Abstract
Purpose
Genome-wide association studies (GWAS) have identified dozens of single nucleotide polymorphisms (SNPs) associated with breast cancer. Few studies focused on young-onset breast cancer, which exhibits etiologic and tumor-type differences from older-onset disease. Possible confounding by prenatal effects of the maternal genome has also not been considered.
Methods
Using a family-based design for breast cancer before age 50, we assessed the relationship between breast cancer and 77 GWAS-identified breast cancer risk SNPs. We estimated relative risks (RR) for inherited and maternally-mediated genetic effects. We also used published RR estimates to calculate genetic risk scores and model joint effects.
Results
Seventeen of the candidate SNPs were nominally associated with young-onset breast cancer in our 1,296 non-Hispanic white affected families (uncorrected p-value<0.05). Top-ranked SNPs included rs3803662-A (TOX3, RR=1.39; p=7.0×10−6), rs12662670-G (ESR1, RR=1.56; p=5.7×10−4), rs2981579-A (FGFR2, RR=1.24; p=0.002), and rs999737-G (RAD51B, RR=1.37; p=0.003). No maternally-mediated effects were found. A risk score based on all 77 SNPs indicated that their overall relationship to young-onset breast cancer risk was more than additive (additive-fit p=2.2×10−7) and consistent with a multiplicative joint effect (multiplicative-fit p=0.27). With the multiplicative formulation, the case sister’s genetic risk score exceeded that of her unaffected sister in 59% of families.
Conclusions
The results of this family-based study indicate that no effects of previously-identified risk SNPs were explained by prenatal effects of maternal variants. Many of the known breast cancer risk variants were associated with young-onset breast cancer, with evidence that TOX3, ESR1, FGFR2, and RAD51B are important for young-onset disease.
Keywords: young-onset breast cancer, genome-wide association study, single nucleotide polymorphism, family-based genetic methods, log-linear models, genetic risk score
Introduction
In the search for genetic risk variants associated with breast cancer, dozens of genome-wide association studies (GWAS) have identified more than a hundred single nucleotide polymorphisms (SNPs) [1–33]. Many of these susceptibility loci have been confirmed in other study samples and across racial groups [34–39], but relatively few studies were designed to examine whether these GWAS ‘hits’ are associated with young-onset breast cancer [1, 16, 23, 40–43]. Given evidence that age modifies the association between breast cancer and some non-genetic risk factors [44–47], and that younger cases are more likely to have strong family histories of the disease [48], additional studies of the genetic determinants of young-onset breast cancer are warranted.
Young-onset breast cancer is often defined as breast cancer before the age of 50, as this age is both a pro×y for menopausal status and an inflection point for incidence trends in US women [44]. Young-onset disease tends to be relatively more aggressive and difficult to treat [49, 50], and includes a higher fraction of triple-negative breast cancers [51], which may have distinct genetic risk factors [52–56].
An early GWAS of young-onset breast cancer identified one risk-associated SNP in GLG1 [16]. In a larger and more recent GWAS of young-onset breast cancer, Ahsan et al. [1] identified 96 SNPs with genome-wide significant p-values, all of which were located in six regions previously linked to breast cancer risk unrestricted by age. In total, the authors found that 32 of 83 previously identified GWAS hits were associated with young-onset disease (p<0.05).
Family-based designs are feasible for diseases that occur at young ages and offer certain advantages over case-control studies, including robustness to bias due to population stratification and the ability to assess maternally-mediated or imprinting effects [57, 58]. We previously conducted a family-based GWAS of young-onset breast cancer, in which we identified 9 SNPs with unadjusted p-values <10−5, including several novel loci [59].
Here, we further investigate the role of 77 known risk variants by reporting their individual associations with young-onset breast cancer and exploring whether their joint effects follow additive or multiplicative risk models. The selected risk variants were previously identified by Mavaddat et al. [60] as having an association with breast cancer at p<5×10−8. We also examined maternally-mediated effects [58] for these same 77 SNPs to assess the influence of the mother’s genotype acting prenatally on her daughter, controlling for the daughter’s genotype. Because mother’s and daughter’s genotypes are correlated, maternally-mediated genetic effects could confound inherited-gene effects and may have biased some of the previously-observed GWAS associations.
Methods
Study participants
Young-onset breast cancer cases and their families were recruited as part of the Sister Study and Two Sister Study. The Sister Study is a prospective cohort of women who had one or more sisters diagnosed with breast cancer, but had never had breast cancer themselves at enrollment (2003–2009). It includes 50,884 US and Puerto Rican women aged 35–74. Because they all have a first-degree family history of breast cancer, participants have, on average, approximately twice the risk of developing breast cancer compared to women with no family history. Sister Study participants who developed breast cancer before age 50 (n=235) were included as cases for this analysis.
When we enrolled women in the Sister Study, we asked them the age and date of diagnosis of their affected sister. Proband sisters whose breast cancer was diagnosed before age 50 and within the previous four years were eligible for inclusion in the Two Sister Study. We developed a family-based genetic study by asking an unaffected sister to forward a study invitation to her eligible affected sister and then asking all of the participating young-onset cases to forward a letter from us to any living parents, asking them to provide saliva samples. If one or both parents were unavailable, we genotyped DNA from the blood or saliva from unaffected sister(s) participating in the Sister Study. In total, 3,331 individuals from 1,477 families were genotyped.
All participants provided written or verbal consent and the study was approved by the National Institute of Environmental Health Sciences and the Copernicus Group Institutional Review Boards. The GWAS data is publically accessible (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000678.v1.p1). None of the Sister Study or Two Sister Study participants were included in any previously published pooled or meta-analyses.
In addition to providing DNA samples, participants completed computer-assisted telephone interviews about various health-related and lifestyle factors. All cases were asked about their breast cancer diagnosis and treatment and to authorize release of their medical records. Tumor information, including invasiveness and estrogen receptor (ER) status was extracted from the medical records for most cases (85–90%). We relied on self-report for the remainder after observing that Sister Study participants could recall their status with reasonable accuracy (positive predictive values of 99%, 64%, 99% and 84% for invasive, in situ, ER+ and ER− breast cancer, respectively). We also reviewed medical records for BRCA1/2 findings and asked participants to report the results of any BRCA1/2 mutation tests. A case was assumed to be BRCA1/2 positive if she had a positive test or if her sister had a positive test, but the case had not been tested.
Genotyping analysis
DNA samples were collected from saliva (80%), whole blood (19%) or blood clot (1%) and shipped to the Johns Hopkins University Center for Inherited Disease Research for genotyping, with subsequent quality control carried out by the Genetics Coordinating Center at the University of Washington. After extraction, DNA samples were processed using 96-well plates, with family members assigned to the same plate. We included 76 HapMap controls and 74 duplicate samples, balanced across plates. All samples were analyzed using the Illumina OmniExpress plus HumanExome-8v1-2 array, which included 964,193 SNPs.
Genotyping revealed that 11 of the sister pairs were half-sisters. The 11 unaffected half-sisters were excluded. Because numbers were inadequate for sufficiently well-parameterized analyses of minority categories, we limited analyses to the majority category of 1,296 non-Hispanic white families (Table 1). No additional participants were excluded, as individuals’ missing call rates were all <2%, and duplicate discordance and Mendelian inconsistency rates were low (7.9×10−6 per SNP per duplicate pair, and 0.0003 per SNP, respectively). Individuals with chromosomal anomalies (n=6) were assigned missing values for affected regions.
Table 1.
Non-Hispanic white participants included in the young-onset breast cancer genotyping analysis.
| Group Description | Number of Families |
Number of Cases |
|---|---|---|
| Trios (affected sister, both parents) | 416 | 416 |
| Sister-pairs (1 affected, 1 unaffected), father | 81 | 81 |
| Sister-pairs | 353 | 353 |
| Affected sister and 1 parent | 321 | 321 |
| Unaffected sister and father | 2 | 0 |
| Parents only | 2 | 0 |
| Affected sister only | 108 | 108 |
| Unaffected sister only | 11 | 0 |
| Mother only | 2 | 0 |
| TOTAL | 1296 | 1279 |
Forty of the 77 candidate SNPs were directly genotyped on our arrays. All met the inclusion criteria in non-Hispanic whites: call rates ≥97%, ≤1 discordant call in 74 study duplicates, ≤5 Mendelian errors, Hardy-Weinberg equilibrium p-values ≥ 1×10−6 among founders. Two SNPs with a minor allele frequency <1% were excluded from our assessment of individual SNP effects, but were retained in the genetic risk score analysis.
Imputation analysis
Imputation analyses were conducted at the University of Washington’s Genetics Coordinating Center using data from the 1000 Genomes Project [61]. Haplotypes were pre-phased using SHAPEIT2 [62] to improve efficiency and make use of known family structures. Imputation was then conducted using IMPUTE (v2.3.0) [63]. The imputation was highly accurate, with good concordance between measured and masked but imputed as most-probable genotypes (99.7% and 98.0% when minor allele frequencies were <5% and ≥5%, respectively).
In total, 22 million loci were imputed, including 37 of the non-genotyped SNPs from our list of 77. We imputed based on the most probable imputed genotype. If none of an individual’s estimated genotype probabilities exceeded 90%, the genotype for that locus was considered missing.
Statistical analysis
Individual SNP effects
For each of the 75 typed candidate SNPs (38 genotyped, 37 imputed), we recorded the odds ratio (OR) reported in Mavaddat et al. [60] and noted which allele was associated with increased breast cancer risk. We then tested the association between each of the candidate SNPs and young-onset breast cancer using a likelihood-based log-linear model to assess transmission distortion within families [58]. Briefly, this conditional model examines whether the relative frequencies of particular offspring genotypes at a di-allelic locus are consistent with Mendelian inheritance. Expected frequencies are modeled using a multinomial distribution with six mating type parameters, which impose conditioning on parental genotypes, and fifteen possible case-parent outcomes. Using likelihood ratio tests (χ2 distribution), we tested the association between each SNP and young-onset breast cancer by comparing models with and without a term for the relative risk of the offspring’s genetic effect. We estimated the relative risks (RR) using Poisson regression. If either or both parents were unavailable, an expectation-maximization algorithm was applied to maximize the likelihood [64, 65]. Because we selected these SNPs based on evidence that they were associated with breast cancer, we did not correct p-values for multiple comparisons, but used α=0.05 to evaluate statistical significance.
To facilitate comparisons with Mavaddat et al. [60] and other prior studies, we estimated RRs for each SNP in relation to the previously-established risk allele. We assumed a log-additive model, coding genotype as the number of copies of the risk allele carried by the affected offspring. All analyses used LEM (http://members.home.nl/jeroenvermunt/#Software) and R (v3.2.1). We performed t-tests to compare the risk ratio observed in our study (representative of the young-onset breast cancer effect estimate) and the pooled odds ratio reported by Mavaddat et al. [60] (representative of the overall breast cancer effect estimate).
We also assessed the effects of each SNP in family subsets limited to invasive, ER+, or premenopausal breast cancer and in the subset of families not known to carry BRCA1/2 mutations. Small numbers precluded assessments for the complementary categories. We also estimated RRs for maternally-mediated effects [58].
As secondary analyses, we considered 89 other SNPs known to be associated with breast cancer risk. Additional details of the SNP selection process and results can be found in the Supplementary Materials.
Genetic Risk Scores
In addition to assessing their main effects, we also assessed the efficacy of our 77-SNP set by calculating both multiplicative and additive scores and comparing their model fits and predictive properties. The multiplicative genetic risk score was calculated as: where ORi is the previously reported OR for one copy of the risk variant at SNP i and xi is the number of copies of that risk variant carried by the individual. The summation is over k=77 candidate SNPs. Therefore, if 1) the effect size for each SNP in our study is equal to the previously-reported effect size, 2) the joint effect of more than one copy is multiplicative, and 3) the joint effect of multiple SNPs is multiplicative, then the risk score will equal the ln-OR for the 77-SNP set. Thus, the coefficient of is 1.0 under a multiplicative joint effects model. To the extent that the multiplicative genetic risk score and the ln-odds of developing young-onset breast cancer differ (as when the β coefficient differs from 1), there is a departure from multiplicity. The multiplicative joint effects model fit can be tested by comparing β to 1.0 using a Wald test (β-1 divided by standard error). One can also test whether the multiplicative genetic risk score is associated with young-onset breast cancer by comparing β to 0, again using a Wald test.
The additive genetic risk score was calculated as: Here, if 1) the effect size for each SNP in our study is equal to the previously-reported effect size, 2) the effect of having more than one copy of the risk allele is additive, and 3) the joint effect of multiple SNPs is additive, then that risk score equals the ln-OR for young-onset breast cancer. Thus, the true coefficient of is 1.0 under a fully additive joint effects model. As with the multiplicative model, the additive model fit can be tested by comparing β to 1.0 using a Wald test, and the association between the additive risk score and young-onset breast cancer can be tested by comparing β to 0.
We calculated the effect estimate (β) per unit increase in risk score using a conditional logistic regression model to compare cases to unaffected sister controls. When both sisters were genotyped, we compared them directly. If the unaffected sister was not genotyped but both parents were, we compared cases to an equally-likely, complementary pseudo-sister whose genotype was defined by her parents’ non-transmitted alleles. Altogether, we included 850 case-sister or case-pseudo-sister pairs in the risk scores analyses. For sporadic missing genotypes, we filled in the expected risk allele counts based on the allele frequencies in the parents. As both sets of risk score models were able to accommodate genotypes for imputed SNPs that were not whole numbers, we assigned individual genotypes for imputed SNPs based on expected allele counts.
The relative risk estimates for the 77 SNPs included in the risk score were taken from Mavaddat et al. [60]. These estimates were calculated using data from a pooled analysis with 33,673 cases. None of the 77 selected SNPs was in high linkage disequilibrium (LD) with another included SNP (all r2<0.80) [60].
To assess model fit and risk score utility, we calculated four p-values for each SNP set: an additive score testing β=0; an additive-fit score testing β=1; a multiplicative score testing β=0; and a multiplicative-fit score testing β=1. Additionally, we compared scores between sister pairs to assess their ability to predict risk. We also tested for maternally-mediated genetic effects by comparing scores of mothers to scores of fathers in families where both parents were genotyped (n=418).
As a natural extension and a means to further examine risk score utility, we also used logistic regression to examine whether the mothers’ scores were associated with their own breast cancer risk. Cases included 119 mothers with breast cancer at any age (cases) versus 599 mothers with no history of breast cancer (controls).
Results
At diagnosis, most of the 1,279 cases from the 1,296 non-Hispanic white families included in our analysis were aged 40–49 (89%) and premenopausal (93%) (Table 2). The proportions of invasive, ER+ and recognized BRCA1/2 mutation positive cases were 84%, 81% and 8%, respectively.
Table 2.
Characteristics of young-onset breast cancer casesincluded in the genotyping analysis (n=1279).
| N (%) | ||
|---|---|---|
| Age at diagnosis | ||
| <40 | 136 (11) | |
| 40–49 | 1143 (89) | |
| Menopausal Status at Diagnosis | ||
| Premenopausal | 1186 (93) | |
| Postmenopausal | 86 (7) | |
| Missing | 7 | |
| Invasive Status | ||
| Ductal carcinoma in situ | 206 (16) | |
| Invasive | 1057 (84) | |
| Missing | 16 | |
| Estrogen Receptor Status | ||
| Positive | 1013 (81) | |
| Negative | 239 (19) | |
| Missing | 27 | |
| BRCA1/2 statusa | ||
| Case carries BRCA1/2 mutation | 100 (8) | |
| Case not known to have | 1179 (92) | |
| BRCA1/2 mutation | ||
Families were categorized as BRCA1 or BRCA2 mutation positive if (1) the case sister reported that she had had a positive test or (2) the case sister was not tested but the unaffected sister reported that she had had a positive test.
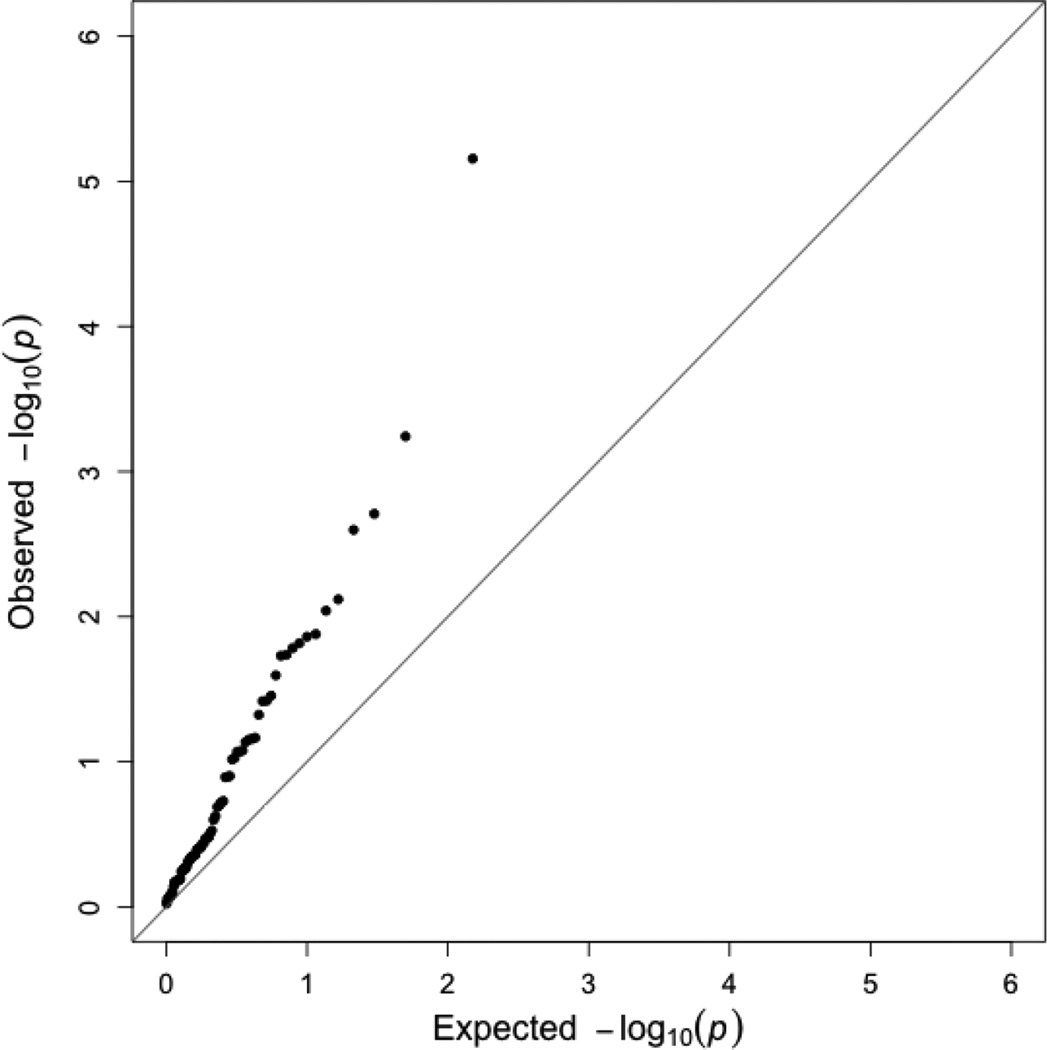
Seventeen of the candidate SNPs were associated with increased risk of young-onset breast cancer (p-value<0.05; Table 3). As demonstrated by the quantile-quantile plot (Figure 1), the distribution of p-values for the candidate SNPs was markedly shifted relative to the uniform distribution expected under a global null for the set. The SNP with the smallest p-value was rs3803662-A, which is located upstream of TOX3 (RR=1.39, 95% CI:1.20–1.60; p=7.0×10−6). The next smallest p-values were for rs12662670-G in ESR1 (RR=1.56, 95% CI: 1.20–2.03; p=5.7×10−4), rs2981579-A in FGFR2 (RR=1.24, 95% CI: 1.08–1.42; p=0.002), and rs999737-G in RAD51B (RR=1.27, 95% CI: 1.09–1.48; p=0.003).
Table 3.
Relative risks and 95% confidence intervals for the association between candidate SNPs and young-onset breast cancer
| Rank | SNPa | Gene/ Region |
Risk Allele |
RAF | RR (95% CI) | p-value | Reported ORb |
|---|---|---|---|---|---|---|---|
| 1 | rs3803662 | TOX3 | A | 0.30 | 1.39 (1.20, 1.60) | 7.0×10−6 | 1.23 |
| 2 | rs12662670 | ESR1 | G | 0.08 | 1.56 (1.20, 2.03) | 5.7×10−4 | 1.14 |
| 3 | rs2981579 | FGFR2 | A | 0.44 | 1.24 (1.08, 1.42) | 0.002 | 1.25 |
| 4 | rs999737 | RAD51B | G | 0.77 | 1.27 (1.09, 1.48) | 0.003 | 1.09 |
| 5 | rs2046210 | ESR1 | A | 0.35 | 1.21 (1.05, 1.39) | 0.008 | 1.05 |
| 6 | rs4849887 | 2q14.2 | C | 0.89 | 1.31 (1.07, 1.60) | 0.009 | 1.09 |
| 7 | rs7726159 | TERT | A | 0.32 | 1.28 (1.05, 1.55) | 0.01 | 1.04 |
| 8 | rs720475 | ARHGEF5 | G | 0.75 | 1.20 (1.04, 1.39) | 0.01 | 1.06 |
| 9 | rs11199914 | 10q26.12 | C | 0.69 | 1.20 (1.04, 1.38) | 0.02 | 1.06 |
| 10 | rs554219 | CCND1 | G | 0.13 | 1.27 (1.04, 1.55) | 0.02 | 1.12 |
| 11 | rs6001930 | MKL1 | G | 0.10 | 1.29 (1.04, 1.59) | 0.02 | 1.13 |
| 12 | rs10759243 | 9q31.2 | A | 0.27 | 1.21 (1.03, 1.41) | 0.02 | 1.05 |
| 13 | rs10941679 | MRPS30 | G | 0.25 | 1.20 (1.02, 1.40) | 0.03 | 1.12 |
| 14 | rs13281615 | 8q24.21 | G | 0.42 | 1.16 (1.01, 1.32) | 0.04 | 1.10 |
| 15 | rs616488 | PEX14 | A | 0.66 | 1.16 (1.01, 1.33) | 0.04 | 1.06 |
| 16 | rs11780156 | 8q24.21 | T | 0.18 | 1.19 (1.01, 1.41) | 0.04 | 1.07 |
| 17 | rs6828523 | ADAM29 | C | 0.90 | 1.24 (1.00, 1.54) | 0.05 | 1.10 |
| 18 | rs2943559 | HNF4G | G | 0.08 | 1.24 (0.98, 1.57) | 0.07 | 1.13 |
| 19 | rs12493607 | TGFBR2 | C | 0.34 | 1.14 (0.99, 1.32) | 0.07 | 1.05 |
| 20 | rs4973768 | SLC4A7 | A | 0.50 | 1.13 (0.99, 1.28) | 0.07 | 1.09 |
| 21 | rs75915166 | FGF3 | A | 0.06 | 1.29 (0.97, 1.72) | 0.07 | 1.02 |
| 22 | rs17356907 | 12q22 | A | 0.73 | 1.14 (0.98, 1.32) | 0.08 | 1.10 |
| 23 | rs2736108 | TERT | T | 0.73 | 1.17 (0.98, 1.39) | 0.09 | 1.07 |
| 24 | rs7072776 | MLLT10 | A | 0.31 | 0.88 (0.77, 1.02) | 0.09 | 1.06 |
| 25 | rs3903072 | 11q13.1 | G | 0.53 | 1.12 (0.98, 1.27) | 0.09 | 1.06 |
| 26 | rs941764 | CCDC88C | G | 0.35 | 1.12 (0.98, 1.29) | 0.10 | 1.06 |
| 27 | rs10472076 | RPL5P15 | G | 0.36 | 1.11 (0.97, 1.27) | 0.13 | 1.04 |
| 28 | rs1292011 | 12q24.21 | A | 0.60 | 1.11 (0.97, 1.26) | 0.13 | 1.08 |
| 29 | rs1011970 | CDKN2B | A | 0.17 | 1.14 (0.96, 1.36) | 0.13 | 1.05 |
| 30 | rs11242675 | FOXQ1 | A | 0.63 | 1.10 (0.96, 1.26) | 0.19 | 1.06 |
| 31 | rs78540526 | CCND1 | T | 0.08 | 1.19 (0.92, 1.55) | 0.19 | 1.18 |
| 32 | rs1045485 | CASP8 | C | 0.88 | 1.14 (0.93, 1.39) | 0.20 | 1.04 |
| 33 | rs12710696 | LINC01376 | T | 0.37 | 1.09 (0.95, 1.24) | 0.20 | 1.04 |
| 34 | rs9693444 | 8p12 | A | 0.44 | 1.09 (0.95, 1.25) | 0.24 | 1.07 |
| 35 | rs11075995 | FTO | A | 0.24 | 0.92 (0.79, 1.06) | 0.25 | 1.04 |
| 36 | rs6762644 | ITPR1 | G | 0.39 | 0.93 (0.82, 1.06) | 0.30 | 1.07 |
| 37 | rs527616 | 18q11.2 | G | 0.68 | 1.09 (0.92, 1.29) | 0.31 | 1.04 |
| 38 | rs17817449 | FTO | A | 0.61 | 1.07 (0.94, 1.22) | 0.33 | 1.08 |
| 39 | rs4245739 | MDM4 | C | 0.27 | 1.08 (0.93, 1.25) | 0.33 | 1.03 |
| 40 | rs889312 | MAP3K1 | C | 0.31 | 1.07 (0.93, 1.23) | 0.34 | 1.12 |
| 41 | rs1436904 | CHST9 | A | 0.60 | 1.06 (0.93, 1.21) | 0.36 | 1.06 |
| 42 | rs11249433 | EMBP1 | G | 0.42 | 1.06 (0.93, 1.22) | 0.37 | 1.10 |
| 43 | rs704010 | ZMIZ1 | A | 0.41 | 1.06 (0.93, 1.21) | 0.39 | 1.07 |
| 44 | rs7904519 | TCF7L2 | G | 0.47 | 0.94 (0.82, 1.08) | 0.39 | 1.06 |
| 45 | rs2236007 | PAX9 | G | 0.88 | 1.07 (0.92, 1.25) | 0.40 | 1.09 |
| 46 | rs6472903 | 8q21.11 | A | 0.83 | 1.08 (0.91, 1.28) | 0.40 | 1.10 |
| 47 | rs11820646 | 11q24.3 | C | 0.61 | 0.95 (0.82, 1.09) | 0.44 | 1.05 |
| 48 | rs2363956 | ANKLE1 | A | 0.51 | 0.95 (0.83, 1.08) | 0.44 | 1.03 |
| 49 | rs3817198 | LSP1 | G | 0.34 | 1.05 (0.92, 1.21) | 0.45 | 1.07 |
| 50 | rs2016394 | DLX2 | G | 0.53 | 1.05 (0.92, 1.20) | 0.45 | 1.05 |
| 51 | rs865686 | RPL31P43 | A | 0.63 | 1.05 (0.92, 1.21) | 0.46 | 1.11 |
| 52 | rs17529111 | 6q14.1 | G | 0.22 | 1.06 (0.90, 1.26) | 0.48 | 1.05 |
| 53 | rs2823093 | CYYR1 | G | 0.73 | 1.05 (0.91, 1.21) | 0.49 | 1.08 |
| 54 | rs132390 | EMID1 | C | 0.02 | 1.16 (0.74, 1.81) | 0.52 | 1.11 |
| 55 | rs10771399 | 12p11.22 | G | 0.89 | 1.07 (0.87, 1.31) | 0.54 | 1.16 |
| 56 | rs9790517 | TET2 | A | 0.24 | 1.05 (0.90, 1.22) | 0.55 | 1.05 |
| 57 | rs6504950 | STXBP4 | G | 0.73 | 1.05 (0.90, 1.22) | 0.55 | 1.07 |
| 58 | rs3760982 | KCNN4 | A | 0.47 | 1.04 (0.91, 1.19) | 0.57 | 1.06 |
| 59 | rs13387042 | TNP1 | G | 0.54 | 1.04 (0.91, 1.18) | 0.57 | 1.14 |
| 60 | rs12422552 | 12p13.1 | C | 0.28 | 0.97 (0.83, 1.12) | 0.64 | 1.03 |
| 61 | rs6678914 | LGR6 | G | 0.59 | 0.97 (0.85, 1.11) | 0.65 | 1.01 |
| 62 | rs11552449 | AP4B1 | A | 0.15 | 0.96 (0.81, 1.14) | 0.65 | 1.08 |
| 63 | rs1550623 | CDCA7 | A | 0.85 | 1.04 (0.87, 1.24) | 0.66 | 1.06 |
| 64 | rs10069690 | TERT | A | 0.27 | 1.03 (0.89, 1.19) | 0.67 | 1.02 |
| 65 | rs11814448 | 10p12.31 | C | 0.02 | 0.92 (0.60, 1.39) | 0.68 | 1.22 |
| 66 | rs204247 | RANBP9 | G | 0.45 | 1.03 (0.90, 1.17) | 0.68 | 1.05 |
| 67 | rs13329835 | CDYL2 | G | 0.23 | 0.97 (0.83, 1.14) | 0.72 | 1.08 |
| 68 | rs4808801 | ELL | A | 0.68 | 1.02 (0.88, 1.17) | 0.80 | 1.07 |
| 69 | rs2588809 | RAD51B | T | 0.17 | 0.98 (0.82, 1.16) | 0.80 | 1.07 |
| 70 | rs1432679 | EBF1 | C | 0.46 | 0.99 (0.86, 1.13) | 0.84 | 1.07 |
| 71 | rs10995190 | ZNF365 | G | 0.86 | 1.02 (0.85, 1.23) | 0.84 | 1.17 |
| 72 | rs16857609 | DIRC3 | T | 0.28 | 0.99 (0.85, 1.15) | 0.87 | 1.07 |
| 73 | rs2380205 | GDI2 | G | 0.58 | 0.99 (0.87, 1.13) | 0.88 | 1.02 |
| 74 | rs8170 | BIBAM1 | A | 0.17 | 0.99 (0.83, 1.17) | 0.88 | 1.03 |
| 75 | rs1353747 | PDE4D | T | 0.91 | 1.01 (0.81, 1.25) | 0.95 | 1.09 |
Abbreviations: CI= Confidence Interval; RR = risk ratio; NR = not reported; RAF= Risk allele frequency
Imputed SNPs are italicized
Odds ratio for the log-additive model reported in Mavaddat et al. [60] Original ORs<1.00 were transformed to reflect the measured effect of the risk allele.
Figure 1.
Quantile-quantile plot for the association between 75 candidate SNPs and young-onset breast cancer in the Two Sister Study (2008–2012).
The remaining 13 SNPs with statistically-significant associations are located in the following loci: ESR1, 2q14.2, TERT, ARHGEF5, 10q26.12, CCND1, MKL1, 9q31.2, MRPS30, 8q24.21, PEX14 and ADAM29. The ESR1 and 8q24.21 regions both had two statistically-significant SNPs which were not in LD with one another. When we compared our observed effect estimates for young-onset breast cancer to those observed for overall breast cancer [60], the distribution of p-values for those tests did not deviate from the expected distribution under the null of no difference (Supplementary Figure S1).
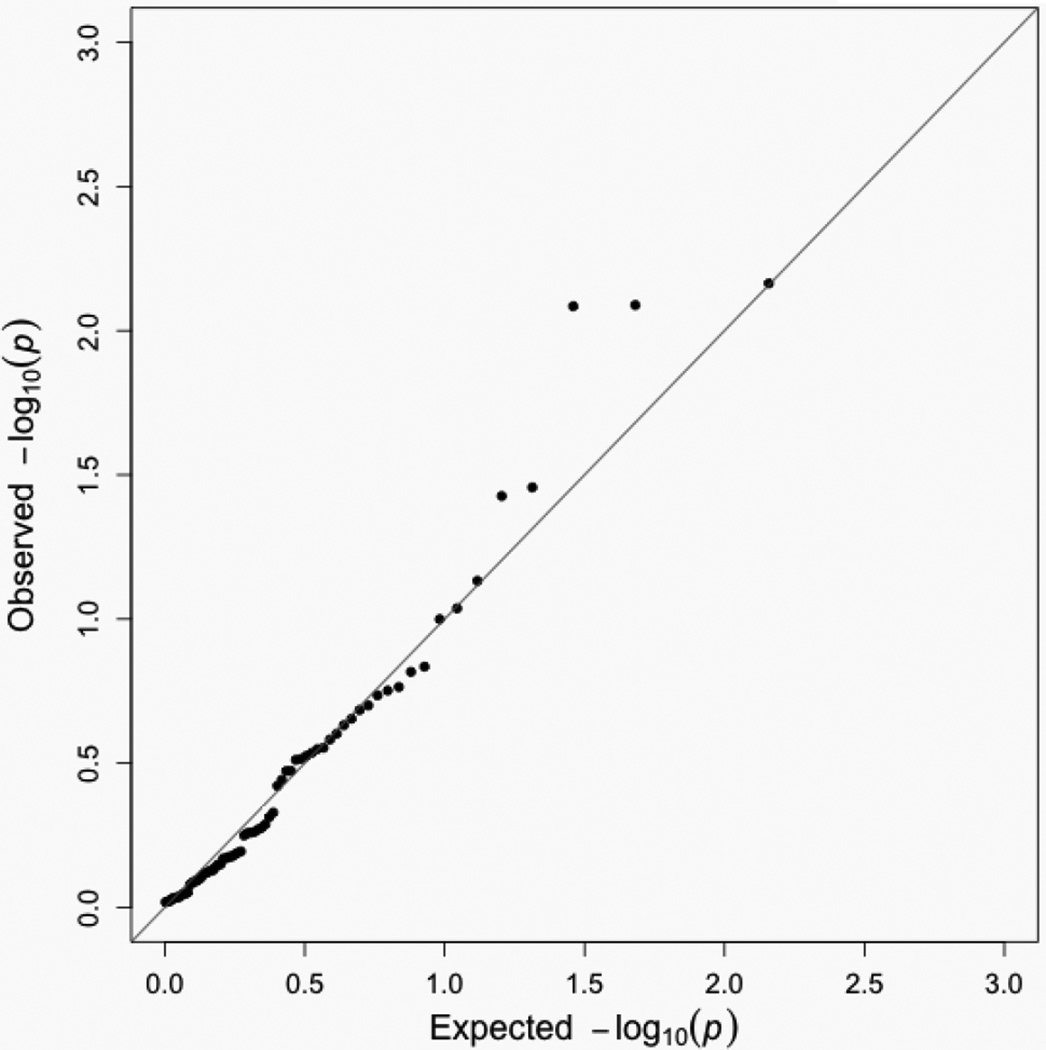
Analyses restricted to families with invasive, ER+, premenopausal cancer or not known to carry risk-related BRCA1/2 mutations showed similar patterns, with small p-values again seen for rs3803662, rs12662670, rs2981579, rs999737, and other top-ranked SNPs from the overall analysis (Supplementary Tables S1–S4). For maternally-mediated effects, we found only five SNPs associated (p<0.05) with young-onset breast cancer, a number compatible with random chance (73 included SNPs, Table 4 and Figure 2). None of those SNPs were also identified as significant in our primary analysis.
Table 4.
Effect estimates for maternally-mediated genetic effects of previous breast cancer genome-wide association study risk variants on the risk of young-onset breast cancer
| Rank | SNPa | Gene | RR (95% CI) | p-value |
|---|---|---|---|---|
| 1 | rs9790517 | TET2 | 0.78 (0.66, 0.94) | 0.007 |
| 2 | rs10995190 | ZNF365 | 0.75 (0.61, 0.93) | 0.008 |
| 3 | rs9693444 | 8p12 | 0.81 (0.70, 0.93) | 0.008 |
| 4 | rs2236007 | PAX9 | 1.21 (1.01, 1.45) | 0.03 |
| 5 | rs2016394 | DLX2 | 0.85 (0.73, 0.99) | 0.04 |
| 6 | rs2588809 | RAD51B | 0.84 (0.69, 1.02) | 0.07 |
| 7 | rs2943559 | HNF4G | 0.80 (0.62, 1.04) | 0.09 |
| 8 | rs6504950 | STXBP4 | 1.15 (0.97, 1.35) | 0.10 |
| 9 | rs6828523 | ADAM29 | 1.19 (0.94, 1.49) | 0.15 |
| 10 | rs2981579 | FGFR2 | 0.90 (0.78, 1.04) | 0.15 |
| 11 | rs10771399 | 12p11.22 | 0.84 (0.66, 1.08) | 0.17 |
| 12 | rs720475 | ARHGEF5 | 0.89 (0.75, 1.05) | 0.18 |
| 13 | rs8170 | BIBAM1 | 0.87 (0.71, 1.07) | 0.18 |
| 14 | rs2046210 | ESR1 | 1.10 (0.95, 1.28) | 0.20 |
| 15 | rs12422552 | 12p13.1 | 0.90 (0.76, 1.06) | 0.21 |
| 16 | rs4245739 | MDM4 | 1.11 (0.94, 1.32) | 0.22 |
| 17 | rs1045485 | CASP8 | 0.87 (0.70, 1.09) | 0.23 |
| 18 | rs7904519 | TCF7L2 | 0.92 (0.79, 1.06) | 0.25 |
| 19 | rs3817198 | LSP1 | 0.91 (0.77, 1.07) | 0.26 |
| 20 | rs865686 | RPL31P43 | 1.09 (0.93, 1.27) | 0.28 |
| 21 | rs13281615 | 8q24.21 | 1.09 (0.93, 1.27) | 0.28 |
| 22 | rs3903072 | 11q13.1 | 1.09 (0.93, 1.27) | 0.29 |
| 23 | rs11242675 | FOXQ1 | 1.09 (0.93, 1.27) | 0.30 |
| 24 | rs10759243 | 9q31.2 | 0.91 (0.76, 1.09) | 0.31 |
| 25 | rs11820646 | 11q24.3 | 0.93 (0.80, 1.07) | 0.31 |
| 26 | rs12662670 | ESR1 | 0.88 (0.67, 1.15) | 0.34 |
| 27 | rs2380205 | GDI2 | 0.93 (0.79, 1.08) | 0.34 |
| 28 | rs13387042 | TNP1 | 1.07 (0.92, 1.25) | 0.36 |
| 29 | rs999737 | RAD51B | 0.93 (0.78, 1.10) | 0.38 |
| 30 | rs4849887 | 2q14.2 | 0.92 (0.72, 1.16) | 0.47 |
| 31 | rs17356907 | 12q22 | 0.94 (0.80, 1.12) | 0.49 |
| 32 | rs13329835 | CDYL2 | 1.06 (0.88, 1.28) | 0.52 |
| 33 | rs6762644 | ITPR1 | 0.95 (0.82, 1.11) | 0.53 |
| 34 | rs204247 | RANBP9 | 0.95 (0.82, 1.11) | 0.54 |
| 35 | rs1292011 | 12q24.21 | 0.95 (0.81, 1.12) | 0.55 |
| 36 | rs616488 | PEX14 | 0.95 (0.82, 1.11) | 0.55 |
| 37 | rs4973768 | SLC4A7 | 0.95 (0.82, 1.11) | 0.55 |
| 38 | rs10069690 | TERT | 0.95 (0.80, 1.13) | 0.56 |
| 39 | rs11814448 | 10p12.31 | 0.90 (0.57, 1.41) | 0.64 |
| 40 | rs10941679 | MRPS30 | 0.96 (0.80, 1.15) | 0.65 |
| 41 | rs16857609 | DIRC3 | 0.96 (0.81, 1.14) | 0.66 |
| 42 | rs1550623 | CDCA7 | 0.96 (0.78, 1.18) | 0.67 |
| 43 | rs2736108 | TERT | 0.96 (0.79, 1.17) | 0.67 |
| 44 | rs6678914 | LGR6 | 1.03 (0.88, 1.21) | 0.68 |
| 45 | rs527616 | 18q11.2 | 1.04 (0.87, 1.25) | 0.68 |
| 46 | rs12493607 | TGFBR2 | 0.97 (0.83, 1.13) | 0.71 |
| 47 | rs704010 | ZMIZ1 | 0.97 (0.83, 1.14) | 0.71 |
| 48 | rs78540526 | CCND1 | 1.05 (0.78, 1.42) | 0.73 |
| 49 | rs3803662 | TOX3 | 0.97 (0.82, 1.15) | 0.74 |
| 50 | rs889312 | MAP3K1 | 0.97 (0.83, 1.15) | 0.75 |
| 51 | rs11075995 | FTO | 0.97 (0.82, 1.15) | 0.76 |
| 52 | rs75915166 | FGF3 | 1.05 (0.77, 1.42) | 0.76 |
| 53 | rs1436904 | CHST9 | 1.02 (0.87, 1.20) | 0.77 |
| 54 | rs1353747 | PDE4D | 0.97 (0.75, 1.24) | 0.79 |
| 55 | rs1011970 | CDKN2B | 0.97 (0.79, 1.19) | 0.80 |
| 56 | rs11199914 | 10q26.12 | 0.98 (0.83, 1.16) | 0.81 |
| 57 | rs2363956 | ANKLE1 | 0.98 (0.85, 1.14) | 0.81 |
| 58 | rs4808801 | ELL | 0.98 (0.84, 1.15) | 0.82 |
| 59 | rs941764 | CCDC88C | 0.98 (0.84, 1.16) | 0.83 |
| 60 | rs1432679 | EBF1 | 1.01 (0.87, 1.17) | 0.89 |
| 61 | rs11249433 | EMBP1 | 0.99 (0.85, 1.15) | 0.89 |
| 62 | rs12710696 | LINC01376 | 1.01 (0.86, 1.19) | 0.90 |
| 63 | rs17817449 | FTO | 0.99 (0.85, 1.16) | 0.90 |
| 64 | rs11552449 | AP4B1 | 0.99 (0.81, 1.21) | 0.92 |
| 65 | rs7726159 | TERT | 0.99 (0.82, 1.20) | 0.92 |
| 66 | rs554219 | CCND1 | 1.01 (0.82, 1.25) | 0.92 |
| 67 | rs10472076 | RPL5P15 | 1.01 (0.87, 1.17) | 0.93 |
| 68 | rs132390 | EMID1 | 1.02 (0.62, 1.69) | 0.93 |
| 69 | rs11780156 | 8q24.21 | 0.99 (0.81, 1.22) | 0.95 |
| 70 | rs2823093 | CYRR1 | 1.00 (0.85, 1.17) | 0.95 |
| 71 | rs6001930 | MKL1 | 1.01 (0.79, 1.29) | 0.95 |
| 72 | rs7072776 | MLLT10 | 1.00 (0.85, 1.16) | 0.96 |
| 73 | rs3760982 | KCNN4 | 1.00 (0.86, 1.16) | 1.00 |
Abbreviations: CI= Confidence Interval; RR = risk ratio
Imputed SNPs are italicized
Figure 2.
Quantile-quantile plot for maternally-mediated genetic effects of 73 candidate SNPs and young-onset breast cancer in the Two Sister Study (2008–2012).
For the polygenic risk score, both the multiplicative and additive genetic risk scores were associated with young-onset breast cancer (p=2.7×10−10 and p=1.3×10−10, respectively, testing β=0). The coefficient for the additive risk score was different from 1.0 (β= 5.1, additive-fit-p=2.2×10−7), but the coefficient for the multiplicative risk score was not (β=0.85, multiplicative-fit-p=0.27). This indicates that the overall joint effect of these risk SNPs was more than additive and consistent with multiplicative and that each one-unit increase in score corresponds to a relative risk increase of 2.34 (e0.85). For both scores, the case sister had a higher score than her control/pseudo-control sister in 59% of pairs, suggesting there is within-family predictive power.
There was no evidence that these genetic effects act prenatally through the mother’s phenotype, as there was no difference between mothers’ and fathers’ genetic scores on either the multiplicative or additive scale (p=0.19 and p=0.18, for paired testing, respectively). When we tested the association between the polygenic risk score and breast cancer in mothers, the score preformed similarly to our original analysis, with associations seen for both multiplicative (p=4.6×10−7) and additive (p=4.0×10−7) models. The effects were again more than additive and consistent with multiplicative (multiplicative β=0.88, multiplicative-fit p=0.50; additive β=5.4, additive-fit p=3.5×10−5), with an estimated area under the receiver operating characteristics curve of 0.65 for both formulations.
Discussion
The purpose of this family-based genetic study was to examine whether previously-identified breast cancer risk variants were associated with young-onset disease through inherited or maternally-mediated effects. We focused on 77 common polymorphisms identified in published GWAS [60]. Seventeen of those candidate SNPs were nominally associated with young-onset breast cancer and a genetic risk score consisting of all 77 SNPs was related to the risk of young-onset breast cancer in daughters and to a history of breast cancer at any age in their mothers. There was no evidence that the independent or combined effects of these candidate SNPs was due to a prenatal effect mediated by the maternal genome.
The SNPs with the lowest p-values were located in well-studied susceptibility genes – TOX3, ESR1, FGFR2, and RAD51B. The results were similar when we restricted analyses to include only families with invasive, ER+, or premenopausal young-onset breast cancer, or to families without known deleterious BRCA1/2 variants.
TOX3 encodes a nuclear protein that regulates calcium-dependent transcription in neurons [66]. Its link to breast cancer is unclear, though there is some evidence that it is a tumor suppressor [66, 67]. Our top hit, rs3803662, was first identified by Easton et al., [7], with several subsequent GWAS confirming the region’s importance [11, 18, 22, 23, 30–32]. Presence of this variant allele has been linked to lower TOX3 expression levels in breast tumor tissue [68]. Several candidate gene studies also reported risks of similar magnitude for rs3803662-T and young-onset breast cancer [1, 38, 40, 43, 69]. In Ahsan et al.’s [1] young-onset GWAS, TOX3 was one of the identified susceptibility regions, and rs3803662 had the smallest p-value for that region. For a more direct comparison of our results to those of Ahsan et al., see Supplementary Table S5 (a comparison of p-values for the 83 previously-established susceptibility loci selected by Ahsan et al. and our results for the same SNPs) and Supplementary Table S6 (an assessment of other established susceptibility loci not included in Mavaddat et al. [60], including the novel GWAS hits from Ahsan et al.).
The SNPs with the second and fifth smallest p-values - rs12662670 and rs2046210 - are located in the 6q25 region just upstream of ESR1. ESR1 encodes Estrogen Receptor α, a ligand-activated transcription factor crucial to sexual development and reproduction [70]. rs12662670 was previously linked to increased risk of triple-negative breast cancer, a subtype more common in younger women [71]. We did not have enough cases of triple-negative cancer for a separate analysis. Carriers of the other SNP, rs2046210-A, have lower ESR1 levels in both tumor and normal breast tissue [72]. The 6q25 region was not one of the regions with genome-wide significant p-values in Ahsan et al. [1], though rs2046210-A was positively associated with young-onset breast cancer at p<0.05.
FGFR2 encodes a fibroblast growth factor receptor, the overexpression of which may contribute to carcinogenesis through increased cell proliferation, migration, and resistance to apoptosis [73]. Our FGFR2 SNP, rs2981579, was first identified by Thomas et al. [31] and independently replicated in two other GWAS [23, 32]. Other GWAS found stronger associations with other SNPs in the same gene [1, 7, 8, 10, 12, 15, 19, 22, 26]. Ahsan et al. [1], for example, reported genome-wide significant p-values for 4 FGFR2 SNPs and young-onset breast cancer, with the smallest p-value seen for rs2981579. Other studies have also observed associations between FGFR2 SNPs and young-onset breast cancer [41, 42, 69, 74].
RAD51B is a homologous recombination repair gene related to BRCA1 and BRCA2 [75]. Thomas et al. [31] were the first to link rs999737-G to breast cancer risk. This was confirmed in a second GWAS [23] and subsequent meta-analysis [76]. Although RAD51B was not one of the genome-wide significant regions identified in Ahsan et al. [1], rs999737 was associated with young-onset breast cancer at p<0.05.
In addition to TOX3, ESR1, FGFR2, and RAD51, we corroborated Ahsan et al.’s findings that SNPs in 8q24.21 and 11q13.3 (CCND1) may be important for young-onset breast cancer. However, we did not see statistically significant effects for SNPs in SLC4A7 or MAP3K1. It is not clear whether these differences are due to chance or heterogeneity across studies. We had limited power to detect small effects, with approximately 80% power to detect RRs of 1.24 or 1.20 for allele frequencies of 20% or 40%, respectively for α=0.05. This modest power limits our ability to detect small effects and age-at-onset interaction effects. Given this caveat, the results of the statistical tests comparing our effect estimates to those of Mavaddat et al. [60] showed no evidence that effect sizes differed by age at breast cancer onset.
Dite et al. [77] also applied the 77-SNP risk score developed by Mavaddat et al. [60] to evaluate young-onset breast cancer. They reported an area under the curve of 0.61 for the risk score alone, which is consistent with the predictive capability we report here. We additionally found that the 77-SNP risk score was predictive of breast cancer in the mothers of cases (which were predominantly older-onset), and that similar coefficients were seen for the two generations.
Our use of previously reported effect measures helped us avoid over-fitting the prediction model to our data [78] and enabled independent replication. Several previous studies have used similar approaches, but most have considered only models with multiplicative joint effects [18, 22, 60, 77, 79]. Like us, Joshi et al. [80] constructed scores for both multiplicative and additive polygenic effects using externally-reported effect estimates. We both found evidence that the joint effects were super-additive, but our results showed consistency with a multiplicative model, while Joshi et al. found that the observed associations were sub-multiplicative. Future studies should consider both additive and multiplicative models to see which mathematical form of the risk score best fits the data and to determine if the same model is appropriate for different subgroups (e.g. younger versus older women). Ideally, investigators will reach a consensus regarding what SNPs to include in the score and how to correctly specify their combined effects. Ultimately, we hope a risk score can be utilized as a tool to classify women’s individual breast cancer risk and identify who should be selected for more or less frequent screening.
A major strength of this study is its family-based design, which is robust to population stratification and amenable to missing-data imputation methods [64, 65]. However, we restricted our analysis to non-Hispanic whites, which somewhat limits the generalizability of our findings. There is also the potential for survival bias if some SNPs are related to survival after breast cancer. However, short-term survival rates for breast cancer are high (91% 5-year relative survival for invasive breast cancer before age 50 [81]), suggesting the effects of such attrition would be minimal.
In this family-based genetic study, we saw little evidence of maternally-mediated genetic effects, but found that many of the known breast cancer risk variants were also associated with young-onset breast cancer. Our analyses provide further evidence that certain loci, including TOX3, ESR1, FGFR2, and RAD51B, are important for the development of breast cancer at any age.
Supplementary Material
Acknowledgments
Funding support: This work was supported by the Intramural Research Program of the NIH, the National Institute of Environmental Health Sciences (project Z01-ES044005 to DPS and project Z01-ES102245 to CRW) and Susan G. Komen for the Cure (grant FAS0703856 to CRW).
The authors would like to thank Stephanie London and Sophia Harlid for their comments on an early draft of this paper. Genotyping, quality control analysis and imputation were completed at the Center for Inherited Disease Research, the Genetics Coordinating Center at the University of Washington (Cecelia Laurie, Quenna Wong, Sarah Nelson, and Cathy Laurie).
Abbreviations
- CI
confidence interval
- ER
estrogen receptor
- GWAS
genome-wide association study
- LD
linkage disequilibrium
- OR
odds ratio
- RR
relative risk
- SNP
single nucleotide polymorphism
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Ethics statement: All participants provided written or verbal consent and the study was approved by the National Institute of Environmental Health Sciences and the Copernicus Group Institutional Review Boards. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable clinical standards.
References
- 1.Ahsan H, Halpern J, Kibriya MG, Pierce BL, Tong L, Gamazon E, et al. A genome-wide association study of early-onset breast cancer identifies PFKM as a novel breast cancer gene and supports a common genetic spectrum for breast cancer at any age. Cancer Epidemiol Biomarkers Prev. 2014;23(4):658–669. doi: 10.1158/1055-9965.EPI-13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniou AC, Wang X, Fredericksen ZS, McGuffog L, Tarrell R, Sinilnikova OM, et al. A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet. 2010;42(10):885–892. doi: 10.1038/ng.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Q, Long J, Lu W, Qu S, Wen W, Kang D, et al. Genome-wide association study identifies breast cancer risk variant at 10q21.2: results from the Asia Breast Cancer Consortium. Hum Mol Genet. 2011;20(24):4991–4999. doi: 10.1093/hmg/ddr405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai Q, Zhang B, Sung H, Low SK, Kweon SS, Lu W, et al. Genome-wide association analysis in East Asians identifies breast cancer susceptibility loci at 1q32.1, 5q14.3 and 15q26.1. Nat Genet. 2014;46(8):886–890. doi: 10.1038/ng.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen F, Chen GK, Stram DO, Millikan RC, Ambrosone CB, John EM, et al. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132(1):39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, Kuchenbaecker KB, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS Genet. 2013;9(3):e1003212. doi: 10.1371/journal.pgen.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elgazzar S, Zembutsu H, Takahashi A, Kubo M, Aki F, Hirata K, et al. A genome-wide association study identifies a genetic variant in the SIAH2 locus associated with hormonal receptor-positive breast cancer in Japanese. J Hum Genet. 2012;57(12):766–771. doi: 10.1038/jhg.2012.108. [DOI] [PubMed] [Google Scholar]
- 9.Fejerman L, Ahmadiyeh N, Hu D, Huntsman S, Beckman KB, Caswell JL, et al. Genome-wide association study of breast cancer in Latinas identifies novel protective variants on 6q25. Nat Commun. 2014;5:5260. doi: 10.1038/ncomms6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fletcher O, Johnson N, Orr N, Hosking FJ, Gibson LJ, Walker K, et al. Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J Natl Cancer Inst. 2011;103(5):425–435. doi: 10.1093/jnci/djq563. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45(4):392–398. doi: 10.1038/ng.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudet MM, Kirchhoff T, Green T, Vijai J, Korn JM, Guiducci C, et al. Common genetic variants and modification of penetrance of BRCA2-associated breast cancer. PLoS Genet. 2010;6(10):e1001183. doi: 10.1371/journal.pgen.1001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold B, Kirchhoff T, Stefanov S, Lautenberger J, Viale A, Garber J, et al. Genome-wide association study provides evidence for a breast cancer risk locus at 6q22.33. Proc Natl Acad Sci U S A. 2008;105(11):4340–4345. doi: 10.1073/pnas.0800441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haiman CA, Chen GK, Vachon CM, Canzian F, Dunning A, Millikan RC, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011;43(12):1210–1214. doi: 10.1038/ng.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39(7):870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kibriya MG, Jasmine F, Argos M, Andrulis I, John EM, Chang-Claude J, et al. A pilot genome-wide association study of early-onset breast cancer. Breast Cancer Res Treat. 2009;114(3):463–467. doi: 10.1007/s10549-008-0039-9. [DOI] [PubMed] [Google Scholar]
- 17.Kim HC, Lee JY, Sung H, Choi JY, Park SK, Lee KM, et al. A genome-wide association study identifies a breast cancer risk variant in ERBB4 at 2q34: results from the Seoul Breast Cancer Study. Breast Cancer Res. 2012;14(2):R56. doi: 10.1186/bcr3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Humphreys K, Darabi H, Rosin G, Hannelius U, Heikkinen T, et al. A genome-wide association scan on estrogen receptor-negative breast cancer. Breast Cancer Res. 2010;12(6):R93. doi: 10.1186/bcr2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Humphreys K, Heikkinen T, Aittomaki K, Blomqvist C, Pharoah PD, et al. A combined analysis of genome-wide association studies in breast cancer. Breast Cancer Res Treat. 2011;126(3):717–727. doi: 10.1007/s10549-010-1172-9. [DOI] [PubMed] [Google Scholar]
- 20.Long J, Cai Q, Shu XO, Qu S, Li C, Zheng Y, et al. Identification of a functional genetic variant at 16q12.1 for breast cancer risk: results from the Asia Breast Cancer Consortium. PLoS Genet. 2010;6(6):e1001002. doi: 10.1371/journal.pgen.1001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long J, Cai Q, Sung H, Shi J, Zhang B, Choi JY, et al. Genome-wide association study in east Asians identifies novel susceptibility loci for breast cancer. PLoS Genet. 2012;8(2):e1002532. doi: 10.1371/journal.pgen.1002532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low SK, Takahashi A, Ashikawa K, Inazawa J, Miki Y, Kubo M, et al. Genome-wide association study of breast cancer in the Japanese population. PLoS One. 2013;8(10):e76463. doi: 10.1371/journal.pone.0076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45(4):353–361. doi: 10.1038/ng.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murabito JM, Rosenberg CL, Finger D, Kreger BE, Levy D, Splansky GL, et al. A genome-wide association study of breast and prostate cancer in the NHLBI's Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S6. doi: 10.1186/1471-2350-8-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purrington KS, Slager S, Eccles D, Yannoukakos D, Fasching PA, Miron P, et al. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35(5):1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rinella ES, Shao Y, Yackowski L, Pramanik S, Oratz R, Schnabel F, et al. Genetic variants associated with breast cancer risk for Ashkenazi Jewish women with strong family histories but no identifiable BRCA1/2 mutation. Hum Genet. 2013;132(5):523–536. doi: 10.1007/s00439-013-1269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sehrawat B, Sridharan M, Ghosh S, Robson P, Cass CE, Mackey JR, et al. Potential novel candidate polymorphisms identified in genome-wide association study for breast cancer susceptibility. Hum Genet. 2011;130(4):529–537. doi: 10.1007/s00439-011-0973-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiq A, Couch FJ, Chen GK, Lindstrom S, Eccles D, Millikan RC, et al. A meta-analysis of genome-wide association studies of breast cancer identifies two novel susceptibility loci at 6q14 and 20q11. Hum Mol Genet. 2012;21(24):5373–5384. doi: 10.1093/hmg/dds381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song C, Chen GK, Millikan RC, Ambrosone CB, John EM, Bernstein L, et al. A genome-wide scan for breast cancer risk haplotypes among African American women. PLoS One. 2013;8(2):e57298. doi: 10.1371/journal.pone.0057298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey SN, Manolescu A, Sulem P, Rafnar T, Gudmundsson J, Gudjonsson SA, et al. Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet. 2007;39(7):865–869. doi: 10.1038/ng2064. [DOI] [PubMed] [Google Scholar]
- 31.Thomas G, Jacobs KB, Kraft P, Yeager M, Wacholder S, Cox DG, et al. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat Genet. 2009;41(5):579–584. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, et al. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. 2010;42(6):504–507. doi: 10.1038/ng.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng W, Long J, Gao YT, Li C, Zheng Y, Xiang YB, et al. Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet. 2009;41(3):324–328. doi: 10.1038/ng.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, et al. Interactions between genetic variants and breast cancer risk factors in the breast and prostate cancer cohort consortium. J Natl Cancer Inst. 2011;103(16):1252–1263. doi: 10.1093/jnci/djr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hutter CM, Young AM, Ochs-Balcom HM, Carty CL, Wang T, Chen CT, et al. Replication of breast cancer GWAS susceptibility loci in the Women's Health Initiative African American SHARe Study. Cancer Epidemiol Biomarkers Prev. 2011;20(9):1950–1959. doi: 10.1158/1055-9965.EPI-11-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Brien KM, Cole SR, Poole C, Bensen JT, Herring AH, Engel LS, et al. Replication of breast cancer susceptibility loci in whites and African Americans using a Bayesian approach. Am J Epidemiol. 2014;179(3):382–394. doi: 10.1093/aje/kwt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reeves SG, Meldrum C, Groombridge C, Spigelman A, Suchy J, Kurzawski G, et al. DNA repair gene polymorphisms and risk of early onset colorectal cancer in Lynch syndrome. Cancer Epidemiol. 2012;36(2):183–189. doi: 10.1016/j.canep.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Slattery ML, Baumgartner KB, Giuliano AR, Byers T, Herrick JS, Wolff RK. Replication of five GWAS-identified loci and breast cancer risk among Hispanic and non-Hispanic white women living in the Southwestern United States. Breast Cancer Res Treat. 2011;129(2):531–539. doi: 10.1007/s10549-011-1498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng W, Cai Q, Signorello LB, Long J, Hargreaves MK, Deming SL, et al. Evaluation of 11 breast cancer susceptibility loci in African-American women. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2761–2764. doi: 10.1158/1055-9965.EPI-09-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elematore I, Gonzalez-Hormazabal P, Reyes JM, Blanco R, Bravo T, Peralta O, et al. Association of genetic variants at TOX3, 2q35 and 8q24 with the risk of familial and early-onset breast cancer in a South-American population. Mol Biol Rep. 2014;41(6):3715–3722. doi: 10.1007/s11033-014-3236-0. [DOI] [PubMed] [Google Scholar]
- 41.Fu F, Wang C, Huang M, Song C, Lin S, Huang H. Polymorphisms in Second Intron of the FGFR2 Gene Are Associated with the Risk of Early-Onset Breast Cancer in Chinese Han Women. The Tohoku Journal of Experimental Medicine. 2012;226(3):221–229. doi: 10.1620/tjem.226.221. [DOI] [PubMed] [Google Scholar]
- 42.Jara L, Gonzalez-Hormazabal P, Cerceno K, Di Capua GA, Reyes JM, Blanco R, et al. Genetic variants in FGFR2 and MAP3K1 are associated with the risk of familial and early-onset breast cancer in a South-American population. Breast Cancer Res Treat. 2013;137(2):559–569. doi: 10.1007/s10549-012-2359-z. [DOI] [PubMed] [Google Scholar]
- 43.Tapper W, Hammond V, Gerty S, Ennis S, Simmonds P, Collins A, et al. The influence of genetic variation in 30 selected genes on the clinical characteristics of early onset breast cancer. Breast Cancer Res. 2008;10(6):R108. doi: 10.1186/bcr2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson WF, Matsuno RK, Sherman ME, Lissowska J, Gail MH, Brinton LA, et al. Estimating age-specific breast cancer risks: a descriptive tool to identify age interactions. Cancer Causes Control. 2007;18(4):439–447. doi: 10.1007/s10552-006-0092-9. [DOI] [PubMed] [Google Scholar]
- 45.Michels KB, Xue F. Role of birthweight in the etiology of breast cancer. Int J Cancer. 2006;119(9):2007–2025. doi: 10.1002/ijc.22004. [DOI] [PubMed] [Google Scholar]
- 46.Trentham-Dietz A, Sprague BL, Hampton JM, Miglioretti DL, Nelson HD, Titus LJ, et al. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145(1):165–175. doi: 10.1007/s10549-014-2905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 48.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. The Lancet. 2001;358(9291):1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 49.Althuis MD, Brogan DD, Coates RJ, Daling JR, Gammon MD, Malone K, et al. Breast cancers among very young premenopausal women (United States) Cancer Causes & Control. 2003;14:151–160. doi: 10.1023/a:1023006000760. [DOI] [PubMed] [Google Scholar]
- 50.Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26(20):3324–3330. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 51.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 52.Broeks A, Schmidt MK, Sherman ME, Couch FJ, Hopper JL, Dite GS, et al. Low penetrance breast cancer susceptibility loci are associated with specific breast tumor subtypes: findings from the Breast Cancer Association Consortium. Hum Mol Genet. 2011;20(16):3289–3303. doi: 10.1093/hmg/ddr228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan M, Ji SM, Liaw CS, Yap YS, Law HY, Yoon CS, et al. Association of common genetic variants with breast cancer risk and clinicopathological characteristics in a Chinese population. Breast Cancer Res Treat. 2012;136(1):209–220. doi: 10.1007/s10549-012-2234-y. [DOI] [PubMed] [Google Scholar]
- 54.Han W, Woo JH, Yu JH, Lee MJ, Moon HG, Kang D, et al. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol Biomarkers Prev. 2011;20(5):793–798. doi: 10.1158/1055-9965.EPI-10-1282. [DOI] [PubMed] [Google Scholar]
- 55.O'Brien KM, Cole SR, Engel LS, Bensen JT, Poole C, Herring AH, et al. Breast cancer subtypes and previously established genetic risk factors: a bayesian approach. Cancer Epidemiol Biomarkers Prev. 2014;23(1):84–97. doi: 10.1158/1055-9965.EPI-13-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rebbeck TR, DeMichele A, Tran TV, Panossian S, Bunin GR, Troxel AB, et al. Hormone-dependent effects of FGFR2 and MAP3K1 in breast cancer susceptibility in a population-based sample of post-menopausal African-American and European-American women. Carcinogenesis. 2009;30(2):269–274. doi: 10.1093/carcin/bgn247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laird NM, Lange C. Family-based designs in the age of large-scale gene-association studies. Nat Rev Genet. 2006;7(5):385–394. doi: 10.1038/nrg1839. [DOI] [PubMed] [Google Scholar]
- 58.Weinberg CR, Wilcox AJ, Lie RT. A log-linear approach to case-parent-triad data: Assessing effects of disease genes that act either directly or through maternal effects and that may be subject to parental imprinting. Am J Hum Genet. 1998;62:969–978. doi: 10.1086/301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Brien KM, Shi M, Sandler DP, Taylor JA, Zaykin DV, Keller J, et al. A family-based, genome-wide association study of young-onset breast cancer: inherited variants and maternally mediated effects. Eur J Hum Genet. 2016 doi: 10.1038/ejhg.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107(5) doi: 10.1093/jnci/djv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2012;9(2):179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 63.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi M, Umbach DM, Weinberg CR. Case-sibling studies that acknowledge unstudied parents and permit the inclusion of unmatched individuals. Int J Epidemiol. 2013;42(1):298–307. doi: 10.1093/ije/dys212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg CR. Allowing for missing parents in genetic studies of case-parent triads. Am J Hum Genet. 1999;64:1186–1193. doi: 10.1086/302337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dittmer S, Kovacs Z, Yuan SH, Siszler G, Kogl M, Summer H, et al. TOX3 is a neuronal survival factor that induces transcription depending on the presence of CITED1 or phosphorylated CREB in the transcriptionally active complex. J Cell Sci. 2011;124(Pt 2):252–260. doi: 10.1242/jcs.068759. [DOI] [PubMed] [Google Scholar]
- 67.Cowper-Sal lari R, Zhang X, Wright JB, Bailey SD, Cole MD, Eeckhoute J, et al. Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat Genet. 2012;44(11):1191–1198. doi: 10.1038/ng.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riaz M, Berns EM, Sieuwerts AM, Ruigrok-Ritstier K, de Weerd V, Groenewoud A, et al. Correlation of breast cancer susceptibility loci with patient characteristics, metastasis-free survival, and mRNA expression of the nearest genes. Breast Cancer Res Treat. 2012;133(3):843–851. doi: 10.1007/s10549-011-1663-3. [DOI] [PubMed] [Google Scholar]
- 69.Barzan D, Veldwijk MR, Herskind C, Li Y, Zhang B, Sperk E, et al. Comparison of genetic variation of breast cancer susceptibility genes in Chinese and German populations. Eur J Hum Genet. 2013;21(11):1286–1292. doi: 10.1038/ejhg.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ESR1 estrogen receptor 1 [Homo sapiens (human)] [Internet] [cited December 22, 2015]; [Google Scholar]
- 71.Stevens KN, Vachon CM, Lee AM, Slager S, Lesnick T, Olswold C, et al. Common breast cancer susceptibility loci are associated with triple-negative breast cancer. Cancer Res. 2011;71(19):6240–6249. doi: 10.1158/0008-5472.CAN-11-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun Y, Ye C, Guo X, Wen W, Long J, Gao YT, et al. Evaluation of potential regulatory function of breast cancer risk locus at 6q25.1. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jain VK, Turner NC. Challenges and opportunities in the targeting of fibroblast growth factor receptors in breast cancer. Breast Cancer Res. 2012;14:208. doi: 10.1186/bcr3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawase T, Matsuo K, Suzuki T, Hiraki A, Watanabe M, Iwata H, et al. FGFR2 intronic polymorphisms interact with reproductive risk factors of breast cancer: results of a case control study in Japan. Int J Cancer. 2009;125(8):1946–1952. doi: 10.1002/ijc.24505. [DOI] [PubMed] [Google Scholar]
- 75.Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2012;12(1):68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dong H, Gao Z, Li C, Wang J, Jin M, Rong H, et al. Analyzing 395,793 samples shows significant association between rs999737 polymorphism and breast cancer. Tumour Biol. 2014;35(6):6083–6087. doi: 10.1007/s13277-014-1805-4. [DOI] [PubMed] [Google Scholar]
- 77.Dite GS, MacInnis RJ, Bickerstaffe A, Dowty JG, Allman R, Apicella C, et al. Breast Cancer Risk Prediction Using Clinical Models and 77 Independent Risk-Associated SNPs for Women Aged Under 50 Years: Australian Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2015 doi: 10.1158/1055-9965.EPI-15-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kraft P. Curses--winner's and otherwise--in genetic epidemiology. Epidemiology. 2008;19(5):649–651. doi: 10.1097/EDE.0b013e318181b865. discussion 57-8. [DOI] [PubMed] [Google Scholar]
- 79.Machiela MJ, Chen CY, Chen C, Chanock SJ, Hunter DJ, Kraft P. Evaluation of polygenic risk scores for predicting breast and prostate cancer risk. Genet Epidemiol. 2011;35(6):506–514. doi: 10.1002/gepi.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joshi AD, Lindstrom S, Husing A, Barrdahl M, VanderWeele TJ, Campa D, et al. Additive interactions between susceptibility single-nucleotide polymorphisms identified in genome-wide association studies and breast cancer risk factors in the Breast and Prostate Cancer Cohort Consortium. Am J Epidemiol. 2014;180(10):1018–1027. doi: 10.1093/aje/kwu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.National Cancer Institute, Surveillance, Epidemiology, and End Results. [January 24, 2016]; http://seercancergov/ Accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.