bethesda units), and no allergic reaction or anaphylaxis to FVIII products. Patients with acquired hemophilia, bleeding disorders other than hemophilia A, with an inhibitor, or symptomatic HCV or HIV disease or life expectancy less than 5 years were excluded. Subjects were recruited from six hemophilia treatment centers, and all provided written informed consent. Randomization was within 24 hours of screening to once-weekly vs. three-time-weekly recombinant factor VIII (rFVIII) prophylaxis at a dose of 40 IU/kg given by intravenous injection. The minimum dose was 40 IU/kg and the maximum dose was up to 5% higher, or 42 IU/kg. After 26 weeks, subjects were crossed over to the alternate arm, beginning after a 72-hour washout period. Subjects used their own prescribed clotting factor. Rescue doses were allowed for breakthrough bleeds on study.
The primary efficacy outcome was joint bleed frequency by patient diary. Secondary outcomes included factor usage, including rescue FVIII for breakthrough bleeds. Range of motion of eight joints, including bilateral hips, knees, elbows, and ankles was measured by digital cameras at 26 and 52 weeks, using summary measures reflecting proportionate reduction from full range of motion for all eight joints, compared to reference values from normal males [9]. Quality-of-life by Haemo-QoL-A was assessed at 26 and 52 weeks, using summary measures for physical, psychological, social, and treatment-related scales, as described [10]. Coagulation factors II, V, VII, VIII, IX, and X, were assessed at weeks 8 and 34, i.e. 8 weeks after initiating each regimen by one-stage clotting assays; anti-thrombin activity by chromogenic assay; total tissue factor pathway inhibitor by ELISA; and thrombin generation on frozen citrated platelet-poor plasma samples, as previously described [11].
Since this pilot study was small and lacked power to test for differences between arms or non-inferiority, no hypothesis testing was conducted. The analyses were descriptive and focused on estimation and confidence intervals (CI), and summary measures including the mean, median, standard deviation for continuous data and frequency and percentages for categorical data. This pilot study was not powered to test the non-inferiority of three-times weekly with once-weekly rFVIII prophylaxis in adults with severe hemophilia A; rather it tested the feasibility of a future larger phase III randomized trial [12]. Prior to conducting this pilot study, preliminary sample size calculations indicated a sample size of 106 subjects, inflated to 124 for 15% attrition, would be required for a phase III randomized, crossover, non-inferiority trial [13]. For this pilot study, a sample size of 20 subjects was considered sufficient for clinical reasons to determine feasibility of approach and practicality of processes, e.g. randomization, web-based data entry, digital imaging joint assessment, quality of life tools, and assay on frozen samples.
Of 172, screened, 168 (98.7%) [95% CI: 94.2%, 99.4%] were excluded: these included 15 who were ineligible, 14 with inhibitors and 1 with thrombocytopenia; 108 who declined to participate, including 62 due to fear of bleeds on once-weekly dosing or fear of intravenous access if three-times weekly dosing, 35 due to competing time/work demands, 6 due to competing studies, 5 due to lack of free factor; and 45 for other reasons (Figure 1). Four subjects (2.3%) [95% CI: 0.7%, 5.8%] were randomized and enrolled between 07/02/12 and 10/29/12, of whom one (25.0%) [95% CI; 0.6%, 80.5%] dropped out at week 8, and was lost to follow-up, leaving three who completed the trial. All three subjects had severe hemophilia A, FVIII < 0.01 U/ml, one randomized to 40 IU/kg rFVIII once-weekly prophylaxis crossed over at week 26 to three-times weekly, and two randomized to 40 IU/kg rFVIII three-times weekly prophylaxis crossed over at week 26 to once-weekly prophylaxis, one of whom was lost to follow-up before cross-over. Because of lagging enrollment, ten additional HTCs were recruited between 01/15/13 and 01/30/13, and by 06/19/13 four had completed and six awaited regulatory (IRB) approval. Contractual approvals for the former four HTCs were delayed until 08/15/13 due to research office staffing and priorities, resulting in early study closure by the NIH-appointed DSMB on 11/08/13. None of the 10 sites enrolled any study subjects.
Figure 1. Hemophilia Adult Prophylaxis Trial Flow Diagram.
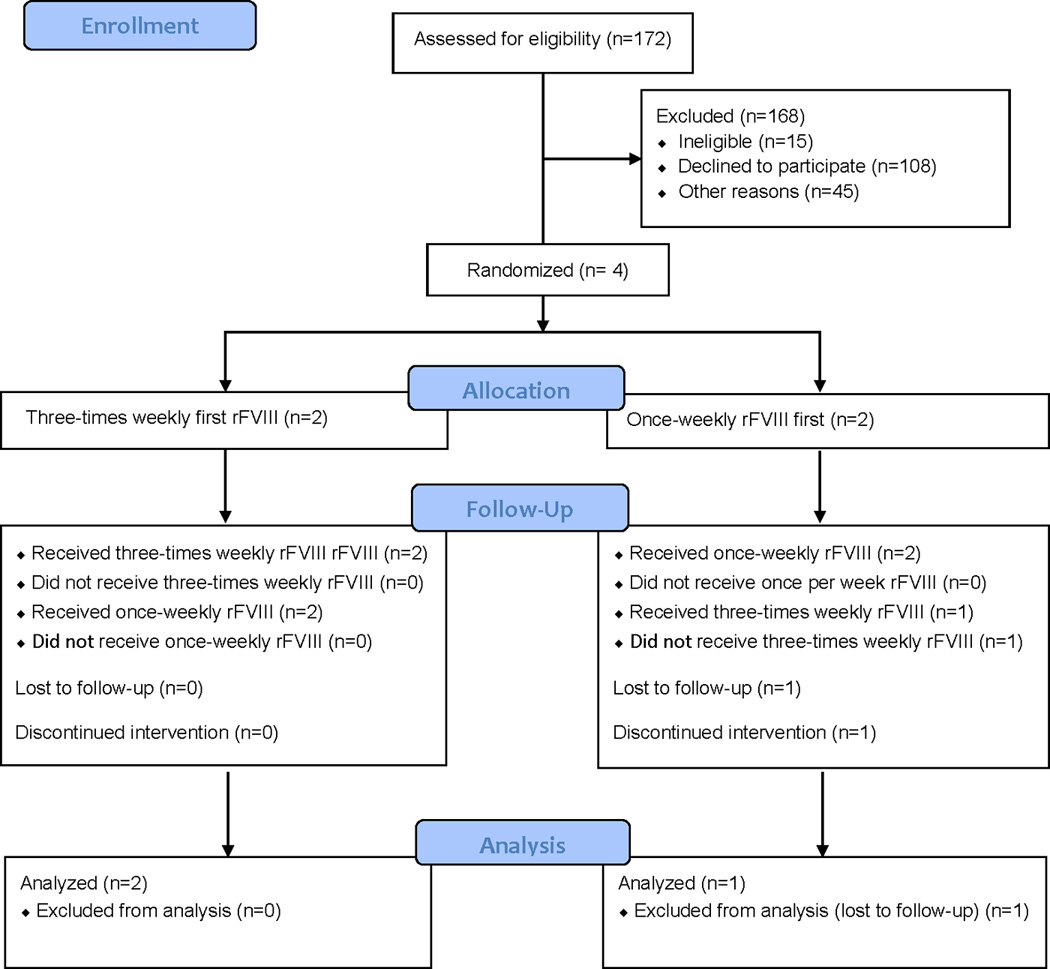
The flow diagram includes numbers of subjects screened, excluded, randomized, completed treatment, and included in analysis.
Traumatic and spontaneous bleeding frequency was low in both prophylaxis regimens, with a trend to fewer bleeds in those treated three-times weekly. None of the subjects had severe bleeding, thrombosis, other adverse events, or serious adverse events. Joint range of motion, quality of life, coagulation levels, and thrombin generation were shown to be feasible and did not distinguish between once-weekly and three-times weekly regimens. The findings of this R34 pilot study proved a larger phase III multi-site trial was not feasible. The low enrollment and delays in adding ten new sites because of regulatory and contractual delays led to early study closure, which was unexpected, and confirm the difficulties of rare disease research [14]. Yet, the lessons learned have been helpful in planning other multi-site clinical trials. Barriers to enrollment, including competing studies and lack of free study factor, were expected and typical of investigator-initiated rare disease trials [14]; other enrollment barriers were unexpected, including patient unwillingness to change prophylaxis frequency, constituting 37% of enrollment exclusions. These findings suggest that bleed severity and infusion difficulty do influence prophylaxis adherence in adults [13], and while the question of whether once-weekly prophylaxis is non-inferior to three-times weekly prophylaxis is unlikely to be answered, it does confirm the potential benefit of novel therapies that reduce treatment frequency.
From a design perspective, one disadvantage of the NIH R34 mechanism was the requirement for a pilot study, which in a rare disease requires numerous sites to achieve enrollment of a small number of subjects in a 2-year timeframe. By contrast, the NIH U34 design allows for planning a larger trial by gathering preliminary data without a pilot study, which we incorporated in two subsequent studies by conducting structured patient and physician interviews regarding acceptability of trial design, piloting assays and web-based endpoints; and surveying physicians for potentially eligible subjects. From a resource perspective, although lagging enrollment was recognized in the first year and led to rapid identification of 10 new sites, slow regulatory approvals by multiple IRBs [15] and slow contract execution by the research office led to early trial closure. We have since instituted a central IRB to coordinate multi-site studies [16] and hired a part-time grant coordinator to expedite multi-site contracting for two planned multi-site trials. From a scientific standpoint, efficacy and safety analyses were limited by low enrollment and analyzed by descriptive statistics [12], but the results of the pilot study proved the lack of feasibility for a larger phase III trial, considered a successful R34 outcome as it avoided wasting scare resources on a study destined to fail.
Acknowledgments
We acknowledge the guidance and oversight of Dr. Andrei Kindzelski, PhD, NHLBI program officer, and Dr. Gary E. Raskob, PhD, University of Oklahoma, who served as Chair of the Data and Safety Monitoring Committee for this R34 study. We also acknowledge the expertise and dedication of Kristen Jaworski, BSN, RN, former research coordinator, Hemophilia Center of Western PA, who coordinated study sites and cared for the local HTC study subjects, and who is now affiliated with Biogen, Inc., Boston MA.
Sources of Funding: This work was supported by Grant NHLBI R34-HL105870 from the Heart Blood and Lung Institute, National Institutes of Health, Bethesda MD (to MVR).
Footnotes
Author Contribution: MVR designed the study, obtained funding, enrolled subjects, interpreted the data, and wrote the manuscript. NCJ enrolled study subjects, contributed to study design, and reviewed the manuscript. PFF, CMK, ATN, and LR contributed to study design and reviewed the manuscript. JGY performed data analysis, interpreted the data, and reviewed the manuscript. CGM contributed to the design, interpreted the data, and reviewed the manuscript. KBZ performed the assays, interpreted the data, and edited the manuscript.
Disclosure of Conflict of Interest: MVR has received research funding from Alnylam, Baxalta, Biogen, CSL Behring, Dimensions, Genentech/Roche, Pfizer, Shire, SPARK; and honoraria for consulting for Alnylam, Baxalta, Biogen, Biomarin, and Tacere Benitec. PFF has received research funding from Baxter, Bayer, Biogen, CSL Behring, Pfizer; and SPARK Therapeutics; and advisory board fees from Bayer, Baxter/Baxalta, Biogen, Chugai, CSL Behring, Novo Nordisk, and Pfizer; and is an employee of Pfizer. CMK has received research funding from Bayer, NovoNordisk, Octapharma, Roche, and Shire; and acted as a paid consultant for Bayer, Biogen, Grifols, Octapharma, Pfizer, Roche Shire. ATN has received fees as a member of an advisory board for Shire. NCJ, LR, JGY, KBZ, and CGM declare no competing interests.
References
- 1.Rosendaal G, Lafeber FP. Pathogenesis of haemophilic arthropathy. Haemophilia. 2006;12(Suppl 3):117–121. doi: 10.1111/j.1365-2516.2006.01268.x. [DOI] [PubMed] [Google Scholar]
- 2.Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, Ingram JD, Manco-Johnson L, Funk S, Jacobon L, Valentino LA, Hoots WK, Buchanan GR, DiMichele D, Recht M, Brown D, Leissinger C, Bleak S, Cohen A, Mathew P, Matsunaga A, Medeiros d, Nugent D, Thomas GA, Thompson AA, McRedmond K, Soucie JM, Austin H, Evatt BL. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. [DOI] [PubMed] [Google Scholar]
- 3.National Hemophilia Foundation. Medical and Scientific Advisory Council (MASAC) Recommendation No. 179. MASAC Recommendation concerning prophylaxis: regular administration of clotting factor concentrate to prevent bleeding. http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007.
- 4.Tagliaferri A, Franchini M, Coppola A, Rivolta GF, Santoro C, Rossetti G, Feola G, Zanon E, Dragani A, Iannaccaro P, Radossi P, Mannucci PM. Effects of secondary prophylaxis started in adolescent and adult haemophiliacs. Haemophilia. 2008;14:945–951. doi: 10.1111/j.1365-2516.2008.01791.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [accessed March 15, 2010];Bleeding and clotting disorders surveillance. Summary report of UDC activity. http://www2a.cdc.gov/ncbddd/htcweb/index.asp.
- 6.Walsh CE, Valentino LA. Factor VIII prophylaxis for adult patients with severe haemophilia A: results of a US survey of attitudes and practices. Haemophilia. 2009;15:1014–1021. doi: 10.1111/j.1365-2516.2009.02036.x. [DOI] [PubMed] [Google Scholar]
- 7.Richards M, Altisent C, Batorova A, Chambost H, Dolan G, de Moerloose P, Fraga M, Hermans C, Karafoulidou A, Klamroth R, Lassial R, Rothschild C. Should prophylaxis be used in adolescent and adult patients with severe haemophilia? A European survey of practice and outcome data. Haemophilia. 2007;13:473–479. doi: 10.1111/j.1365-2516.2007.01478.x. [DOI] [PubMed] [Google Scholar]
- 8.Valentino LA, Mamonov V, Hellmann A, Quon DV, Cybicka A, Schroth P, Patrone L, Wong WY. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2011;10:359–367. doi: 10.1111/j.1538-7836.2011.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soucie JM, Wang C, Siddiqi A, Kulkarni R, Recht M, Konkle BA. The longitudinal effect of body adiposity on joint mobility in young males with hemophilia A. Haemophilia. 2011;17:196–203. doi: 10.1111/j.1365-2516.2010.02400.x. [DOI] [PubMed] [Google Scholar]
- 10.von Mackensen S, Bullinger M. Development and testing of an instrument to assess the quality of life of children with haemophilia in Europe (Haemo-QoL) Haemophilia. 2004;10(Suppl 1):17–25. doi: 10.1111/j.1355-0691.2004.00875.x. [DOI] [PubMed] [Google Scholar]
- 11.Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in haemophilia A. Haemophilia. 2009;15:1118–1125. doi: 10.1111/j.1365-2516.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore CG, Carter RE, Nietert PJ, Stewart PW. Recommendations for planning pilot studies in clinical and translational research. Clin Transl Sci. 2011;4:332–337. doi: 10.1111/j.1752-8062.2011.00347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragni MV. Rationale for a randomized controlled trial comparing two prophylaxis regimens in adults with severe hemophilia A: The Hemophilia Adult Prophylaxis Trial. Expert Rev Hematol. 2011;4:495–507. doi: 10.1586/ehm.11.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ragni MV, Moore CG, Bias V, Key NS, Kouides PA, Francis CW for the Hemostasis Thrombosis Research Society (HTRS) Challenges of rare disease research: limited patient and competing priorities. Haemophilia. 2012;18:e192–e194. doi: 10.1111/j.1365-2516.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 15.Menikoff J. The paradoxical problem with multiple-IRB review. N Engl J Med. 2010;363:1591–1593. doi: 10.1056/NEJMp1005101. [DOI] [PubMed] [Google Scholar]
- 16.Department of Health and Human Services. [Accessed May 31, 2016];Clinical coordinating center for multi-site investigator-initiated clinical trials. https://grants.nih.gov/grants/guide/pa-files/PAR-16-300.html.


