Abstract
Rationale
Individuals prone to attribute incentive salience to food-associated stimuli (“cues”) are also more sensitive to cues during drug-seeking and drug-taking. This may be due in part to a difference in sensitivity to the affective or other stimulus properties of the drug. In rats, these properties are associated with 50 kHz ultrasonic vocalizations (USVs), in that they are elicited during putative positive affective and motivational states, including in response to drugs of abuse.
Objectives
We sought to determine whether individual differences in the tendency to attribute incentive salience to a food cue (as measured by approach) were associated with differences in cocaine-induced USVs. We also tested whether the food cue would elicit USVs, and if this response was related to approach to the food cue.
Methods
In experiment 1, rats underwent Pavlovian Conditioned Approach (PavCA) training where they learned to associate a cue (an illuminated lever) with the delivery of a food pellet into a food cup. Subjects were categorized based on their approach to the cue (“sign-trackers”), or to the food cup (“goal-trackers”). Rats subsequently underwent nine testing days in which they were given saline or cocaine (10 mg/kg i.p), and placed into a locomotor chamber.
In experiment 2, rats were first tested in the locomotor chambers for one saline-treated day followed by one cocaine-treated day, and then trained in PavCA. USVs were recorded from a subset of individuals during the last day of PavCA to determine if the food cue would elicit USVs.
Results
Sign-trackers produced 5 - 24 times more cocaine-induced 50 kHz USVs compared to goal-trackers for all days of experiment 1, and this response sensitized with repeated cocaine, only in sign-trackers. Similarly in experiment 2, individuals that produced the most cocaine-induced USVs on a single exposure also showed the greatest tendency to sign-track during PavCA. Lastly, while sign-trackers produced more USVs during PavCA generally, the cue itself did not elicit additional USVs in sign- or goal-trackers.
Conclusions
These results indicate a robust and consistent relationship between approach to a food cue and cocaine-induced USV production. Thus, these USVs may index the neurobiological differences underlying the behavioral distinctions of sign- and goal-trackers.
Keywords: Autoshaping, Cocaine, Incentive Salience, Pavlovian Conditioning, Positive Affect, Self-Report, Sensitization, Ultrasonic Vocalizations
Introduction
Cues are environmental stimuli that signal significant events, and can act as predictors for imminent aversive or appetitive outcomes. When paired with a reward such as food or drug (unconditioned stimuli; US), initially neutral cues (conditioned stimuli; CS) can prompt a conditioned response which is consistent with the response produced by the reward (Pavlov 1927). Further, due to their relationship with the reward, cues can acquire incentive salience, and thus prompt emotional and motivational states that generate new, sometimes maladaptive, behavior (Berridge 2001; Bindra 1978; Robinson et al. 2014). For example, food and drug cues have been shown to facilitate reinstatement of operant behaviors that are indicative of reward-seeking after prolonged abstinence (Grimm et al. 2001; Kruzich et al. 2001; Shaham et al. 2003; Yager and Robinson 2010). However, there is considerable individual variation in the degree to which cues influence behavior, and whether these cues become incentive stimuli (Mahler and de Wit 2010; Robinson and Flagel 2009; Robinson et al. 2014).
In rodent models, individual variability in the propensity to attribute incentive salience to a food cue can be operationalized using a Pavlovian Conditioned Approach paradigm (Flagel et al. 2007; Meyer et al. 2012a). During this task, an illuminated lever predicts the delivery of a food pellet into a food cup. For a subset of individuals trained in this task (“sign-trackers”, STs), the cue acquires incentive value and elicits cue-approach behavior (Flagel et al. 2007; Hearst and Jenkins 1974; Tomie et al. 2008). For others (“goal-trackers”, GTs) the cue instead elicits approach to the reward delivery location (Boakes 1977). In addition to these differences in cue-elicited approach, cues are more effective conditioned reinforcers, and elicit food-seeking to a greater degree in sign-trackers, compared to goal-trackers (Robinson and Flagel 2009; Yager and Robinson 2013). Thus, these cues acquire incentive salience to a greater degree in sign-trackers compared to goal-trackers (Robinson et al. 2014)
Sign- and goal-tracker differences also generalize to drug cues. Previous work has shown that tendency to sign-track a food cue is associated with different patterns of drug-related behavior, including higher progressive ratio break-points during cocaine self-administration, more robust cocaine and nicotine cue-induced reinstatement, and stronger cocaine conditioned place preference (Meyer et al. 2012b; Saunders and Robinson 2010; 2011; Versaggi et al. 2016; Yager and Robinson 2013). For this reason, sign-tracking is considered a model for drug-cue responsivity, in that individuals displaying this behavior may be more vulnerable to the effects of cues on relapse to drug-taking (Flagel et al. 2009; Saunders and Robinson 2013).
A few studies have examined the acute unconditioned responses to drugs of abuse such as cocaine, in sign- and goal-trackers, but only subtle differences in drug-induced locomotion have been reported (Beckmann et al. 2011; Flagel et al. 2008). However, drugs including cocaine have other unconditioned effects, including the ability to elicit 50 kHz ultrasonic vocalizations (USVs; Browning et al. 2011; Ma et al. 2010; Maier et al. 2012; Meyer et al. 2012b). These USVs have been proposed to have a communicative purpose, and also to serve as an expression of motivational and affective states in rodents (Knutson et al. 2002; Portfors and Perkel 2014; Radziwon and Dent 2014; Brudzynski 2013; Wohr and Schwarting 2013). In particular, USVs in the upper frequency range near 50 kHz predominate in positive contexts including mating, exploration, play, and also in the anticipation of rewards such as food and electrical stimulation (Bialy et al. 2000; Burgdorf et al. 2006; Knutson et al. 1998; 1999). As a result, 50 kHz USVs have been proposed to be an index of affective state and further, as a preclinical analogue to human “self-report” of subjective states (Mahler et al. 2013; Panksepp et al. 2002). Given the potential motivational significance of these USVs, it is possible that they index an aspect of drug sensitivity distinct from locomotion, and may thus distinguish sign- and goal-trackers, especially after administration of addictive drugs.
The current study assessed the potential relationship between the USV response to acute and repeated cocaine and the attribution of incentive value to a food cue. Since previous research suggests that sign-trackers may be more sensitive to the motivational components of drug-taking and drug cues, we hypothesized that individuals who show sign-tracking behavior during Pavlovian Conditioned Approach (PavCA) would be more likely to produce cocaine-induced 50 kHz USVs as a representation of their enhanced positive affect in response to the drug. To this end, we conducted two experiments; the first measured approach to a food cue during PavCA prior to measuring cocaine-induced USVs; the second measured cocaine-induced USVs before PavCA testing. In addition to conditioned approach behaviors during PavCA, we assessed whether the food cue elicited USVs, and further, whether this response differed among sign- and goal-trackers.
Methods & Materials
Subjects and housing
Male Sprague Dawley rats (experiment 1: n = 48; experiment 2: n = 48, all rats 250 - 275g) were purchased from Envigo (Indianapolis, IN). Upon arrival, subjects were pair-housed in a temperature- and humidity-controlled room on a reverse 12 h light-dark cycle (lights off at 0730), and handled daily for one week. All testing occurred during the dark phase of this cycle. In their home cages, food and water were available ad libitum throughout the study. All experimental procedures followed the guidelines of laboratory animal care specified and approved by the Institutional Animal Care and Use Committee (IACUC) at the University at Buffalo.
Pavlovian Conditioned Approach
Apparatus
Med-Associates conditioning chambers (20.5×24.1 cm floor area, 29.2 cm high; St. Albans, VT) were equipped with LED-illuminated retractable levers located on either the left or right side (counterbalanced across rats) of a central food cup (3 cm above a stainless steel grid floor). An illuminated red house light was located on the chamber wall opposite the food cup. Banana-flavored food pellets (45 mg, BioServ, #F0059, Frenchtown, NJ) were delivered into the cup by an automatic feeder. The food cup was equipped with an infrared photobeam that detected head entries.
PavCA Procedure
Rats were given ~50 food pellets in their home cages on the two days prior to Pavlovian Conditioned Approach (PavCA; Flagel et al. 2007; Meyer et al. 2012a). Following this, rats first completed a food cup training session, during which 25 food pellets were dispensed into the food cup on a variable time 30 (1-60 s) schedule. The lever was retracted and the house light remained on for this session. Beginning on the subsequent day, subjects underwent five sessions of PavCA (one session/day). Each session consisted of 25 trials in which an 8-s illuminated lever presentation (CS) was followed by the delivery of a food pellet (US). Trials were separated according to a variable time 90 (30-150 s) schedule. It is important to note that the delivery of a food pellet was never contingent on the subject's behavior. Sessions lasted 37.5 min on average.
PavCA Index
Rats were categorized as sign- and goal-trackers based on a PavCA index previously described (Meyer et al., 2012a). Briefly, the PavCA index is a score ranging from −1.0 to 1.0 that is calculated from the average of three factors; response bias [(lever presses minus CS food cup entries) / (lever presses plus food cup entries)], approach probability difference [(# of trials with at least one lever press minus # of trials with at least one food cup entry during the CS) / 25 trials], and latency difference [(latency to enter the food cup during the CS minus latency to lever press) / 8]. The average of this PavCA index from the last 2 sessions is used to determine an overall index, thus characterizing rats as STs (PavCA Index ranging from 0.3 to 1.0) or GTs (−0.3 to −1.0). Intermediate rats (PavCA Index −0.29 to 0.29) were not included in any analyses.
Ultrasonic vocalizations
Apparatus
Locomotor chambers were constructed with black acrylic walls (47.5 cm length × 15.5 cm width × 30 cm height) and smooth matte black flooring. Positioned directly above each chamber were infrared video cameras hooked up to a 16-channel DVR (Swann Communications, Inc., Santa Fe Springs, CA). Locomotion was captured by the cameras and analyzed by Topscan video tracking software (Clever Sys., Inc., Reston, VA; Flagel and Robinson, 2007; Meyer et al., 2012b). Condenser microphones with a flat frequency range up to 250 kHz at 8-bit resolution (Model CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany), were placed 8 cm above each chamber and connected to an UltraSoundGate 416H recorder with four balanced analog inputs (Avisoft Bioacoustics, Berlin, Germany). USV recordings were converted to spectrograms and analyzed by an experimenter in Adobe Audition (Adobe Systems Inc, San Jose, CA).
Ultrasonic vocalization analysis
A trained experimenter, blind to the PavCA index scores, analyzed the recordings (experiment 1: nine 30 min treatment days; experiment 2: two 30 min treatment days, one ~37.5 min PavCA task) using Adobe Audition. USVs falling within the 50 kHz range (35 – 100 kHz) were individually counted and time-stamped. All USVs produced during the experiments fell within this range. USVs produced on day 16 and day 23 of experiment 1 were further examined to identify the various vocalization categories produced by cocaine-treated subjects. Seven categories of USVs were selected prior to this analysis, modeled after Wright et al. (2010). These include flat (near-constant frequency, mean slope between −0.2 and 0.2 kHz/ms), ramp (monotonically increasing or decreasing in frequency, mean slope > 0.2 kHz/ms), inverse-U (a monotonic increase followed by a monotonic frequency decrease, each at least 5 kHz), short (duration less than 12 ms), multi-step (one or more frequency changes), trill (rapid frequency oscillations), and complex (miscellaneous, not otherwise specified).
Experiment 1: PavCA performance as a predictor for cocaine-induced USVs
Procedure
In this experiment, rats (n = 48) were first tested in the PavCA procedure described above. Rats categorized as sign- and goal-trackers (n = 10, n = 14) were then tested for nine 30-min sessions (one session/day) where saline- and cocaine-induced locomotion as well as USVs were recorded. Prior to each session, rats were removed from their home cages, weighed, and then transported to the testing room. Here, they received an injection and were immediately placed in a locomotor chamber. For the first two consecutive days, subjects were injected with 0.9% saline (1 ml/kg, i.p.). During the next six testing days, occurring Monday, Wednesday and Friday for two weeks, rats were given an injection of 10 mg/kg cocaine (i.p.). A final cocaine testing day took place one week later, to test whether the USV response was altered after a prolonged drug-free period. Cocaine HCl (Nat. Inst. of Drug Abuse, Bethesda, MD) was dissolved in saline. One sign-tracker died prior to the completion of testing and thus, was excluded from the USV analysis.
Experiment 2: Cocaine-induced USVs as a predictor for PavCA performance
Procedure
For this experiment, rats (n = 48) underwent two 30-min sessions in a locomotor chamber as previously described. In brief, rats were injected with 0.9% saline (1 ml/kg, i.p.) on the first day and a 10 mg/kg cocaine (i.p.) on the second day. Rats were then tested under the PavCA paradigm described above, and Pavlovian index scores were calculated for all 48 subjects. In addition, a sixth day of PavCA was added to the procedure for a subset of randomly selected rats (n = 36). On this day, the condenser microphones were placed 8 cm above the Med-Associates conditioning chambers and USV recordings were collected. Five rats were dropped from this USV analysis due to a procedural error that led to some recordings being truncated, preventing analysis.
Statistics
For experiment 1, approach behaviors were measured using repeated-measures analysis of variance (ANOVA), using Phenotype (ST, GT) as the between-groups variable, and Day (1-5) as the within-groups variable. Similarly, USVs were analyzed using repeated measures ANOVA with Phenotype (ST, GT) as the between-subjects variable and a within-subjects variable of Day (1, 2, 5, 7, 9, 12, 14, 16, 23). Mann-Whitney U tests determined whether STs and GTs differed in the proportion of USVs per category on day 16 and day 23 of treatment.
In experiment 2, saline- and cocaine-induced USVs were analyzed using Phenotype as the between-subjects variable and Day (1-2) as the within-subjects variable. Further, USVs recorded during PavCA were analyzed using Period (pre-CS, CS, US) and Trial (1-25) as within-subjects variables. All significant main effects and interactions were then investigated using Fisher's Least Significant Difference post-hoc test. Statistical comparisons were considered significant at p < 0.05.
Results
Experiment 1
Pavlovian Conditioned Approach
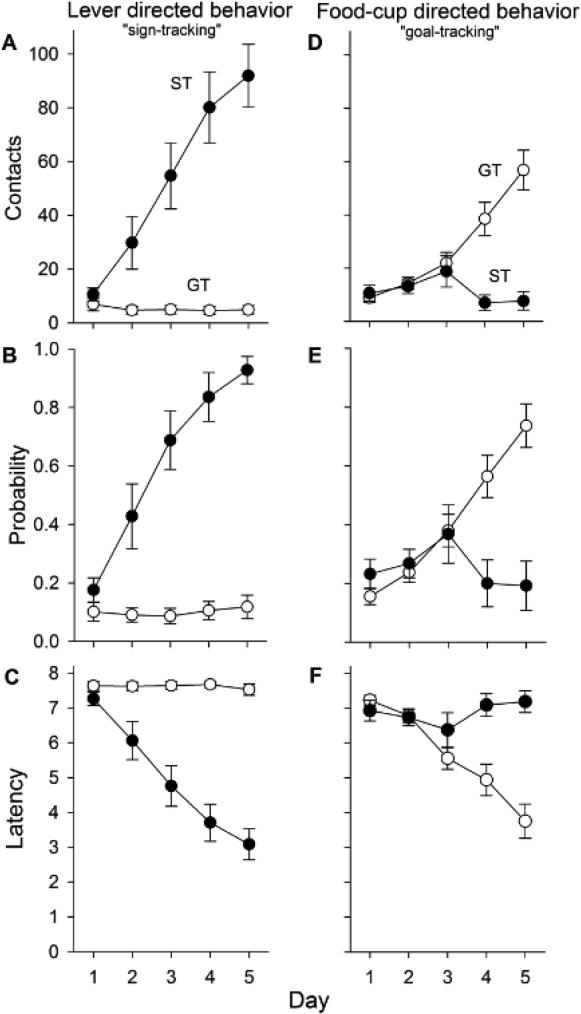
In line with previous findings (Flagel et al. 2007; Meyer et al. 2012a), subjects showed substantial variation in their approach responses as a result of PavCA training. Repeated measures ANOVA verified significant Phenotype × Day interactions [Fs (4,108) > 18.2, ps < 0.001] for all measures of lever- and food cup-directed behaviors including contacts, probability to approach and latency to approach (Fig. 1). For example, over successive days, sign-trackers increased lever contacts and probability to approach the lever while decreasing their latency to approach the lever. Analyses also revealed main effects of Phenotype [Fs (1,27) > 6.1, ps < 0.05] for all measures except food cup probability, as well as main effects of Day [Fs (4,108) > 9.8, ps < 0.001] for all measures. Post-hoc analyses indicated differences between sign- and goal-trackers in all lever-directed measures on days 2-5 (p < 0.001), and all food cup-directed behaviors on days 4-5 (p < 0.001).
Fig. 1.
Sign-tracking (left) and goal-tracking (right) behaviors during Pavlovian Conditioned Approach. Contacts indicate the mean number of lever contacts (A) and food cup entries (D) each day. Probability displays the mean number of trials in which a lever contact (B) or food cup entry (E) occurred. Latencies indicate the mean elapsed time (s) before either contacting the lever (C) or entering the food cup (F). Data are represented as the mean (+/− SEM). Rats were categorized as STs (n = 10) or GTs (n = 18) based on calculated PavCA index (see “Methods and Materials”). See text for the description of statistical results.
Repeated cocaine-induced USVs and categorization
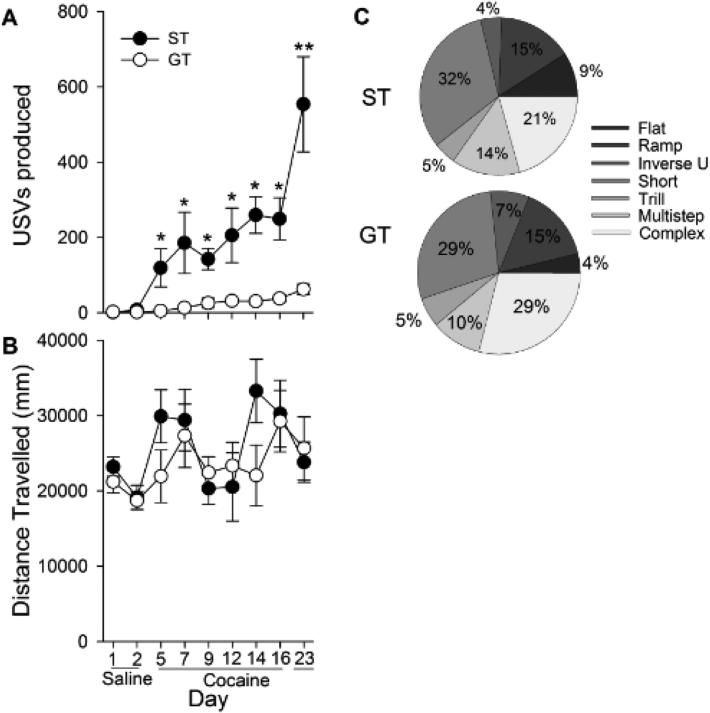
Repeated measures ANOVA revealed main effects of Phenotype [F (1, 22) = 35.1, p < 0.001] and Day [F (8, 176) = 14.4, p > 0.001] on USV production. An interaction of these variables (Phenotype × Day) on USVs [Fig. 2A; F (8, 176) = 8.7, p < 0.001] was also found, and post-hoc analysis indicated that sign-trackers produced more USVs than goal-trackers on all cocaine-treated days (ps < 0.05), but not saline. On any given day, sign-trackers emitted at least five times more cocaine-induced USVs than goal-trackers. Furthermore, the USV response to cocaine sensitized in sign-trackers, as demonstrated by a significant increase in cocaine-induced USVs from the first administration of the drug to the last (e.g., day 3 vs. day 16, and day 3 vs. day 23; ps < 0.05). This sensitization in the USV response to cocaine was not statistically significant in goal-trackers (ps = 0.39, 0.14). In addition, sign-trackers, but not goal-trackers, emitted significantly more USVs on day 23 compared to day 16 (p < 0.01), indicating that the drug-free period further sensitized the response in sign-trackers. In contrast, there was no statistical difference in cocaine-induced locomotion between the two phenotypes (Fig. 2B). Finally, while sign-trackers made proportionally more flat USVs than goal-trackers on day 16 (13% vs 5%; p < 0.001) as well as day 23 (9% vs 4%; p < 0.05), there were no differences in any other subtype of cocaine-induced USVs (Fig. 2C).
Fig. 2.
USVs produced (A) and locomotor activity (B) of STs (n = 9) and GTs (n = 14) during saline and cocaine treatment across days in experiment 1. Single asterisks (*) denote differences between STs and GTs on the indicated cocaine-treated days. Double asterisks (**) denote a ST and GT difference on the indicated day as well as a ST within-group difference between Day 16 and Day 23. Data are represented as the mean (+/− SEM). Percentages of occurrence for each of the seven USV categories during Day 23 are shown for both groups (C). Categories were previously based on Wright et al. (2010).
Experiment 2
Acute cocaine-induced USVs
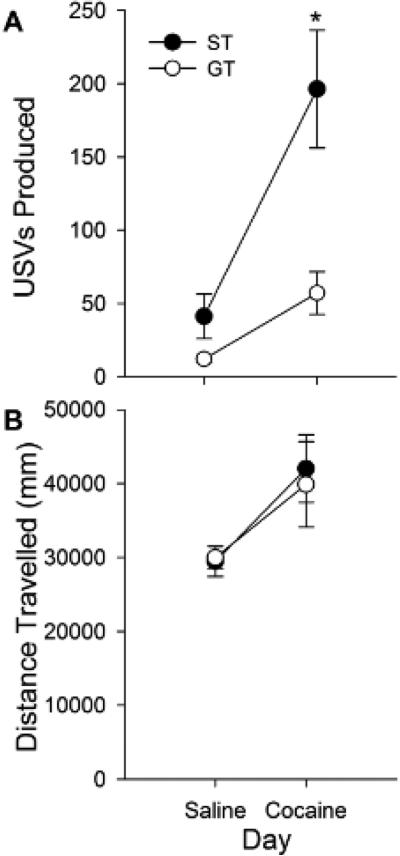
In experiment 2, sign-trackers (n = 19) again produced more cocaine-induced USVs compared to goal-trackers (n = 19) after a single treatment. Repeated measures ANOVA revealed main effects of Phenotype [F (1, 36) = 10.4, p < 0.001] and Day [F (1, 36) = 27.6, p < .001] as well as a Phenotype × Day interaction for USV production [F (1,36) = 7.7, p > 0.01]. Post-hoc analysis of this interaction suggested that the difference in USVs between sign- and goal-trackers was specific under cocaine-treatment, and not saline (Fig. 3A; p < 0.001). Sign- and goal-trackers showed no difference in locomotion for either day (Fig. 3B).
Fig. 3.
USV production (A) and locomotor activity (B) of STs (n = 19) and GTs (n = 19) during saline- and cocaine-treated days of experiment 2. Asterisk (*) indicates a significant ST/GT difference in USVs on the cocaine-treated day. Data are represented as the mean (+/− SEM).
PavCA USVs
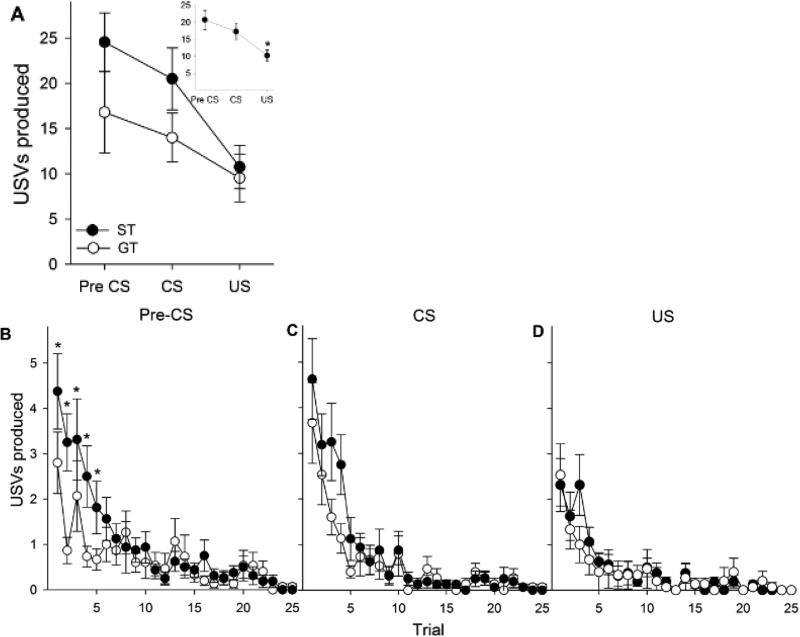
USVs were recorded in a subset of randomly selected subjects (n = 31) during PavCA to determine whether the food cue would elicit USVs and if USV production would differ between sign- (n = 16) and goal-trackers (n = 15) in this task (Fig. 4A). We analyzed the eight second periods of time prior to (Fig 4B; pre-CS), during (Fig 4C; CS), and after (Fig 4D; US) CS presentation to determine the USV response to no stimuli, the lever stimuli and the food stimuli, respectively. Repeated measures ANOVA revealed a significant main effect of Period [F (2, 58) = 18.4, p < 0.001], and post-hoc analyses indicated that more USVs were produced during the pre-CS and CS periods compared to the US period (Fig. 4A inlet; ps < 0.001), but there was no main effect of Phenotype or Phenotype × Period interaction. However, when the pre-CS and CS periods were analyzed separately, where Trial was included as a repeated measure, we found that sign-trackers produced significantly more USVs compared to goal-trackers during the pre-CS period [main effect of Trial: F (24, 696) = 12.5, p <0.001; Phenotype × Trial interaction: F (24, 696) = 2.2, p < 0.001]. This interaction was not observed in the CS period, although there was a main effect of Trial [F (24, 696) = 20.5, p < 0.001]. Post-hoc analysis indicated that sign-trackers produced more USVs during the pre-CS than goal-trackers, but only during the first five trials (p < 0.05). The US period could not be analyzed in this manner because no USVs were made by either group during later trials. In summary, during PavCA, sign- and goal-trackers only differed in USV production during the eight-second pre-CS period of the first five trials, and both phenotypes showed a decrease in USV production during all periods over the course of the session.
Fig. 4.
USVs produced by STs (n = 16) and GTs (n = 15) during each eight-second period summed over the 25 trials of PavCA in experiment 2 (A). Inset depicts mean USVs during each period collapsed across phenotype (n=31). Asterisk (*) indicates a significant decrease (p < 0.05) of in USVs produced during the US period compared to pre-CS and CS periods. Pre-CS (B), CS (C) and US (D) panels show USVs produced by STs and GTs during the 25 trials of PavCA. Asterisks (*) indicates a significant ST/GT difference for designated trials. Data are represented as the mean (+/− SEM).
Discussion
In summary, we found that sign-trackers consistently produced five or more time the cocaine-induced 50 kHz USVs than goal-trackers on all treatment days. This behavioral distinction between phenotypes was evident even on the first day of cocaine exposure. In addition, repeated cocaine sensitized the USV response in sign-trackers, but not significantly in goal-trackers, and this sensitization difference became most pronounced after a weeklong drug-free period. Further, during PavCA, sign-trackers emitted more USVs than goal-trackers, but only at the beginning of the session. This difference was specifically observed during the first five pre-CS periods. Together, these results indicate a strong relationship between cue responsivity and cocaine-induced vocalizations, implying that these two behaviors may rely on overlapping neurobehavioral mechanisms. Previous work has indicated other phenotypic differences between sign- and goal-trackers including how individuals respond to drugs of abuse and their associated cues during various paradigms such as cocaine self-administration, cue-induced reinstatement, and conditioned place preference (Meyer et al. 2012b; Saunders and Robinson 2010; 2011; Yager and Robinson 2013). Thus, our results suggest that these established distinctions may be due, in part, to differences in the unconditioned responses to cocaine.
Cocaine-induced USVs
In experiment 1, sign-trackers produced significantly more cocaine-induced USVs than goal-trackers on the first day of administration. This was also true in experiment 2 when rats were treated with cocaine prior to PavCA training, thus showing that the difference in cocaine-induced USVs does not rely on training for expression. This phenotypic difference in USVs was similarly seen in a conditioned place preference (CPP) paradigm, during which cocaine-treated sign-trackers produced more USVs than cocaine-treated goal-trackers for all days of cocaine-pairings (Meyer et al. 2012b). Thus, these results suggest that sign-trackers are more sensitive to the unconditioned, affective effects of cocaine relative to goal-trackers. Further, this may be related to the reinforcing effect of cocaine. For example, Browning et al. (2011) measured USVs during cocaine self-administration and found that USV production on the first day of acquisition was positively correlated with the speed at which cocaine self-administration was acquired. Moreover, sign-trackers that showed novelty-induced place preference also acquired cocaine self-administration more rapidly (Beckmann et al. 2011). Taken together with the current findings, the initial USV response to cocaine in sign-trackers may reflect their sensitivity to the motivational properties of cocaine and thus could serve as a predictor of the tendency to engage in drug-taking behavior.
The current study also showed that with repeated administration, the USV response to cocaine sensitized in sign-trackers, but not significantly in goal-trackers, and the USV response to cocaine was further sensitized in sign-trackers after a week-long drug-free period. In other studies, subjects that exhibit behavioral phenotypes related to sign- and goal-tracking showed a comparable distinction in the sensitized USV response to cocaine. For example, Mu et al. (2009) used rat lines that had been selectively bred for high and low USV production in response to socially rewarding “tickling” (HL and LL, respectively) which is related to high and low responsiveness to rewarding stimuli (Panksepp and Burgdorf 2000). While both groups increase USV production with a 15 mg/kg (i.p.) cocaine treatment, the magnitude of sensitization of the USV response to repeated cocaine was much larger in HL rats than LL rats, with HL rats making more than two times the USVs of LL rats by the fifth day of treatment. This effect was further enhanced in HL rats by a cocaine challenge following two days of abstinence, which demonstrates not only a greater sensitivity to the sensitizing effects of cocaine in the HL line, but also the possible development of a conditioned response to the drug-associated context (Mu et al. 2009). In line with this idea, additional studies have shown that individuals exhibiting USV sensitization to psychostimulant drugs will also display a conditioned escalation in USVs in response to the drug-associated context alone (Ahrens et al. 2009; Meyer et al. 2012b; Simola and Morelli 2015). Although our study did not specifically test context-induced USVs, it can be noted that some sign-trackers immediately began producing USVs upon entering the chamber on all days following the first cocaine treatment, even though peak levels of cocaine in the brain are not achieved until 10 minutes after the injection (Javaid and Davis 1993; Maier et al. 2012). These USVs could be a result of the conditioned effects of the context itself, possibly in anticipation of cocaine's effects (Ma et al. 2010). This evidence further substantiates the idea that the sign-tracking phenotype is sensitive to the motivational salience of drug-related cues and contexts.
The differences in the development of sensitization also suggest that sign-trackers may be more sensitive to cocaine-induced neuroplasticity, which involves alterations in the mesolimbic dopamine system (Berridge 2001; Berridge et al. 2009; Pierce and Kalivas 1997; Vanderschuren and Kalivas 2000). For example, individuals prone to sign-tracking have more cue-induced increases in dopamine in the nucleus accumbens, and are sensitive to dopamine receptor antagonism (Flagel et al. 2011; Saunders and Robinson 2012; Tomie et al. 2000). Dopamine release in the nucleus accumbens has been linked to the production of 50 kHz USVs, and thus sensitization of drug-induced USVs may be a result of sensitization of dopamine transmission in this circuit (Burgdorf et al. 2001; Burgdorf et al. 2008; Simola and Morelli 2015; Willuhn et al. 2014). Thus, our findings corroborate the idea that sign- and goal-trackers differ in terms of cocaine-induced adaptations of the dopamine system.
Interestingly, while there were large differences in the rates of USV production between sign- trackers and goal-trackers on day 16 and day 23, only the proportions of flat USVs were greater in sign-trackers compared to goal-trackers. Previously, Wright et al. (2010) found that trill USVs increased when rats were paired together or when they were given amphetamine, and suggested that these calls are “reward associated”. However, we found no differences in the proportions of trill USVs produced by sign-trackers and goal-trackers. Wright et al. (2010) also found an increase in flat USVs when rats were paired together, suggesting a social function for this call type, and Wohr et al. (2008) suggested that flat USVs are more likely to be produced in a social context for establishing or maintaining contact between individuals. In contrast, Mahler et al. (2013), found that rats made more flat USVs during multiple forms of methamphetamine cue-induced reinstatement, and suggested that the flat USVs have a non-specific function (Brudzynski 2005; Burgdorf et al. 2008; Schwarting et al. 2007). Thus, the difference in flat USVs warrants further investigation, and may provide insight into both the behavioral disposition of these phenotypes, as well as the behavioral significance of flat USVs.
2. Cocaine-induced locomotion
In both experiments, locomotor activity for all subjects increased as a result of cocaine treatment compared to saline treatment, however there were no differences found between sign- and goal-trackers on the first day of administration, nor on any subsequent treatment day. This indicates that acute locomotor response to cocaine may not be as sensitive as USVs in revealing underlying neurobiological differences between the sign- and goal-tracker phenotypes. This is in line with Flagel et al. (2008), who measured acute locomotor response and head movements after repeated cocaine. Similar to our results, they did not find a difference in locomotion, although they did see modest increases in head movements among sign-trackers after repeated cocaine. Studies utilizing other phenotypes related to sign- and goal-tracking have shown similar dissociation between drug-induced USVs and locomotion. For example, Garcia and Cain (2016) measured amphetamine induced-locomotion and USVs in high and low novelty-seeking individuals and found that while USVs were positively correlated with novelty seeking, locomotor activity was not. Additionally, Mu et al. (2009) showed a difference in sensitization of USVs between HL and LL rats, without showing the same effect in locomotion after repeated administration, indicating that while neuroplastic changes may have been occurring in HL rats, it was not indexed by locomotor activity. Finally, Maier et al. (2012) found that while the USV response to cocaine during self-administration increased following the drug-free weekend periods, locomotor activity and lever responding did not. Overall, these studies suggest that drug induced-locomotor activity and USV production are dissociable and represent different behavioral responses. It appears that drug-induced USVs are unique in identifying positive appetitive states and thus reflect different neurobehavioral processes than those expressed by drug-induced locomotion (Knutson et al. 2002; Ma et al. 2010; Simola and Morelli 2015).
3. Food cue-induced USVs
USV results from the last day of PavCA showed a marginal difference between sign- and goal-trackers, with sign-trackers emitting more USVs during the pre-CS period of the first five trials of the task, but not during the CS itself. In this instance, the production of USVs seems to occur independently from approach and consummatory behaviors. These results were unexpected, particularly for sign-trackers, because previous studies have shown increases in 50 kHz USVs during approach to rewarding stimuli (Burgdorf et al. 2000; Knutson et al. 2002). In another example, Browning et al. (2011) measured USVs during acquisition, extinction and cue-induced reinstatement phases of sucrose self-administration in which ad lib fed rats nose-poked for delivery of a sucrose pellet. In this procedure, USV production was relatively stable, with no significant difference in the rate of USVs in each phase. However, when evaluating the time course of USVs within a 2 h testing session, subjects increase their USV production in the first five minutes, but then decreased to near zero. This initial increase in USVs followed by an appreciable reduction was similar to our results and could be due in part, to the conditioned response to the testing environment, which appears to be heightened in sign-trackers compared to goal-trackers. Importantly, the enhanced USV response during the pre-CS periods of the first five trials in sign-trackers is not a result of “anticipation” for the lever cue due to the variable nature of the ITI separating each CS, which makes its presentation unpredictable. Rather, this increased rate during the pre-CS period may be due to differences in context-induced USVs, which would suggest that the PavCA chamber itself acquired more motivational salience in sign-trackers than goal-trackers. This USV response habituated in both groups over time, perhaps indicating a decreasing appetitive state separable from the approach behaviors observed throughout the session. Additionally, explanation for our low rate of cue-induced USVs from either group may be a result of several factors, including predictability of the cue presentations, (Burgdorf et al. 2000; Burgdorf et al. 2005), food deprivation (Brenes and Schwarting 2014; Knutson et al. 2002), the nature of the CS (auditory vs visual) (Brenes and Schwarting 2015; Meyer et al. 2014), or the form of the approach measured (Brenes and Schwarting 2015). Thus, whether or not a cue elicits the initiation and/or enhancement in USVs is dependent on the specifics of the paradigm, rather than being a consistent response to a broad class of reward-associated stimuli.
In conclusion, the results of the current study show that cocaine-induced USVs are an unconditioned response to cocaine that is separate from locomotion. After repeated cocaine administration, these USVs likely reflect neuroadaptations in response to drug that are unique to individuals who have a propensity to attribute incentive salience to rewards and their cues. These findings add to the growing body of literature that implicates USVs as an important measure of affective and motivational states in response to dopaminergic drugs and their related cues (Knutson et al. 1999; 2002; Panksepp and Burgdorf 2000). As Mahler et al. (2013) has speculated, drug-induced USVs may be a model of subjective “self-report” during acute and repeated cocaine exposure. Thus, USVs may be a particularly useful index of the motivational properties of drugs; one that does not require learning (and can thus be measured in a single session), and also reflects individual variation of other traits associated with pathological motivational states, including drug addiction.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse (P50DA037844). The authors would like to thank Dr. Alexis Thompson, Dr. Matt Paul and Christopher King for their comments on earlier versions of the manuscript.
References
- Ahrens AM, Ma ST, Maier EY, Duvauchelle CL, Schallert T. Repeated intravenous amphetamine exposure: rapid and persistent sensitization of 50-kHz ultrasonic trill calls in rats. Behavioral Brain Research. 2009;197:205–9. doi: 10.1016/j.bbr.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behavioral Brain Research. 2011;216:159–65. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Reward learning: Reinforcement, incentives, and expectations. In: Medin DL, editor. Psychology of Learning and Motivation: Advances in Research and Theory. Academic Press Inc.; New York: 2001. pp. 223–278. [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behavioral Neuroscience. 2000;114:983–90. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Bindra D. How adaptive behavior is produced: A perceptual-motivation alternative to response reinforcement. Behavioral Brain Research. 1978;1:41–91. [Google Scholar]
- Boakes R. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz H, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 67–97. [Google Scholar]
- Brenes JC, Schwarting RK. Attribution and expression of incentive salience are differentially signaled by ultrasonic vocalizations in rats. PLoS One. 2014;9:e102414. doi: 10.1371/journal.pone.0102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenes JC, Schwarting RK. Individual differences in anticipatory activity to food rewards predict cue-induced appetitive 50-kHz calls in rats. Physiol Behav. 2015;149:107–18. doi: 10.1016/j.physbeh.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Browning JR, Browning DA, Maxwell AO, Dong Y, Jansen HT, Panksepp J, Sorg BA. Positive affective vocalizations during cocaine and sucrose self-administration: A model for spontaneous drug desire in rats. Neuropharmacology. 2011;61:268–75. doi: 10.1016/j.neuropharm.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behav Genet. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Current opinion in neurobiology. 2013;23:310–7. doi: 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behavioral Neuroscience. 2000;114:320–7. [PubMed] [Google Scholar]
- Burgdorf J, Knutson B, Panksepp J, Ikemoto S. Nucleus accumbens amphetamine microinjections unconditionally elicit 50-kHz ultrasonic vocalizations in rats. Behavioral Neuroscience. 2001;115:940–4. doi: 10.1037//0735-7044.115.4.940. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–67. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Beinfeld MC, Kroes RA, Moskal JR. Regional brain cholecystokinin changes as a function of rough-and-tumble play behavior in adolescent rats. Peptides. 2006;27:172–7. doi: 10.1016/j.peptides.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Panksepp J, Brudzynski SM, Kroes R, Moskal JR. Breeding for 50-kHz positive affective vocalization in rats. Behav Genet. 2005;35:67–72. doi: 10.1007/s10519-004-0856-5. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology 56 Suppl. 2009;1:139–48. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behavioral Brain Research. 2008;186:48–56. doi: 10.1016/j.bbr.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Garcia EJ, Cain ME. Novelty response and 50 kHz ultrasonic vocalizations: Differential prediction of locomotor and affective response to amphetamine in Sprague-Dawley rats. Psychopharmacology. 2016;233:625–37. doi: 10.1007/s00213-015-4132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins HM. Sign tracking: The stimulus-reinforcer relation and directed action.. Proceedings of the Psychonomic Society; Austin, TX. 1974. [Google Scholar]
- Javaid JI, Davis JM. Cocaine disposition in discrete regions of rat brain. Biopharmaceutics & drug disposition. 1993;14:357–64. doi: 10.1002/bdd.2510140408. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. J Comp Psychol. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. High-frequency ultrasonic vocalizations index conditioned pharmacological reward in rats. Physiol Behav. 1999;66:639–43. doi: 10.1016/s0031-9384(98)00337-0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Burgdorf J, Panksepp J. Ultrasonic vocalizations as indices of affective states in rats. Psychol Bull. 2002;128:961–77. doi: 10.1037/0033-2909.128.6.961. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Congleton KM, See RE. Conditioned reinstatement of drug-seeking behavior with a discrete compound stimulus classically conditioned with intravenous cocaine. Behavioral Neuroscience. 2001;115:1086–92. doi: 10.1037//0735-7044.115.5.1086. [DOI] [PubMed] [Google Scholar]
- Ma ST, Maier EY, Ahrens AM, Schallert T, Duvauchelle CL. Repeated intravenous cocaine experience: development and escalation of pre-drug anticipatory 50-kHz ultrasonic vocalizations in rats. Behavioral Brain Research. 2010;212:109–14. doi: 10.1016/j.bbr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015475. DOI: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Feltenstein MW, Cox BM, Ogburn KB, Bachar M, McGonigal JT, Ghee SM, See RE. A rodent “self-report” measure of methamphetamine craving? Rat ultrasonic vocalizations during methamphetamine self-administration, extinction, and reinstatement. Behavioral Brain Research. 2013;236:78–89. doi: 10.1016/j.bbr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier EY, Abdalla M, Ahrens AM, Schallert T, Duvauchelle CL. The missing variable: ultrasonic vocalizations reveal hidden sensitization and tolerance-like effects during long-term cocaine administration. Psychopharmacology. 2012;219:1141–52. doi: 10.1007/s00213-011-2445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Cogan ES, Robinson TE. The form of a conditioned stimulus can influence the degree to which it acquires incentive motivational properties. PLoS One. 2014;9:e98163. doi: 10.1371/journal.pone.0098163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012a;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Ma ST, Robinson TE. A cocaine cue is more preferred and evokes more frequency- modulated 50-kHz ultrasonic vocalizations in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2012b;219:999–1009. doi: 10.1007/s00213-011-2429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu P, Fuchs T, Saal DB, Sorg BA, Dong Y, Panksepp J. Repeated cocaine exposure induces sensitization of ultrasonic vocalization in rats. Neurosci Lett. 2009;453:31–5. doi: 10.1016/j.neulet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behavioral Brain Research. 2000;115:25–38. doi: 10.1016/s0166-4328(00)00238-2. [DOI] [PubMed] [Google Scholar]
- Panksepp J, Knutson B, Burgdorf J. The role of brain emotional systems in addictions: a neuro- evolutionary perspective and new 'self-report' animal model. Addiction. 2002;97:459–69. doi: 10.1046/j.1360-0443.2002.00025.x. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press; London, UK: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17:3254–61. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Current opinion in neurobiology. 2014;28:115–20. doi: 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon KE, Dent ML. Frequency difference limens and auditory cue trading in CBA/CaJ mice (Mus musculus). Behavioural processes. 2014;106:74–6. doi: 10.1016/j.beproc.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biological Psychiatry. 2009;65:869–73. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76(Part B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biological Psychiatry. 2010;67:730–6. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual Variation in the Motivational Properties of Cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. Individual variation in resisting temptation: Implications for addiction. Neuroscience and Biobehavioral Reviews. 2013;37:1955–1975. doi: 10.1016/j.neubiorev.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting RK, Jegan N, Wohr M. Situational factors, conditions and individual variables which can determine ultrasonic vocalizations in male adult Wistar rats. Behavioral Brain Research. 2007;182:208–22. doi: 10.1016/j.bbr.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M. Repeated amphetamine administration and long-term effects on 50-kHz ultrasonic vocalizations: Possible relevance to the motivational and dopamine-stimulating properties of the drug. European Neuropsychopharmacology. 2015;25:343–355. doi: 10.1016/j.euroneuro.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D. Individual differences in pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav. 2000;65:509–17. doi: 10.1016/s0091-3057(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Research Reviews. 2008;58:121–35. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Versaggi C, King C, Meyer P. The tendency to sign-track predicts cue-induced reinforcement during nicotine self-administration, and is enhanced by nicotine but not ethanol. Psychopharmacology. 2016 doi: 10.1007/s00213-016-4341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Schwarting RK, Wohr M. Phasic dopamine release in the nucleus accumbens in response to pro-social 50kHz ultrasonic vocalizations in rats. J Neurosci. 2014;34:10616–23. doi: 10.1523/JNEUROSCI.1060-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93:766–76. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Wohr M, Schwarting RK. Affective communication in rodents: ultrasonic vocalizations as a tool for research on emotion and motivation. Cell and tissue research. 2013;354:81–97. doi: 10.1007/s00441-013-1607-9. [DOI] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology. 2010;211:1–13. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behavioral Brain Research. 2010;214:30–4. doi: 10.1016/j.bbr.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–28. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]