Abstract
Rationale
Cocaine produces significant aversive/anxiogenic actions whose underlying neurobiology remains unclear. A possible substrate contributing to these actions is the serotonergic (5-HT) pathway projecting from the dorsal raphé (DRN) to regions of the extended amygdala, including the Bed Nucleus of the Stria Terminalis (BNST) which have been implicated in the production of anxiogenic states.
Objectives
The present study examined the contribution of 5-HT signaling within the BNST to the anxiogenic effects of cocaine as measured in a runway model of drug self-administration.
Methods
Male Sprague-Dawley rats were fitted with bilateral infusion cannula aimed at the BNST and then trained to traverse a straight alley once a day for a single 1mg/kg i.v. cocaine infusion delivered upon goal-box entry on each of 16 consecutive days/trials. Intracranial infusions of CP 94,253 (0, 0.25, 0.5, or 1.0μg/side) were administered to inhibit local 5-HT release via activation of 5-HT1B autoreceptors. To confirm receptor specificity, the effects of this treatment were then challenged by co-administration of the selective 5-HT1B antagonist NAS-181.
Results
Intra-BNST infusions of the 5-HT1B autoreceptor agonist attenuated the anxiogenic effects of cocaine as reflected by a decrease in runway approach-avoidance conflict behavior. This effect was reversed by the 5-HT1B antagonist. Neither start latencies (a measure of the subject’s motivation to seek cocaine) nor spontaneous locomotor activity (an index of motoric capacity) were altered by either treatment.
Conclusions
Inhibition of 5-HT1B signaling within the BNST selectively attenuated the anxiogenic effects of cocaine, while leaving unaffected the positive incentive properties of the drug.
Keywords: Anxiety, Cocaine, Self-Administration, Serotonin, Drug Abuse, Operant Runway, Drug Reward, Drug Aversion, 5-HT, Extended Amygdala
Introduction
Cocaine has long been known to produce both an initial euphoria followed typically by a “crash” characterized by dysphoria, irritability, anxiety and cravings (Resnick et al 1977; Gawin 1991; Williamson et al 1997). Both the positive reinforcing and aversive/anxiogenic effects of cocaine have also been demonstrated in laboratory animals (Yang et al 1992; Rogerio and Takahashi 1992; Ettenberg 2004; Hayase et al 2005). These dual and opposing effects of cocaine conceptually fit well with Solomon and Corbit’s “opponent process theory” of motivated behavior (Solomon and Corbit 1974) in which an initial shift in affect (either positive or negative in valence) is counteracted by a delayed “opponent process” whose function is to return the organism to affective homeostasis. Cocaine’s actions closely adhere to this theory in that the initial positive, rewarding state produced by the drug is followed by a delayed anxiogenic state that presumably serves to return the subject to affective homeostasis (Ettenberg et al 1999; Knackstedt et al 2002; Ettenberg 2004). One of the key features of drug addiction involves dysregulation of these oscillations between positive and negative states due to repeated activation of a homeostatic mechanism. As tolerance is built to the initial positive effects of the drug, the delayed negative effects are thought to become sensitized (Kreek and Koob 1998; Ben-Shahar et al 2004; Koob and Le Moal 2008; Su et al 2011; 2013).
While considerable effort has been expended to identifying the underlying neurobiology of cocaine’s rewarding/reinforcing actions, far less is known about the drug’s aversive/anxiogenic effects. In preliminary studies from our laboratory, we have reported that inactivation of the extended amygdala (i.e., the bed nucleus of the stria terminalis [BNST] or the central nucleus of the amygdala [CeA]) dramatically reduced both the approach-avoidance conflict behavior of animals running an alley for i.v. cocaine, as well as prevented the development of cocaine-induced conditioned place aversions while leaving learned drug-place preferences intact (Wenzel et al 2011). Thus functional inhibition of these brain structures reduced the negative/anxiogenic impact of cocaine. Others have similarly identified a role for structures of the extended amygdala in the expression of a variety of fearful, stressful and anxiogenic states (Koob 1999; Walker et al 2003; Davis et al 2010). Of particular relevance to the current study are reports suggesting that the BNST appears to be important in the regulation of sustained anxiety due to stress, rather than in response to specific cue-induced fear (Sullivan et al 2004; Davis et al 2010). Since cocaine seems to potentiate anxiety-like behaviors without necessarily inducing fear or anxiety on its own (Blanchard and Blanchard 1999), the BNST serves as an ideal candidate for putatively contributing to the anxiogenic properties of cocaine.
To further investigate this possibility, the current study examined the impact of alterations in serotonergic (5-HT) signaling within the BNST on the approach-avoidance retreat behaviors of animals running an alley for daily infusions of i.v. cocaine. In this test, animals leave the start box faster and faster over trials (an indication of cocaine’s positive incentive properties), but develop an ambivalence about entering the goal box that strengthens with continued testing as subjects come to associate the drug with its delayed anxiogenic effects (Ettenberg and Geist 1991, 1993). The resulting approach-avoidance conflict behavior (in which animals repeatedly approach, then stop and retreat away from the goal box during runway trials) provides a quantifiable measure of the animal’s dual positive and negative response to cocaine and has been shown to result from the subject’s mixed positive and negative associations with the cocaine experience in the goal box (Ettenberg 2004; 2009). The rationale for examining the functional role of 5-HT with respect to cocaine’s anxiogenic effects stems from the fact that 5-HT has been implicated in the development and expression of anxiety-like behaviors (Watson and Mann 2000; Sena et al 2003; Abram et al 2005) and that cocaine has significant affinity for the 5-HT transporter (Cunningham et al 1992a, 1992b; Walsh and Cunningham 1997; Filip et al 2004). Additionally, in our own prior studies, inactivation of the 5-HT cell bodies within the dorsal raphé nucleus (Ettenberg et al 2011) or treatment with the anxiolytic 5-HT1A partial agonist, buspirone, both reduced approach-avoidance “retreats” in the runway (Ettenberg and Bernardi 2006). Buspirone was also effective at selectively attenuating the delayed negative effects of cocaine without modifying the drug’s initial positive effects in a Conditioned Place Test (Ettenberg and Bernardi 2007).
The decision to focus on the BNST is due to the fact that this region receives dense projections from the dorsal raphé nucleus and expresses multiple inhibitory and excitatory 5-HT receptor subtypes (Guo et al 2009; Hammack et al 2009; Hazra et al 2012). Of particular interest here is the 5-HT1B autoreceptor whose activation serves to decrease 5-HT release from the pre-synaptic serotonergic cell at the synapse (Sari et al 1997; Adell et al 2001; Threlfell et al. 2010). Whole-tissue samples of BNST show relatively high levels of expression of 5-HT1B mRNA, whereas single cells from the same region do not express this transcript (Guo et al 2009). This finding indicates that 5-HT1B receptors within the BNST are likely localized presynaptically and so manipulations targeted at these receptors should be selective to 5-HT projections into the BNST. The current study therefore employed the use of a selective 5-HT1B agonist infused directly into the BNST in an attempt to locally inhibit of 5-HT release without disrupting 5-HT effects in other brain regions. The impact of this putative reduction of 5-HT release within the BNST was examined on the approach-avoidance conflict behavior of animals running an alley for the delivery of i.v. cocaine upon goal box entry.
Materials and methods
Subjects
The subjects were 127 male Sprague–Dawley rats (Charles River Labs, Hollister, CA) weighing approximately 300g at the time of surgery. Rats were pair housed within a temperature-controlled (22°C) vivarium maintained on a reverse 12-h light/dark cycle (lights on at 2000 hours) and had ad libitum access to both food (Purina Rat Chow) and water. Animals were handled daily for at least 7 days prior to surgery. All methods were conducted in strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCSB Institutional Animal Care and Use Committee.
Surgery
Rats were deeply anesthetized with an intramuscular injection of ketamine and xylazine (56.25 and 7.5 mg/kg, respectively; Abbott Laboratories) and fitted with an indwelling intravenous catheter (13 mm of Silastic tubing, 0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning) inserted into the right jugular vein, secured in place by silk sutures, and subcutaneously passed to a threaded guide cannula (catalog #313G; Plastics One) that exited though a 2 mm hole on the animal’s back. The guide cannula was cemented to a 3 cm square piece of Mersiline mesh (Bard) that was laid flat subcutaneously on the animal’s back where it was sutured in place. Each rat was also fitted with bilateral intracranial guide cannulae (22 gauge, 9 mm; Catalog #313GA/SPC; Plastics One) stereotaxically aimed 1 mm above the BNST using the following coordinates relative to bregma: AP −0.4, ML ±3.5, and DV −6.2 from skull surface with a lateral inclination of 15° (Paxinos and Watson 2005). During surgery, subjects received the non-opiate analgesic flunixin meglumine, (2mg/kg s.c. at a concentration of 5 mg/ml in saline) to control for post-surgical pain, and saline for rehydration (3.0 ml s.c.). The catheters were flushed with ticarcillin disodium and clavulanate potassium (Timentin, 50mg/0.25ml i.v.) and heparinized saline (6.25IU, 0.1 ml i.v.).
After surgery, catheter patency was maintained through daily flushing with 10mg in 0.1 ml of Timetin antibiotic followed by 0.1 ml of heparinized 0.9% physiological saline. Animals recovered for at least 7 days prior to behavioral testing. Catheter patency was assessed periodically through observation of the loss of the righting reflex after i.v. injection of the fast-acting barbiturate, methohexital (Brevital, 2.0 mg/kg/0.1 ml). Rats that were unresponsive to Brevital prior to the start of behavioral testing were re-implanted with a new catheter using the left jugular vein and given additional days for recovery. Catheter patency failure during the course of behavioral testing resulted in subject removal from data analysis (12 rats were removed due to catheter failure).
Drugs
Cocaine hydrochloride (provided by the National Institute on Drug Abuse) was dissolved in 0.9% physiological saline and sterile filtered through a 0.2μm filter (ThermoScientific). Cocaine was diluted to a dose of 1 mg/kg delivered in a volume of 0.1 ml over a period of 4.3 s via a 10ml syringe nested in a motorized syringe pump (Razel Scientific Instruments). The dose of 1 mg/kg i.v. cocaine was chosen based upon the results of previous runway work from our laboratory (Raven et al 2000; Ettenberg 2004; Ettenberg and Bernardi 2006; Wenzel et al 2011; 2014).
The 5-HT1B agonist CP 94,253 dihydrochloride (Sigma-Aldrich) was prepared in a vehicle solution of aCSF (l-Ascorbic Acid 0.35g/L, NaCl 8.47g/L, KCl .20g/L, MgCl2 .20g/L, CaCl2 .18g/L, NaH2PO4 .276g/L, Na2HPO4 .5362g/L) for intracranial infusion at the concentrations 0.25, 0.5, or 1.0μg/0.5μl. CP 94,253 was selected as it shows the greatest affinity for 5-HT1B over other receptors in the 5-HT1 family (Koe et al 1992). Utilized doses were determined from prior studies reporting behavioral effects with intracerebral administration (De Almeida et al 2006; Veiga and Miczek 2007). The selective 5-HT1B antagonist NAS-181 (Stenfors et al 2000; De Groote et al 2002; 2003) was prepared in the same vehicle as CP 94,253 and infused at doses of 0.1μg or 1.0μg per 0.5μl/side.
Apparatus
Experimental testing was conducted in two identical wooden straight-arm runways. Each apparatus measured 155cm (L) x 15cm (W) x 40cm (H). On opposite ends of the straight alley were identically sized start and goal boxes (each measuring 24cm x 25cm x 40cm) each separated from the middle runway section of the apparatus by retractable doors. Along the interior length of the alley were 13 infrared photodetector-emitter pairs positioned in the walls 16 cm apart from one another. Input from these photocells was fed through an Any-Maze interface (Stoetling) to a laptop computer running AnyMaze software, which recorded the subjects’ location in the runway in real time throughout each trial. For a more detailed description of the runway apparatus see Geist and Ettenberg (1990).
Procedures
Subjects were acclimated to the apparatus by placing them individually into the start box and permitting them to freely explore the apparatus for 10 min (the goal door remained closed to prevent entry into the goal box). On the next day, the first of 16 single daily runway trials was initiated. Three separate experiments were performed: In Experiment I, CP 94,253 was delivered as a pretreatment (10 min prior to each runway trial); Experiment II was conducted in the same manner, except CP 94,253 was delivered as a post-treatment (5 minutes after each runway trial); and in Experiment III the behavioral effects of the autoreceptor agonist were challenged by co-administration of the 5-HT1B antagonist, NAS-181.
In Experiments I the subjects were administered bilateral intra-BNST infusions (0.5 μl/side) of one of the three doses of CP 94,253 (0.25, 0.5 or 1.0μg/side) or vehicle prior to each runway trial. The infusions were administered slowly over 120 s using a 25μl Hamilton syringe that was seated in a motorized syringe pump (KD Scientific). The syringe was connected via PE20 tubing to 28 gauge internal cannula (catalog #313LI/SPC Plastics One) that, when inserted into the implanted guide cannula on the animal’s head, projected 1mm beyond the tip of the guide cannula. The internal cannulae were left in place for 60 s following each infusion to permit diffusion of the drug away from the injection tip. After 10 min, each subject was moved to the runway apparatus, connected to the i.v. drug delivery system, and placed into the start box where, after 5 s, the start door was opened and the trial initiated. Animals were free to traverse the runway until they entered the goal box at which point the goal door automatically closed behind them (to prevent retracing) and an i.v. infusion of 1.0 mg/kg cocaine (in 0.1 ml) was administered over 4.3 s. After 5 min the subjects were removed from the goal box, disconnected from the drug delivery system, and returned to their home cages. On the rare occasion that an animal did not enter the goal box within 10 min, it was gently encouraged (pushed from behind) to enter the goal box, where it then received an i.v. injection of cocaine. All trials for a given subject were conducted in the same apparatus. To maintain catheter patency, animals were flushed with 10mg/0.1ml Timentin followed by 0.1ml heparinized saline after removal from the apparatus.
Since the anxiogenic effects of i.v. cocaine appear to peak at 15-min post injection (Ettenberg et al 1999; Knackstedt et al 2002; Jhou et al 2013) it was important to examine the impact of CP 94,253 in the BNST after the subjects’ had experienced the initial rewarding effects of the cocaine but before the onset of the drug’s anxiogenic actions–hence the treatment in Experiment II was applied 5-min post-cocaine. Animals first ran to and entered the goal box where they earned an i.v injection of cocaine, and then were removed (after 5-min) and administered either 0.0 or 1.0μg i.c. CP 94,253.
Experiment III was conducted to demonstrate the selectivity of the agonist’s effects to the 5-HT1B receptor. In this protocol, 10min prior to each runway trial, animals were provided intra-BNST bilateral infusions of either the vehicle solution alone, or a 0.5μg dose of the 5-HTB1 agonist, CP 94,253, co-administered with either 0.0, 0.1 or 1.0 μg of the selective 5-HT1B antagonist NAS-181. Both drugs were administered in the same microinjection after which the runway testing was accomplished as described for Experiment I.
In all experiments, three dependent measures were recorded on every trial. “Start latency” -- the time required for the animal to leave the start box once the start door was opened; “Run Time” -- the time required for the animal to enter the goal box after it had left the start box; and “Retreats” -- the number of times an animal halted its forward motion and retreated back toward the start box by the length of at least two photodetector-emitters (i.e., approximately 32cm).
Spontaneous locomotor activity
To ensure that central application of the intra-BNST infusions did not produce nonspecific alterations in the response capacity of the subjects, animals from Experiment I and III were examined in a test of spontaneous locomotor activity following completion of runway testing. Locomotor behavior (distance traveled) was measured in 12 identical Plexiglas chambers each 20cm (L) x 40cm (W) x 20 cm(H) (Kinder Scientific). Each test chamber was each equipped with an array of 15 infrared photodetector-emitter pairs evenly spaced along its long axis and 7 along its narrow axis, all 8 cm above the floor surface. Movement within the chamber produced photobeam interruptions that were recorded by a desktop computer running custom software (Kinder Scientific). At the start of testing, all animals were allowed to acclimate to the locomotor chambers for 60 min. Rats were then removed from the test chambers and administered the same bilateral microinjections that they had received previously during runway testing immediately after which they were returned to the locomotor chambers for an additional 15 min test session.
Histology
After completion of behavioral testing, animals were euthanized with an overdose of sodium pentobarbital and phenytoin sodium solution (Euthasol; Virbac) and perfused with 200mL Phosphate Buffered Saline (PBS) followed by 200mL 4% Paraformaldehyde (PFA) in PBS. Brains were removed and post-fixed in 4% PFA, after which cannula placements were determined from Nissl-stained 40μm frozen sections.
Results
Histology
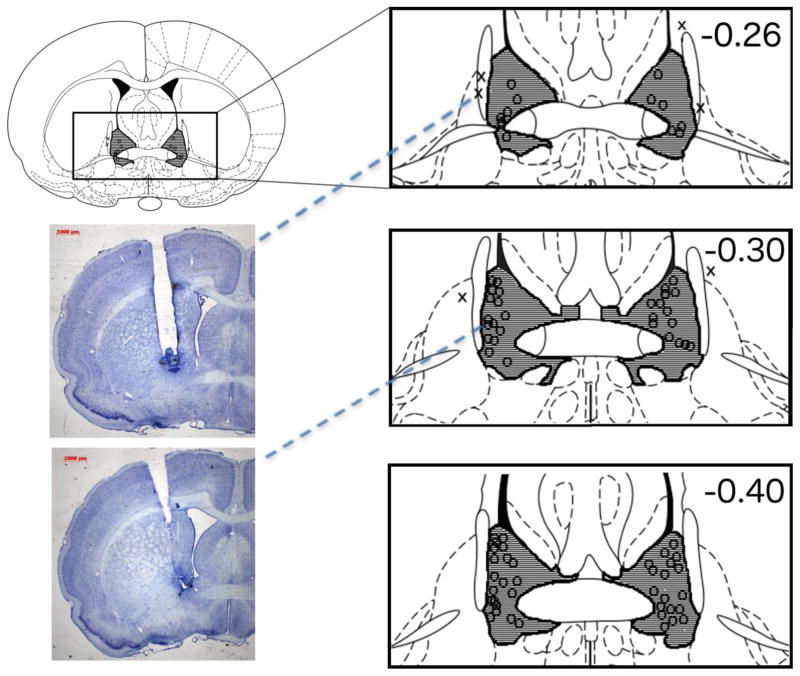
A subject’s inclusion in the study required strict histological confirmation of bilateral placements directly above the target brain areas under investigation (see Fig. 1). A total of 44 animals successfully completed Experiment I, 16 completed Experiment II, and 33 in Experiment III. In order to verify anatomical specificity of our manipulation, an additional group of 16 animals was included in the analysis consisting of the subjects in which cannulae placements were outside the target region. Of the 16 animals in the anatomical control group, N=6 had one or more cannula located within the lateral ventricles and N=4 are not pictured in Fig. 1 as their cannulae were located in regions posterior to the data visualized. Additionally, 6 animals that were found to have evidence of necrosis around the injection site were removed from the data analyses.
Figure 1.
Histological confirmation of cannula placements within the BNST. Shaded areas indicate regions where successful cannula placements were identified. Locations marked with an “O” indicate the placement of cannula tips within the targeted region. The “X’s” indicate placements that missed the target and were separately analyzed as anatomical controls. Not pictured are 6 animals whose cannulae tips were located within the lateral ventricles and 4 animals whose cannulae placements were posterior to the target region. Numbers represent distance of coronal slices (in mm) posterior to bregma. Figure adapted from Paxinos and Watson (2005). The two representative photomicrographs provide an example of a missed cannula placement (top) and a correctly placed cannula (bottom) sitting above the BNST. Dashed lines point to the corresponding cannula placement on the schematic diagram. The darkly-stained areas at the tip of each cannula track reflect the tissue displaced by the cannula insertion. Note that the small diameter internal infusion cannula protruded beyond these tracks infusing drug into a spherical region beginning approximately 1 mm below the end of the cannula track. Both animals in these examples were infused with the 5-HT1B agonist; the subject with the missed cannula (top) made 18 retreats over the final 8 trials while the animal whose cannula was identified as ‘on target’ (bottom) for the BNST made 2 retreats during the final 8 trials. These individual data are reflective of the performance of the groups to which each animal was assigned.
Experiment I
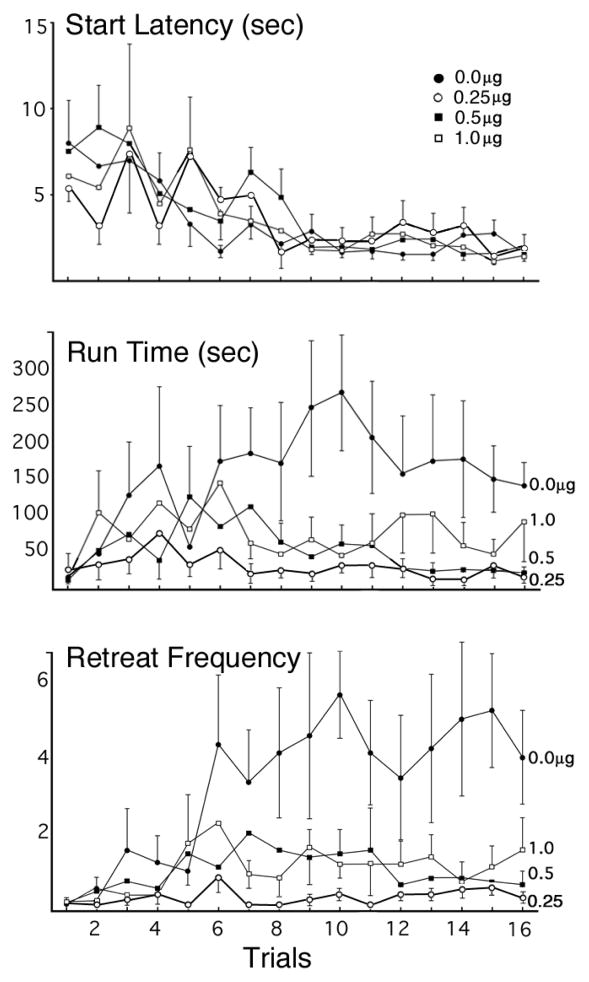
This experiment tested the effect of bilateral intra-BNST infusions of CP 94,253 (0, 0.25, 0.5, or 1.0μg) on the runway behavior of animals approaching and entering a goal-box associated with the administration of 1.0 mg/kg i.v. cocaine. Group sizes were N=12, 8, 12, 12, respectively. Figure 2 depicts the runway performance of the four groups during the 16 days of testing. A two-factor (Group x Trial) ANOVA computed on the Start Latency data (top panel) revealed a significant main effect of Trial (F(15,25)=3.976, p=.001), but no significant effect of Group (F(3,39)=.177, p >.05) and no significant Group x Trial interaction (F(45,81)=.792, p >.05), indicating that all groups reliably and comparably decreased their start latencies over the course of the experiment. CP 94,253 did not, therefore, reliably affect the subjects’ response initiation in the runway.
Figure 2.
Group Mean (±SEM) start latencies (top panel), run times (middle panel) and approach-avoidance retreat behaviors (bottom panel) of animals running a straight alley once each day for single daily infusions of 1.0mg/kg i.v. cocaine after pretreatment with bilateral intra-BNST infusions (0.0, 0.25, 0.5 or 1.0 μg) of the 5HT1B agonist, CP 94,253. Group sizes were N=12, N=8, N=12, N=12, respectively.
The run time data for each group are shown in the middle panel of Figure 2. The two-factor ANOVA computed on these data identified a statistically significant main effect of Group (F(3,40)=4.043, p = 0.013). As the figure illustrates, vehicle animals took the longest to enter the goal box, the low and intermediate doses of CP 94,253 produced the shortest run times, while the high dose group produced intermediate results. Post Hoc analyses confirmed that animals treated with the 0.25μg and 0.5μg dose of the 5-HT1B agonist entered the goal box sooner than subjects in the vehicle group (Fischer’s Least Significant Difference [LSD] Test; p=.015 and p=.047, respectively), while the comparison between the high dose and vehicle did not reach statistical significance (p=.198). There was no reliable difference observed between the doses of the agonist (p>.05). The ANOVA revealed no main effect of Trial; F(15,26)=1.137, p=.374, and no significant Group x Trial interaction; F(45,84)=.908, p=.634.
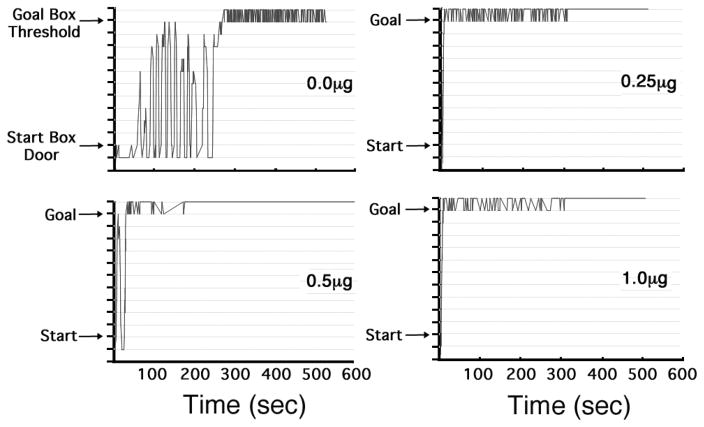
Analysis of the mean (± SEM) retreat frequencies of the four groups (bottom panel of Figure 2) revealed a significant main effect of Group (F(3,40)=5.332, p = .003) and a significant main effect of Trial (F (15,26)=2.093, p= .048). The Group x Trial interaction did not reach statistical significance (F(45,84)=1.010, p = .474). Post-hoc analyses of the retreat data using Fischer’s LSD Tests revealed that animals in each of the three CP 94,253 groups -- the 0.25μg (p=.005), 0.5μg (p=.023) and 1.0μg (p =.034) groups -- emitted fewer retreats than those in the vehicle control group, while the difference between the drug groups themselves was not significant (p >.05). The effect of CP 94,253 on approach-avoidance retreat behavior is also illustrated in Figure 3 which graphically depicts the path that a representative animal from each group took as it proceeded from start box to goal box. The steep slopes of the lines reflect the fact that animals ran quickly toward or away from the goal box while the number of retreats is reflected by the number of times the line changes direction. As the figure clearly shows, the vehicle-treated animal exhibited many more retreat behaviors than did each of the three CP 94,253 representative animals.
Figure 3.
Sample Spatio-Temporal Records from four representative animals -- one from each group as identified by the dose of the 5-HT1B agonist (CP 94,253) that was applied to each subject. The figure depicts the animal’s location within the alley in real time; i.e., the path that the animal took from start box (at the bottom the y-axis) to the goal box (near the top of the y-axis). Crossing the threshold into the goal box closed the goal-door behind the animal (to prevent retracing) and trigged infusion of 1.0mg/kg i.v. cocaine. Each panel depicts the behavior of a representative animal on Trial 15. Peaks in the graph correspond to retreats, i.e., a reversal in direction from approach to avoidance of the goal box.
Experiment II
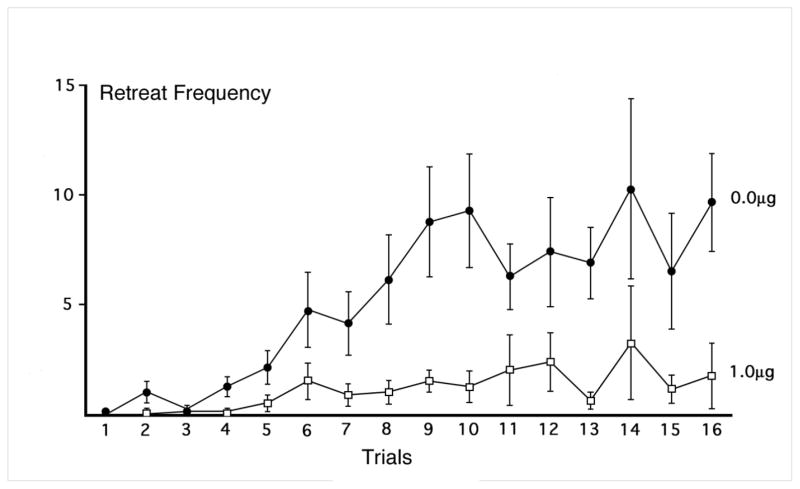
As described above, the second experiment was conducted to determine whether intra-BNST administration of CP 94,253 5-min after goal-box administration of cocaine, could prevent or attenuate the impact of cocaine’s anxiogenic effects which have been shown to occur 15 min post-injection (Ettenberg et al 1999; Knackstedt et al 2002; Jhou et al 2013). Each group was comprised of N=8 animals. Post-cocaine treatment with the 0.5μg infusions of the 5-HT1B agonist immediately after removal from the goal box effectively reduced the frequency of approach-avoidance retreats (Figure 4). There was a significant main effect of Group (F(1,14) = 7.963, p =.014), Trial (F(15,210) = 6.155, p <.001), and a significant Group x Trial interaction (F(15,210) = 2.586, p = .001). As the figure illustrates, while both groups behaved comparably at the outset of testing, the drug group continued to exhibit relatively low levels of retreat behavior while the vehicle-treated animals produced increased retreats as testing progressed.
Figure 4.
Mean (±SEM) retreat frequency of animals treated with bilateral intra-BNST infusions of 0.0 or 1.0 μg/side of the 5HT1B agonist, CP 94,253, 5-min after single daily trials in animals running a straight alley for 1.0 mg/kg i.v. cocaine. N=8 per each treatment group.
The results for Start Latency and Run Time (data not shown) were comparable to those reported for Experiment I. Start latencies decreased as testing progressed (a significant main effect of Trial; F(15,210) = 2.865, p < .001), and did so comparably for both groups (there was no main effect of Group and no Group x Trial interaction (p>.05). Although Run Times tended to increase on average across both groups as retreat frequencies increased (retreating animals take longer to get to the goal box; a main effect of Trial; F(15,210) = 4.458, p<.001), the CP 94,253 group entered the goal box sooner than the vehicle-treated animals (a significant main effect of Group; F(1,14) = 5.246, p = .038). The Group x Trial interaction was not statistically significant (p>.05).
Experiment III
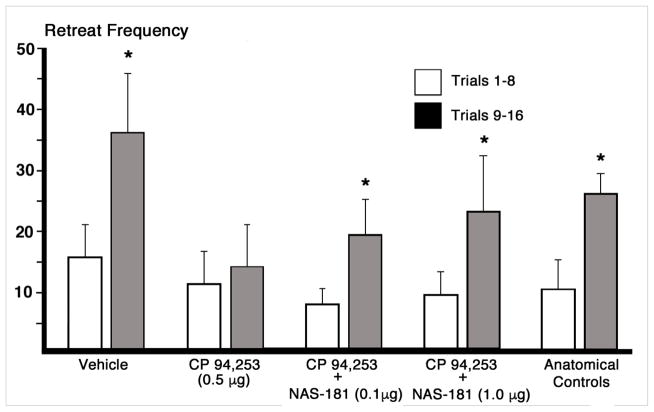
This experiment was conducted to assess the efficacy of a highly selective 5-HT1B antagonist in reversing the effects of CP 94,253 observed in Experiment I. Group sizes were N=8 for vehicle treated animals, N=7 in the CP 94,253 (0.5μg) alone group, N=9 for the agonist + the low dose (0.1μg) of the 5-HT1B antagonist (NAS-181), N=8 for the agonist plus + high dose (1.0μg) of the antagonist, and N=16 for the anatomical control group (i.e., subjects that received the autoreceptor agonist alone across Experiments I and III but whose cannula were determined to be outside the target zone). Figure 5 illustrates the change in retreats across the first and second halves of runway trials for each group. Bonferroni-protected one-tailed repeated measures t-tests were conducted on the changes in retreat frequency over trials for each group. The data analyses identified a significant increase in retreats over trials in the vehicle treated group t(8)= −2.44, p=<.05, an effect prevented by pre-treatment within intra-BNST administration of CP 94,253 alone (t(6)= −0.60, p>.05; confirming of the results of Experiment I). In contrast, co-administration of the autoreceptor agonist with either the low or high dose of the NAS-181 antagonist reversed the effect of the agonist alone–both groups demonstrated increases in retreat frequency over trials [t(8)= −2.140, and t(7)= −2.447, respectively, both p<.05). Additionally, animals in the large anatomical control group performed comparably to vehicle-treated animals exhibiting an increase in retreat frequency over trials (t(15)= −2.57, p<.05).
Figure 5.
Mean (±SEM) retreat frequency of animals running an alley for single daily iv infusions of 1.0 mg/kg cocaine delivered upon goal-box entry. The group designations refer to the bilateral intra-BNST infusions that each group received prior to each trial. Retreats were summed across the first half (trials 1–8) and second half (trials 9–16) of the experiment. Group sizes were N=8 for vehicle treated animals, N=7 in the 5-HT1B autoreceptor agonist CP 94,253 (0.5μg) group, N=9 for the CP 94,253 + the low dose (0.1μg) of the autoreceptor antagonist NAS-181, N=8 for CP 94,253 + high dose of the antagonist (1.0μg), and N=16 for the anatomical control group (which received 0.5μg of CP 94,253 alone but whose cannulae were histologically determined to have missed the BNST target site). The data analyses compared the performance of each group during the first 8 trials to that on the final 8 trials *p<.05.
Spontaneous Locomotor Activity
The effects of CP 94,253 and the combination of CP 94,253 + NAS 181 on spontaneous locomotor behavior were assessed by two (Group x Time) ANOVAs: one computed on the data from the initial 60 min baseline/acclimation period, and another on the data following the drug infusions (data not shown). Although there were the expected reductions in locomotor activity as animals acclimated to the apparatus (i.e., significant main effects of Time during both the baseline and test periods; p<.001) no Group nor Group x Time interactions were identified. Thus, manipulations of the 5-HT1B autoreceptor within the BNST produced no perceivable decrements in the spontaneous ambulatory behavior of subjects relative to vehicle controls.
DISCUSSION
The present study examined the impact of 5-HT1B autoreceptor activation in the BNST on the behavior of animals running a straight alley for a “reward” of i.v. cocaine. As previously reported, cocaine-reinforced animals developed a characteristic pattern of retreat behaviors, reflecting an approach-avoidance conflict about entering a goal box with which the subjects had formed mixed positive and negative associations (see Ettenberg et al 1999; Ettenberg 2004; Raven et al 2000; Jhou et al 2013). Pretreating animals with a selective 5-HT1B agonist delivered into the BNST significantly decreased expression of retreat behaviors, an effect that was reversed by co-administration of a selective 5-HT1B antagonist. These results cannot be easily accounted for by some form of nonspecific motoric or sedative incapacitation produced by the autoreceptor agonist (and reversed by the antagonist) since the drug did not have a significant effect on the animals’ spontaneous locomotor activity. Taken together, the results are consistent with the hypothesis that serotonergic release within the BNST contributes to the aversive/anxiogenic response to cocaine.
Of course, reductions in approach-avoidance conflict (retreats) could conceivably occur in response to a treatment-induced enhancement in the rewarding properties of cocaine as opposed to our hypothesized reduction in the drug’s anxiogenic actions. For example, Parsons et al. (1998) reported that both systemic and intra-ventricular treatment with different 5-HT1B agonists produced changes in cocaine self-administration that mirrored what is seen when the unit dose of cocaine is increased, suggesting that 5-HT1B activation produces an increase in the reward value of cocaine (see also Filip et al 2010 and Miszkiel et al 2011). To account for such results, it has been suggested that activation of 5-HT1B receptors on GABAergic neurons of the VTA could lead to a disinhibition of dopamine release through a reduction in the tonic GABA inhibitory activity within this region (Castanon et al 2000; Filip et al 2003). This would allow for a 5-HT1B mediated potentiation of cocaine’s rewarding effects.
While the results described above might seem in opposition to the current findings and conclusions, there is no a priori reason to assume that the actions of a presynaptic receptor agonist in one brain region would be functionally equivalent to those in another region. Thus, while it is certainly conceivable that the application of a 5-HT1B agonist to the VTA may increase cocaine reward through a dopaminergic mechanism, it is equally conceivable that the same drug applied to the BNST might act on a different neurotransmitter system whose actions serve to enhance the “net” reward value of the drug via a reduction in the anxiogenic/negative actions of cocaine. For example, a great deal of recent attention has been paid to the fact that the ventral subregion of the BNST projects to and thereby modulates the function of cells within the VTA (Aston-Jones et al 2001; Georges and Aston-Jones 2002; Dumont and Williams 2004; Jalabert et al 2009; Jennings et al 2013; Sparta et al 2013; Adhikari 2014; Stamatakis et al 2014). Given the well documented role of the VTA in the performance of reward-related behaviors (e.g., McBride et al 1999; Koob 2003; Stuber et al 2012; George et al 2012; Jennings et al 2013; Koob and Volkow 2016), and the demonstration that the BNST sends excitatory projections to the region (e.g., Aston-Jones et al. 2001; Georges and Aston-Jones 2002) it may be that intra-BNST infusions of a 5-HT1B agonist reduce the serotonergic inhibition of excitatory inputs to the VTA and thereby increase the rewarding effects of cocaine. Consistent with this notion are the results of Sartor and Aston-Jones (2012) demonstrating that disruption of the ventral-BNST-VTA pathway prevents the expression of cocaine-induced conditioned place preferences. However, more recent work by Jennings et al (2013) suggests that the functional relationship between the BNST and VTA is more complex than originally thought. These investigators have shown that the BNST sends both glutametergic and GABAergic projections to the VTA and that each pathway modulates the VTA in opposing ways. More specifically, in vivo photostimulation of BNST-glutametergic projections to the VTA resulted in aversive and anxiogenic behavioral phenotypes, while activation of BNST-GABAergic projections produced rewarding and anxiolytic phenotypes. Thus, the current BNST manipulations of 5-HT may have altered the response of animals seeking cocaine either by increasing the reward signal or decreasing the anxiogenic signal from BNST to VTA. As indicated above, we hypothesize that the latter explanation best fits our results.
Our conclusion is based upon the fact that while start latencies (the time it took for subjects to initiate responding by leaving the start box) dramatically decreased over trials (suggesting that the motivation to seek the drug increased as testing progressed), there were no group differences in the development or magnitude of this effect. Put simply, there was no evidence that the goal box experience was more rewarding (and hence more motivating) in the groups pretreated with of CP 94,253 than in the vehicle control group. The fact that the animals’ start latencies were unaltered by the infusion of CP 94,253 into the BNST suggests that the motivation to seek the cocaine was unaffected, while the reduction in retreats suggests that the negative consequences of cocaine were reduced. It may therefore be that the enhanced cocaine-reward effects observed by others following intra-VTA administration of 5-HT1B agonists might similarly be due to a reduction in the negative/anxiogenic effects of the cocaine as opposed to a direct stimulatory action on reward circuitry.
Experiment II was conducted to obtain further insight into the behavioral mechanism by which intra-BNST administration of CP 94,253 reduced the animal’s approach-avoidance conflict in the runway. Since pretreatment with drugs like diazepam have been shown to reduce approach-avoidance retreats (Ettenberg and Geist 1991), it is possible that the 5-HT1B agonist pretreatment (Experiment I) was acting as a general anxiolytic that reduced retreats not by interfering with the delayed negative consequences of cocaine, but more simply by reducing the animals’ general anxiety prior to each trial. Indeed, the systemic administration of 5-HT1B agonists has been demonstrated to have anxiolytic and antidepressant-like effects (Tatarczyńska et al 2004; 2005). It was therefore of interest to determine whether or not BNST application of CP 94,253 after the runway trial but before the onset of cocaine’s negative effects (which have been shown to reach peak levels in rats 15-min after injection; see Ettenberg et al 1999; Knackstedt et al 2002; Jhou et al 2013) would similarly reduce approach-avoidance retreat behaviors in the runway. The observed reduction in retreat behaviors produced by this treatment could not therefore be accounted for by any indirect or nonspecific action of the treatment on either the animals’ anxiety prior to the start of testing nor their behavioral capacity since the subjects were running prior to the delivery of either the cocaine or the CP 94,253. We therefore conclude that the comparable effects of the pre- and post-treatment application of CP 94,253 suggest that the treatment altered the impact of the delayed negative consequences of cocaine administration.
Finally, the authors recognize that the current study does not decisively identify a presynaptic mechanism of action for CP 94,253. For example, it has been established that 5-HT1B receptors are not exclusively located on 5-HT pre-synaptic elements -- they also exist as heteroreceptors on the terminals of glutamatergic neurons that synapse within the BNST (Guo and Rainnie 2010). Thus the reductions in retreat behavior observed in the present study might be due to CP 94,253’s putative inhibitory effects on 5-HT release as we have concluded, or alternatively to an impaired functioning of a glutamatergic excitatory “driver” input. That being said, there is evidence that the 5-HT1B receptor exists primarily on the presynaptic membrane in regions receiving 5-HT projections (Riad et al. 2000), and other evidence that 5-HT1B hetereoreceptors have a lower sensitivity to 5-HT1B agonists than 5-HT1B autoreceptors (Sarhan and Fillion 1999) again suggesting that the current results were likely due to an action of CP 94,253 on presynaptic elements within the BNST. Research is continuing in our laboratory to further examine the role of 5-HT, as well as other neuronal systems both within the BNST and brain regions to which the BNST projects, in contributing to the anxiogenic response to cocaine.
Acknowledgments
The authors wish to thank Dr. Kerisa Shelton for her assistance throughout the project. This work was funded by NIDA grant DA03370 awarded to AE.
References
- Abrams JK, Johnson PL, Hay-Schmidt a, et al. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience. 2005;133:983–997. doi: 10.1016/j.neuroscience.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Adell A, Celada P, Artigas F. The role of 5-HT 1B receptors in the regulation of serotonin cell firing and release in the rat brain. J Neurochem. 2001;79:172–182. doi: 10.1046/j.1471-4159.2001.00550.x. [DOI] [PubMed] [Google Scholar]
- Adhikari A. Distributed circuits underlying anxiety. Front Behav Neurosci. 2014;8:112. doi: 10.3389/fnbeh.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Biobehav Rev. 1999;23:981–91. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: A comparison of knockouts and antagonists. Pharmacol Biochem Behav. 2000;67:559–566. doi: 10.1016/S0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Serotonin neurotransmission in cocaine sensitization. Ann N Y Acad Sci. 1992a;654:117–27. doi: 10.1111/j.1749-6632.1992.tb25960.x. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992b;11:112–23. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Almeida RMM, Rosa MM, Santos DM, Saft DM, Benini Q, Miczek KA. 5-HT(1B) receptors, ventral orbitofrontal cortex, and aggressive behavior in mice. Psychopharmacology (Berl) 2006;185:441–50. doi: 10.1007/s00213-006-0333-3. [DOI] [PubMed] [Google Scholar]
- De Groote L, Klompmakers AA, Olivier B, Westenberg HGM. An evaluation of the effect of NAS-181, a new selective 5-HT1B receptor antagonist, on extracellular 5-HT levels in rat frontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:89–94. doi: 10.1007/s00210-002-0685-0. [DOI] [PubMed] [Google Scholar]
- De Groote L, Olivier B, Westenberg HG. Extracellular serotonin in the prefrontal cortex is limited through terminal 5-HT1B autoreceptors: A microdialysis study in knockout mice. Psychopharmacology (Berl) 2002;162:419–424. doi: 10.1007/s00213-002-1117-z. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–204. doi: 10.1523/jneurosci.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–7. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi R. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi R. Anxiolytic-like actions of buspirone in a runway model of intravenous cocaine self-administration. Pharmacol Biochem Behav. 2006;85:393–399. doi: 10.1016/j.pbb.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–8. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Animal model for investigating the anxiogenic effects of cocaine. Pharmacol Biochem Behav. 1991;103:455–461. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Ofer O, Mueller C. Inactivation of the dorsal raphe nucleus reduces the anxiogenic response of rats running an alley for intravenous cocaine. Pharmacol …. 2011;97:632–639. doi: 10.1016/j.pbb.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Alenina N, Bader M, Przegaliński E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15:227–49. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar M, Cunningham KA. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol …. 2004;310:1246–1254. doi: 10.1124/jpet.104.068841.effects. [DOI] [PubMed] [Google Scholar]
- Filip M, Papla I, Nowak E, Czepiel K, Przegaliński E. Effects of 5-HT1B receptor ligands microinjected into the ventral tegmental area on cocaine discrimination in rats. Eur J Pharmacol. 2003;459:239–245. doi: 10.1016/S0014-2999(02)02873-X. [DOI] [PubMed] [Google Scholar]
- Gawin FH. Cocaine Psychology and Addiction : Neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying drug reinforcement in a runway. Pharmacol Biochem Behav. 1990;36:703–706. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 2012;106:58–64. doi: 10.1016/j.physbeh.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–87. doi: 10.1523/JNEUROSCI.22-12-05173.2002. 20026472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-D, Hammack SE, Hazra R, Levita L, Rainnie DG. Bi-directional modulation of bed nucleus of stria terminalis neurons by 5-HT: molecular expression and functional properties of excitatory 5-HT receptor subtypes. Neuroscience. 2009;164:1776–93. doi: 10.1016/j.neuroscience.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-D, Rainnie DG. Presynaptic 5-HT1B receptor-mediated serotonergic inhibition of glutamate transmission in the bed nucleus of the stria terminalis. Neuroscience. 2010;165:1390–1401. doi: 10.1016/j.neuroscience.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo J-D, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1309–20. doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayase T, Yamamoto Y, Yamamoto K. Persistent anxiogenic effects of a single or repeated doses of cocaine and methamphetamine: interactions with endogenous cannabinoid receptor ligands. Behav Pharmacol. 2005;16:395–404. doi: 10.1097/00008877-200509000-00012. [DOI] [PubMed] [Google Scholar]
- Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalabert M, Aston-Jones G, Herzog E, et al. Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuro-Psychopharmacology Biol Psychiatry. 2009;33:1336–1346. doi: 10.1016/j.pnpbp.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–8. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Rowley CS, Xu S-P, Wang H, Burnham NW, Hoffman AF, Lupica CR, Ikemoto S. Cocaine drives aversive conditioning via delayed activation of dopamine-responsive habenular and midbrain pathways. J Neurosci. 2013;33:7501–12. doi: 10.1523/JNEUROSCI.3634-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt L, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–936. doi: 10.1016/S0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koe BK, Nielsen JA, Macor JE, Heym J. Biochemical and behavioral studies of the 5-HT{-1B} receptor agonist, CP-94, 253. Drug Dev Res. 1992;26:241–250. [Google Scholar]
- Koob G. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Koob G. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101:129–52. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Filip M, Przegaliński E. Role of serotonin (5-HT) 1B receptors in psychostimulant addiction. Pharmacol Reports. 2011:1310–1315. doi: 10.1016/s1734-1140(11)70695-8. [DOI] [PubMed] [Google Scholar]
- Parsons L, Weiss F, Koob G. Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci. 1998;18:10078–10089. doi: 10.1523/JNEUROSCI.18-23-10078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–124. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Kestenbaum RS, Schwartz LK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195:696–698. doi: 10.1126/science.841307. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. doi: 10.1002/(SICI)1096-9861(20000207)417:2<181::AID-CNE4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–3. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Sari Y, Lefèvre K, Bancila M, Quignon M, Miquel M-C, Langois X, Hamon M, Vergé D. Light and electron microscopic immunocytochemical visualization of 5-HT1B receptors in the rat brain. Brain Res. 1997;760:281–286. doi: 10.1016/S0006-8993(97)00400-9. [DOI] [PubMed] [Google Scholar]
- Sarhan H, Fillion G. Differential sensitivity of 5-HT 1B auto and heteroreceptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:382–390. doi: 10.1007/s002109900067. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of ventral tegmental area by bed nucleus of the stria terminalis is required for expression of cocaine preference. 2012;100:130–134. doi: 10.1016/j.pestbp.2011.02.012.Investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LM, Bueno C, Pobbe RLH, et al. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res. 2003;142:125–133. doi: 10.1016/S0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Jennings JH, Ung RL, Stuber GD. Optogenetic strategies to investigate neural circuitry engaged by stress. Behav Brain Res. 2013;255:19–25. doi: 10.1016/j.bbr.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology. 2014;76(Pt B):320–8. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Britt J, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010.Optogenetic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-I, Wenzel J, Baird R, Ettenberg A. Comparison of self-administration behavior and responsiveness to drug-paired cues in rats running an alley for intravenous heroin and cocaine. Psychopharmacology (Berl) 2011;214:769–778. doi: 10.1007/s00213-010-2088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z-I, Wenzel J, Ettenberg A, Ben-Shahar O. Prior extended daily access to cocaine elevates the reward threshold in a conditioned place preference test. Addict Biol. 2013 doi: 10.1111/adb.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Stenfors C, Yu H, Ross SB. Enhanced 5-HT metabolism and synthesis rate by the new selective r5-HT1B receptor antagonist, NAS-181 in the rat brain. Neuropharmacology. 2000;39:553–560. doi: 10.1016/S0028-3908(99)00173-2. [DOI] [PubMed] [Google Scholar]
- Tatarczyńska E, Antkiewicz-Michaluk L, Kłodzińska A, et al. Antidepressant-like effect of the selective 5-HT1B receptor agonist CP 94253: A possible mechanism of action. Eur J Pharmacol. 2005;516:46–50. doi: 10.1016/j.ejphar.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Greenfield SA, Cragg SJ. 5-HT(1B) receptor regulation of serotonin (5-HT) release by endogenous 5-HT in the substantia nigra. Neuroscience. 2010;165:212–20. doi: 10.1016/j.neuroscience.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Veiga C, Miczek K. Effect of 5-HT1B receptor agonists injected into the prefrontal cortex on maternal aggression in rats. Brazilian J …. 2007;40:825–830. doi: 10.1590/s0100-879x2006005000113. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/S0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Cunningham KA. Serotonergic mechanisms involved in the discriminative stimulus, reinforcing and subjective effects of cocaine. Psychopharmacology (Berl) 1997;130:41–58. doi: 10.1007/s002130050210. [DOI] [PubMed] [Google Scholar]
- Watson S, Man MS. Serotonin, stress and corticoids. J Psychopharmacol. 2000;14:419–421. doi: 10.1177/026988110001400415. [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Cotten SW, Dominguez HM, Lane JE, Shelton K, Su Z-I, Ettenberg A. Noradrenergic β-receptor antagonism within the central nucleus of the amygdala or bed nucleus of the stria terminalis attenuates the negative/anxiogenic effects of cocaine. J Neurosci. 2014;34:3467–74. doi: 10.1523/JNEUROSCI.3861-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, Su Z-I, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011;217:221–30. doi: 10.1007/s00213-011-2267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/S0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Yang XM, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–50. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]