Abstract
There is a pressing need for biomarkers and outcomes that can be used across disease stages in Duchenne muscular dystrophy (DMD), to facilitate the inclusion of a wider range of participants in clinical trials and to improve our understanding of the natural history of DMD. Quantitative magnetic resonance imaging (qMRI) and spectroscopy (MRS) biomarkers show considerable promise in both the legs and forearms of individuals with DMD, but have not yet been examined in functionally important proximal upper extremity muscles such as the biceps brachii and deltoid. The primary objective of this study was to examine the feasibility of implementing qMRI and MRS biomarkers in the proximal upper extremity musculature, and the secondary objective was to examine the relationship between MR measures of arm muscle pathology and upper extremity functional endpoints. Biomarkers included MRS and MRI measures of fat fraction and transverse relaxation time (T2). The MR exam was well tolerated in both ambulatory and nonambulatory boys. qMR biomarkers differentiated affected and unaffected participants and correlated strongly with upper extremity function (r=0.91 for biceps brachii T2 versus Performance of Upper Limb score). These qMR outcome measures could be highly beneficial to the neuromuscular disease community, allowing measurement of the quality of functionally important muscles across disease stages to understand the natural history of DMD and particularly to broaden the opportunity for clinical trial participation.
Keywords: nonambulatory, transverse relaxtion time, biomarker, magnetic resonance spectroscopy
INTRODUCTION
Boys with DMD experience progressive proximal to distal muscle weakness, lose ambulation in late childhood or adolescence, and die due to cardiac or respiratory failure in the 3rd or 4th decade of life [17]. Clinical deficits in arm function typically begin to appear between 9–14 years [4], and progress to the point where the individual no longer has meaningful use of the hands or arms. Whereas mobility aids such as wheelchairs can partially compensate for lost ambulatory function, loss of upper extremity function has a tremendous impact on quality of life, affecting the individual’s ability to eat and drink, perform personal care, and use a computer or power wheelchair.
Over the last decade, tremendous progress has been made in therapeutic development for DMD, with an unprecedented number of promising interventions moving into clinical trials. Unfortunately, current clinical trials rely almost exclusively on ambulatory endpoints, often at the exclusion of non-ambulatory patients, who constitute more than two thirds of the population. Advocacy groups, clinicians, and pharmaceutical companies are quickly recognizing the need for upper extremity outcome measures as well as natural history data, to facilitate the inclusion of non-ambulatory boys into clinical trials and to meet enrollment targets in the face of numerous trials in this rare disease. Several biotechnology companies are already pursuing the inclusion of upper extremity endpoints in clinical trials (eg. NCT01826474, NCT02310763). However, these endpoints are often subjectively scored and the sensitivity to treatment or to change over time may not be known [12].
Magnetic resonance (MR) imaging is quickly emerging as a powerful outcome measure in DMD. Quantitative MR imaging and spectroscopy (MRS) of the lower extremity muscles have proven to be sensitive to disease progression [7, 22], predict ambulatory functional ability [1], and detect the therapeutic response to corticosteroids [11]. Multiple pharmaceutical companies have started to incorporate MR biomarkers of lower extremity muscles as secondary or primary outcomes in clinical trials. Unfortunately, few studies have explored the feasibility of implementing quantitative MR (qMR) strategies to evaluate the upper extremity musculature in boys with DMD. Two groups have monitored muscle pathology in the forearm muscles of both ambulatory and non-ambulatory patients with DMD [10, 18, 20], but to our knowledge no previous MR studies have examined the proximal upper extremity muscles, such as the biceps brachii (BB) or deltoid (DEL).
The primary objective of this study was to examine the feasibility of implementing qMR imaging and MRS biomarkers in the upper extremity musculature, especially in functionally important proximal muscles, such as the BB, triceps brachii (TB), and DEL. Quantitative MR measures of muscle pathology included intramuscular fat fraction (determined by chemical shift encoded MR imaging and single voxel 1H-MRS) as well as proton muscle transverse relaxation time (T2), an index of muscle damage, inflammation, and edema. The second objective was to examine the relationship between qMR measures of arm muscle pathology and upper extremity functional endpoints.
METHODS
Research Design
22 boys with DMD (10.8 ± 2.5 years, 2 nonambulatory) and 6 unaffected control boys (CON; 11.7 ± 2.3 years) participated in this study, which was approved by the institutional review board at the University of Florida. Parents of each boy provided informed consent, and boys provided informed assent. The study consisted of an MR exam lasting less than one hour, which included measurements of the upper arm muscles for all boys. A subset of boys also completed forearm measurements and/or shoulder measurements.
Four MR biomarkers were measured in the upper arm and shoulder: fat fraction (FF) measured using 1H-MRS, 1H2O-T2 measured using 1H-MRS, MRI FF using chemical shift-encoded (also known as Dixon) imaging, and muscle T2 measured using T2 weighted imaging (MRI T2). Only MRI T2 measures were performed in the forearm.
Following MR data acquisition, subjects completed the Performance of Upper Limb test (PUL) as previously described [14, 16], the Brooke Upper Extremity Scale [5], and grip and pinch strength testing using a Jamar handgrip dynamometer [13].
MR Data Acquisition and Analysis
All MR measurements were completed with subjects lying supine within the bore of a 3T MR whole body magnet (Philips Achieva). The arm was stabilized with the forearm in neutral, the elbow in full extension and the shoulder abducted only as far as necessary to prevent respiratory motion impacting the arm (typically ~20 degrees) using a fabricated thermoplastic splint, encompassing part of the forearm and hand. A separate support splint was placed around the shoulder, cupping the deltoid. Either an 8 channel general purpose flexible surface coil (Invivo) or a dual SENSE flex coil (Phillips) were positioned over the different regions of the arm to be imaged. Sandbags were placed over the arm and hand to minimize motion. Boys were encouraged to watch a movie during data acquisition, and a staff member as well as the subject’s parent or guardian was present in the scan room throughout the scans.
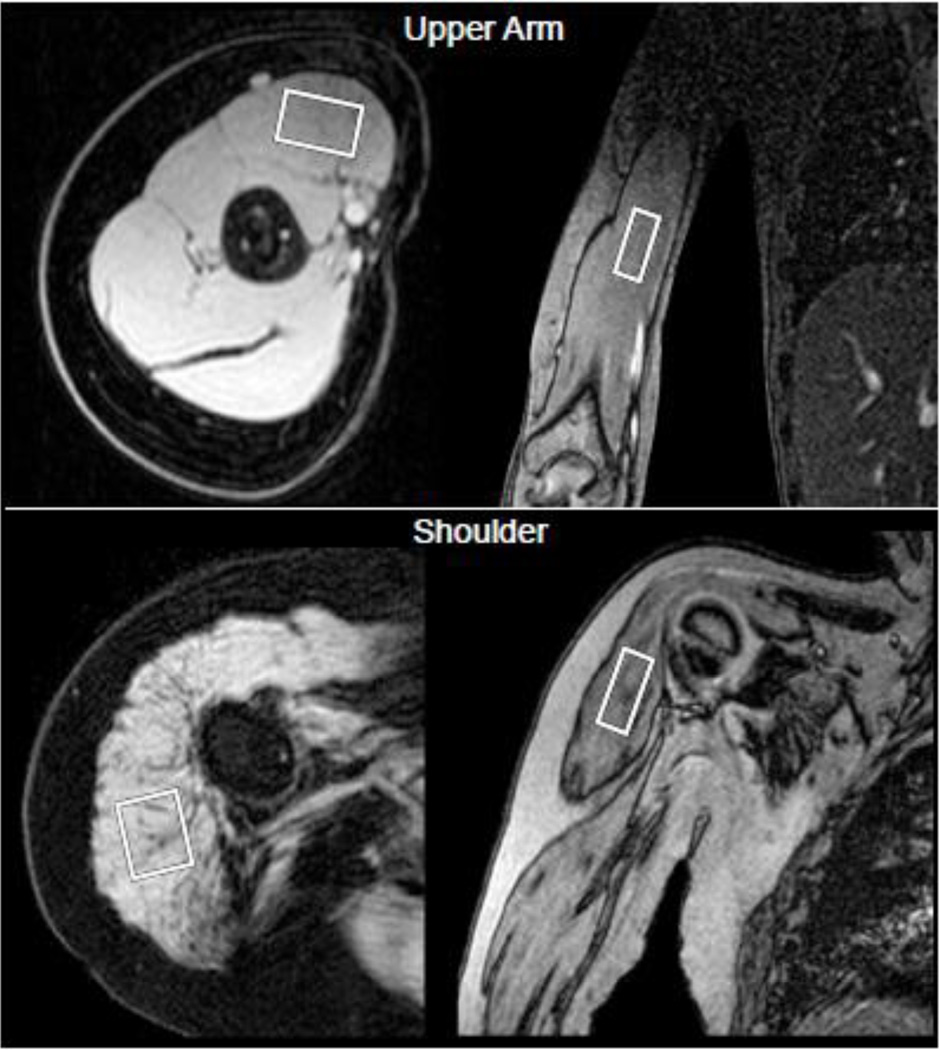
Single voxel 1H MR spectra were acquired from a cuboidal region of interest prescribed in the belly of the BB muscle or in the posterior portion of the DEL muscle (Fig 1A-B). In the distal to proximal direction, the center 1/3th of the muscle was targeted, staying away from the tendon regions. The MR operator maximized the voxel size for each individual while keeping the edges of the voxel at least 3 mm from muscle borders to avoid contamination from subcutaneous fat, bone, or other muscles. Pulse repetition time (TR) was set at 9,000 ms and individual echoes at multiple echo times (TEs) were acquired (TE=11, 27, 54, and 243 ms) to allow the calculation of water T2. MRS data were automatically processed. For the determination of muscle FF, spectra acquired at TE=27 ms were integrated to determine the area under the fat (0.5-2.6 ppm) and water peaks (4.3-5.1 ppm). Peaks were corrected for T1 and T2 weighting as previously described [19] and the ratio of fat:(fat + water) was calculated. For water T2, relative water signal was measured at each TE using complex principal components [6], and a three-parameter monoexponential model was fit to the resulting signal using least absolute deviations (LAD) minimization. LAD objective function minimization was performed using the Nelder-Mead simplex algorithm [15] initialized with a grid search to ensure a global minimum.
Fig. 1.
Axial and coronal images of the upper arm in a CON subject and of the shoulder in a boy with DMD, demonstrating the voxel placement for MR spectroscopy.
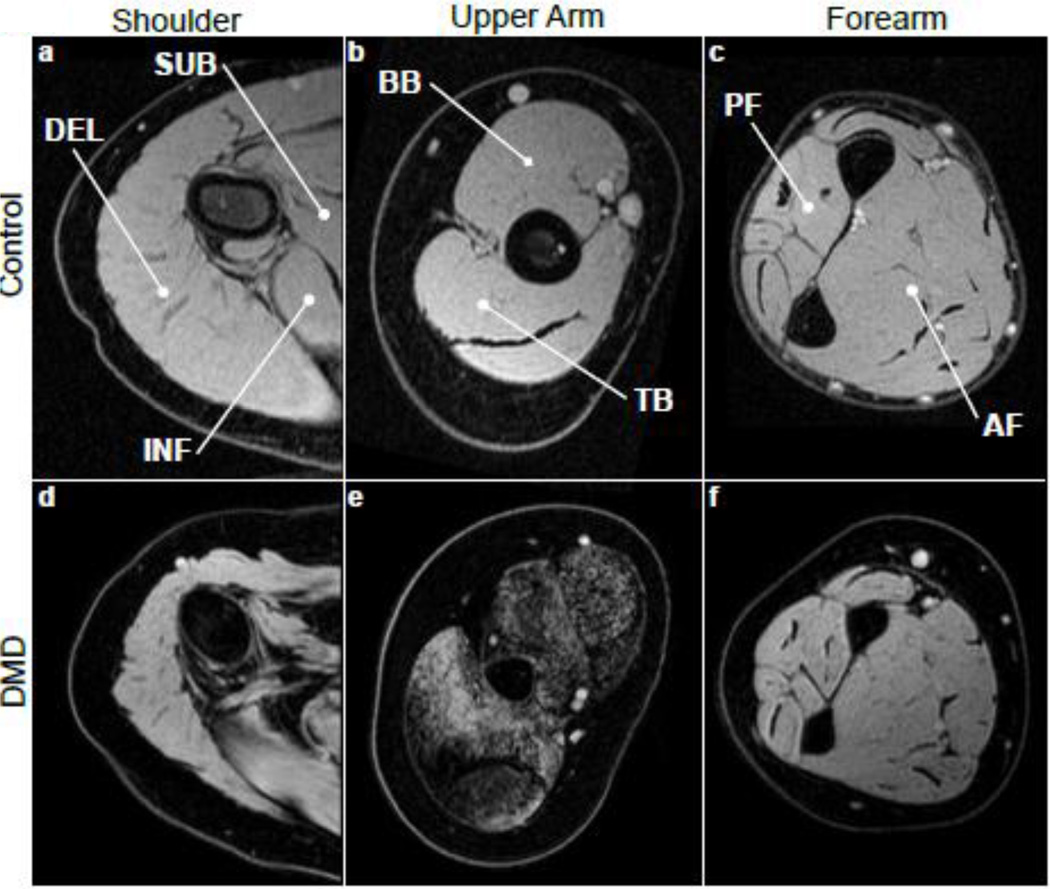
Multipolar gradient echo (GE) images were acquired at 3 different echo times (TR/TE=430/8.06, 9.21, 10.36 ms) over 25 axial slices (4 mm slice thickness, 1 mm gap) with a 20° flip angle using mDixon (Philips) and field of view adjusted depending on subject anatomy [19]. Experienced image analyzers selected slices based on a standardized anatomical landmark, and outlined the BB, TB, DEL, subscapularis (SUB), infraspinatus (INF) posterior compartment of the forearm (PF), and anterior compartment of the forearm (AF). Regions of interest (ROIs) were traced on the water map and applied to the FF map. For each muscle, analyzers took care to avoid inclusion of extramuscular tissue such as bone, subcutaneous fat, or other muscles in the outlined segment. Three consecutive slices in or near the muscle belly were analyzed for each muscle, and the pixel-by-pixel FF values were averaged to give a mean FF for each muscle.
MRI-T2 maps were generated by acquiring T2 weighted images with a TR of 2,000 ms and 5 TE’s evenly spaced from 20–100 ms. The signal intensity in each pixel was fit to a single exponential decay to compute pixel-by-pixel T2 maps. The first TE was excluded from analysis to reduce the bias from stimulated echoes [2, 9, 21]. ROIs were traced on the first TE image and applied to the T2 map in a similar manner to the MRI-FF ROIs. 3 consecutive slices were analyzed for each muscle, and the pixel-by-pixel T2 values were averaged to give a mean T2 for each muscle.
Statistical Analysis
DMD and CON groups were compared statistically using Mann-Whitney tests. Age groups were compared with each other and with the CON group using Kruskal-Wallis tests, with planned follow up Mann- Whitney tests as needed. Relationships between qMR variables and between qMR variables and function were examined using Spearman rank correlations. Statistical significance was specified as p≤0.05. Data are presented as mean ± sd unless otherwise indicated.
RESULTS
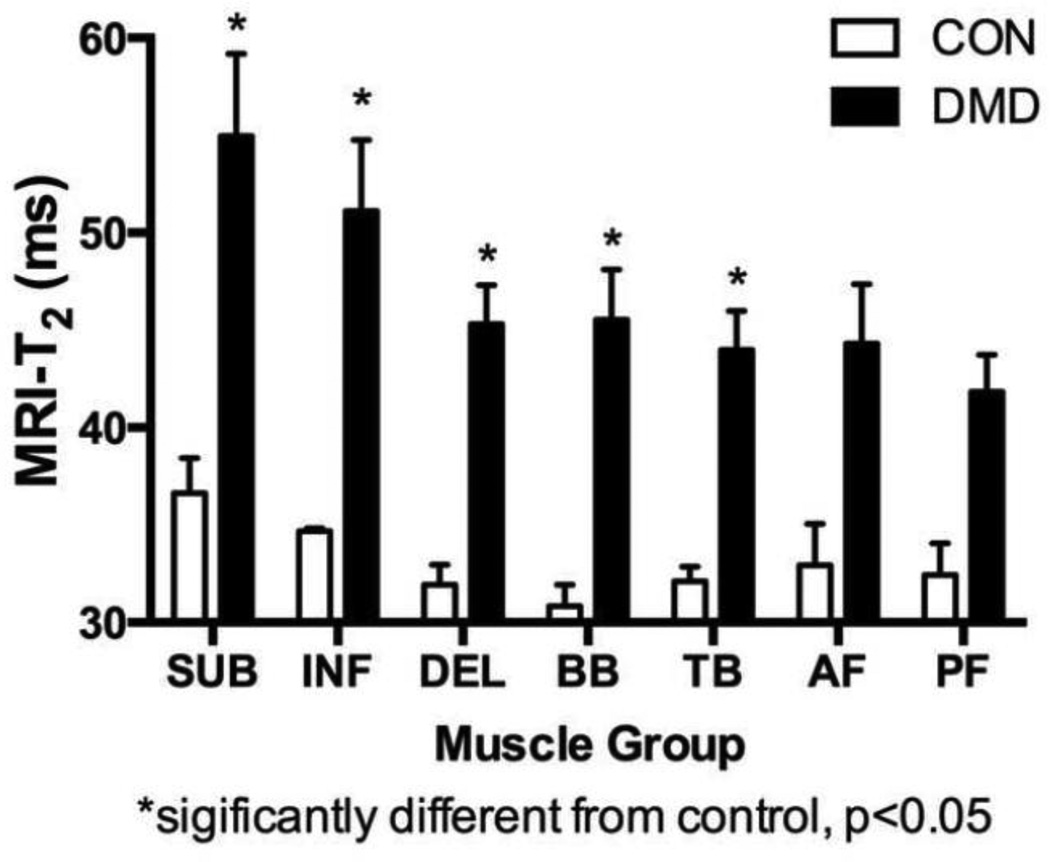
Each subject tolerated the positioning and duration of the scanning procedures well. Representative images and spectra acquired from the upper extremity musculature in unaffected CON subjects and boys with DMD are shown in Fig. 3 & 6. MR biomarkers, specifically MRI T2 (Fig 4) and 1H20 T2 (Table 1), in the upper arm differentiated CON and DMD subjects (p<0.05)). A comparison across muscles showed the highest T2 values in the shoulder muscles, followed by the upper arm, and forearm muscles (Fig 4).
Fig. 3.
MR images (water maps derived from chemical shift based imaging) from the arms of CON (a, b, and c) and DMD (d, e, and f, aged 12, 13, and 13 years) subjects, with muscles of interest labeled.
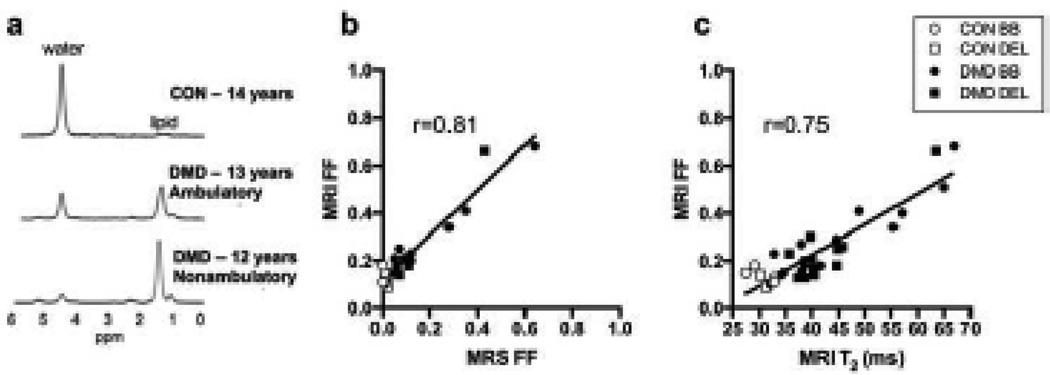
Fig. 6.
(a) Example spectra from the BB demonstrated elevated FF in both ambulatory and nonambulatory boys with DMD. (b) FF measured using Dixon imaging correlated well with FF measured using MRS, the gold standard. (c) A strong correlation was also found between FF and MRI T2 measures
Fig. 4.
MRI T2 measures were significantly elevated in DMD compared with CON in all proximal muscles. Error bars show standard error of the mean.
Table 1.
MR spectroscopy measures of FF and water T2, and MRI measures of FF in CON and DMD
| CON | DMD | p | |
|---|---|---|---|
| Fat Fraction | |||
| BB | 0.01 ± 0.01 | 0.16 ± 0.19 | 0.0008 |
| DEL | 0.02 ± 0.004 | 0.12 ± 0.15 | 0.11 |
| 1H20-T2 | |||
| BB | 26.1 ± 1.0 | 29.9 ± 2.0 | 0.0001 |
| DEL | 27.3 ± 1.2 | 29.2 ± 2.0 | 0.21 |
significantly different from CON, p<0.05
MR derived FF images demonstrated muscle deterioration in the proximal upper extremity muscles in boys aged 11–16 years. The increase in fat fraction measured using chemical shift encoded imaging in the upper arm muscles was confirmed using MR spectroscopy of the BB (Table 1Fig 6a & 6b). FF measures determined by chemical shift encoded imaging showed a strong correlation with MRI-T2 measures (r=0.75; Fig 6c), confirming a predictable relationship between these measures.
In addition to the MR exam, subjects completed functional testing, including the PUL version 1.2, and grip and pinch strength testing. Each of these, except pinch grip strength showed a strong correlation with upper arm MRI biomarkers, specifically MRI-T2 and FF (Table 2). The strongest correlations were seen between total PUL score and MR biomarkers measured in the BB.
Table 2.
Correlations between qMRI biomarkers and functional tests.
| PUL total | PUL high | PUL middle |
PUL distal | Grip | Key Pinch | |
|---|---|---|---|---|---|---|
| BB MRI T2 | −0.91* | −0.60* | −0.71* | −0.81* | 0.61 | 0.10 |
| TB MRI T2 | −0.83* | −0.64* | −0.56* | −0.63* | 0.75* | 0.13 |
| BB MRI FF | −0.83* | −0.47 | −0.75* | −0.74* | 0.48 | 0.14 |
| TB MRI FF | −0.77* | −0.56* | −0.47 | −0.60* | 0.47 | 0.16 |
significant correlation, p<0.05
DISCUSSION
This study demonstrates for the first time that it is feasible to implement quantitative MR imaging measures of muscle pathology in the proximal upper extremity musculature in patients with DMD. MR biomarkers of the proximal upper extremity muscles differentiated control and affected subjects and strongly correlated with functional endpoints. Previous studies have shown that MR biomarkers in the forearm detect disease progression in DMD [10, 18, 20], but to our knowledge, the more proximal muscles necessary for eating and personal care have not yet been examined.
Several challenges are inherent in upper extremity imaging, including field inhomogeneities resulting from the off-center location of the arm, and respiratory motion. Perhaps as a result of these challenges, previous investigations have been limited to the distal forearm muscles [18, 20]. However, we believe that the antigravity muscles of the shoulder and upper arm are critical both to allow measurement of meaningful disease markers across a range of disease stages and to capture muscles that are important for essential activities of daily living, such as eating and personal care, in both ambulatory and nonambulatory boys. Through careful positioning of subjects arm as close to isocenter as possible, and the use of padding and splints to secure the subject’s arm and isolate it from the trunk, we were able to successfully acquire high quality, quantitative MR data throughout the upper extremity in boys with DMD.
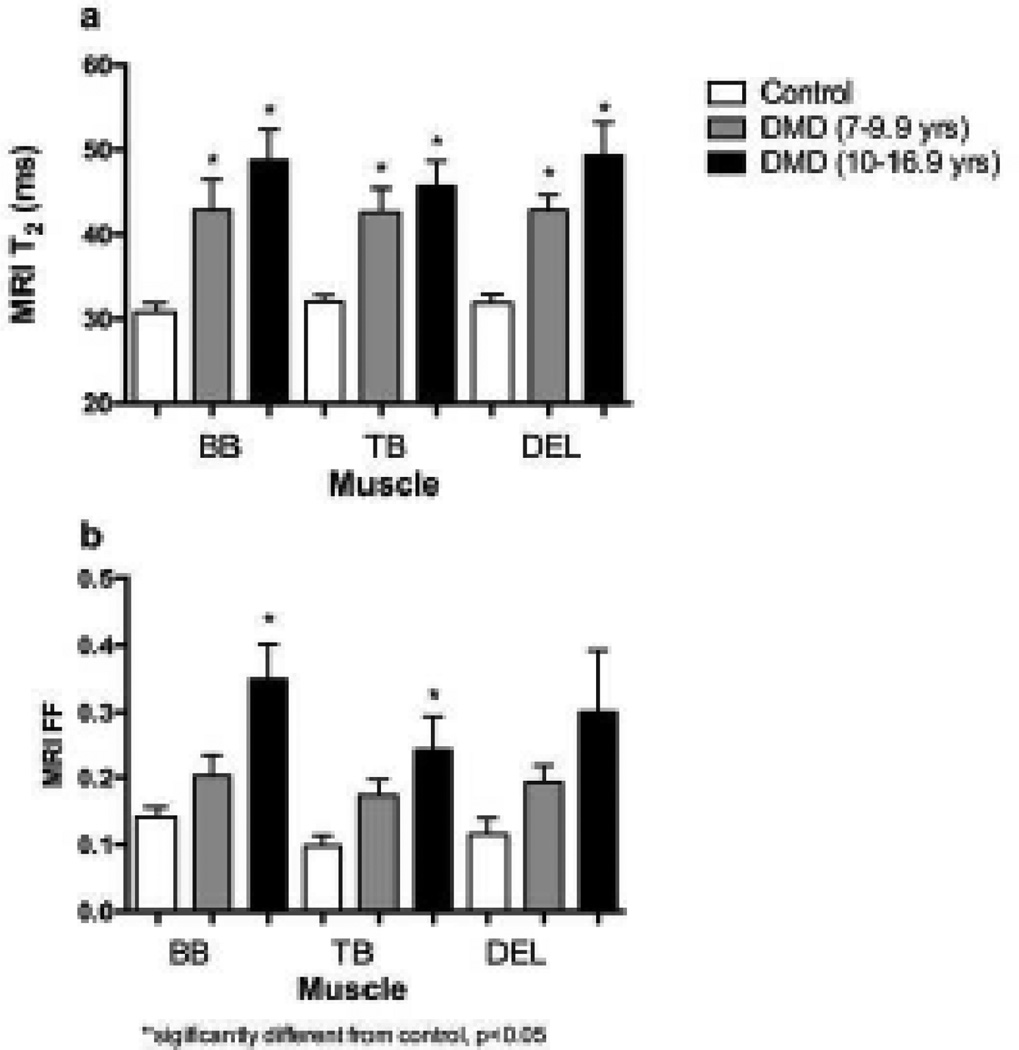
Similar to the lower extremity [8], T2 in the upper extremity muscles was elevated compared to CON, even in 7 year old boys with DMD (the youngest boys studied), making this a potential early disease marker. Both MRI-T2 and MRS-T2 have been previously shown to decrease with steroid treatment [3], indicating that these biomarkers may be particularly suitable for use in clinical trials of anti-inflammatory compounds, including corticosteroids and steroid alternatives. MRI-T2 in the BB was also strongly correlated with functional ability, more than any other variable, indicating that BB MRI-T2 captures disease progression (likely fat infiltration) as well as being sensitive to early disease processes such as muscle damage, edema, and inflammation.
Fat fraction was not elevated in 7–10 year old boys, but was elevated in the BB in boys 11 years of age and older. FF in the BB also correlated significantly with total, middle, and distal PUL score. FF can be measured using single voxel 1H-MRS, which is the gold standard but which suffers from limited spatial resolution. Alternatively, chemical shift encoded MR (Dixon) imaging, which has gained considerable popularity in recent years [20, 23, 24], also has been shown to effectively measure FF in DMD. We have previously shown that these two measurements correlate well with each other [19] in the legs. Here, we have demonstrated that the same is true in the arms, despite challenges resulting from the off-center positioning of the area of interest. The high spatial resolution of MRI-FF measures allowed the comparison of fat fraction across muscles of the proximal upper extremity.
Despite the tremendous progress in therapeutic development for DMD over the last decade, the DMD community has experienced significant frustration, much of it linked to the lack of a sensitive, objective outcome measure meeting the needs of clinical trials. Currently, clinical trials in DMD primarily recruit ambulatory patients, due, in part, to a lack of upper extremity outcome measures. As patients approach the point of loss of ambulation, the frustration of parents and advocates often escalates, with the realization that clinical trial participation opportunities are declining, even if the patient’s genetic mutation makes them a good candidate for a therapy. While the development of novel upper extremity endpoints, such as the PUL and Microsoft Kinect gaming interfaces appear to have potential, non-invasive markers not dependent on subject motivation need to be explored.
This preliminary study data demonstrates the feasibility of measuring quantitative MR biomarkers of muscle pathology in proximal upper extremity muscles essential for activities of daily living in patients with DMD. Quantitative MRI/MRS of the shoulder and upper arm was well-tolerated in both ambulatory and non-ambulatory patients, differentiated affected and non-affected subjects and strongly correlated to upper extremity functional endpoints. The adoption of these qMR outcome measures could help to further facilitate more inclusive clinical trials, which is advantageous both from an ethical standpoint, allowing more boys and men to share the benefits and burdens of trial participation, and from a practical standpoint, reducing the considerable enrollment competition among numerous trials which currently target a narrow cohort of ambulatory school aged boys. The availability of these qMR biomarkers also provides the opportunity to improve our understanding of the natural history of disease in the upper extremity in DMD, including muscle specific disease trajectories and the impact of loss of ambulation on upper extremity muscle quality.
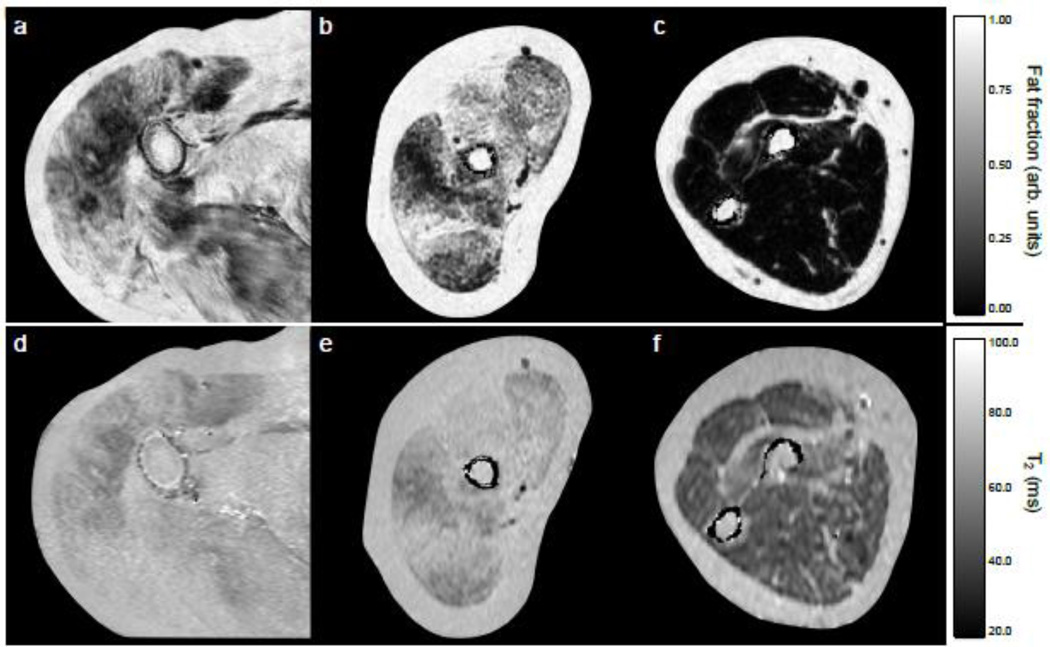
Fig. 2.
Fat fraction maps of the shoulder (a), upper arm (b), and forearm (c) from DMD subjects, and T2 maps from the shoulder (d), upper arm (e), and forearm (c) from the same subjects.
Fig. 5.
MRI T2 (a) and MRI FF measures (b) in the proximal upper extremity muscles in CON, and young versus older boys with DMD. Error bars show standard error of the mean.
Acknowledgments
Research reported in this publication was supported by National Institutes of Neurological Disorders and Stroke and National Institutes of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01AR065943 and R01AR056973, by the Department of Defense under award number W81XWH-12-1-0387, and by the Muscular Dystrophy Association under award number MDA314182. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Department of Defense, or Muscular Dystrophy Association. A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s AMRIS Facility, which is supported by National Science Foundation Cooperative Agreement No. DMR-1157490 and the State of Florida.
Footnotes
The authors declare that they have no conflict of interest.
ETHICAL STANDARDS
This study was approved by the local Institutional Review Board and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Parents and/or guardians of participants provided informed consent for their child’s participation, and participants provided informed assent to participate.
References
- 1.Akima H, Lott D, Senesac C, Deol J, Germain S, Arpan I, Bendixen R, Lee Sweeney H, Walter G, Vandenborne K. Relationships of thigh muscle contractile and non-contractile tissue with function, strength, and age in boys with Duchenne muscular dystrophy. Neuromuscul Disord. 2012;22:16–25. doi: 10.1016/j.nmd.2011.06.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arpan I, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Triplett WT, Deol JK, Sweeney HL, Walter GA, Vandenborne K. T2 mapping provides multiple approaches for the characterization of muscle involvement in neuromuscular diseases: a cross-sectional study of lower leg muscles in 5-15-year-old boys with Duchenne muscular dystrophy. NMR Biomed. 2013;26:320–328. doi: 10.1002/nbm.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arpan I, Willcocks RJ, Forbes SC, Finkel RS, Lott DJ, Rooney WD, Triplett WT, Senesac CR, Daniels MJ, Byrne BJ, Finanger EL, Russman BS, Wang DJ, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Examination of effects of corticosteroids on skeletal muscles of boys with DMD using MRI and MRS. Neurology. 2014;83:974–980. doi: 10.1212/WNL.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Florence J, King WM, Pandya S, Robison J, Schierbecker J. Duchenne muscular dystrophy: patterns of clinical progression and effects of supportive therapy. Neurology. 1989;39:475–481. doi: 10.1212/wnl.39.4.475. [DOI] [PubMed] [Google Scholar]
- 5.Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981;4:186–197. doi: 10.1002/mus.880040304. [DOI] [PubMed] [Google Scholar]
- 6.Elliott MA, Walter GA, Swift A, Vandenborne K, Schotland JC, Leigh JS. Spectral quantitation by principal component analysis using complex singular value decomposition. Magn Reson Med. 1999;41:450–455. doi: 10.1002/(sici)1522-2594(199903)41:3<450::aid-mrm4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Fischmann A, Hafner P, Gloor M, Schmid M, Klein A, Pohlman U, Waltz T, Gonzalez R, Haas T, Bieri O, Fischer D. Quantitative MRI and loss of free ambulation in Duchenne muscular dystrophy. J Neurol. 2013;260:969–974. doi: 10.1007/s00415-012-6733-x. [DOI] [PubMed] [Google Scholar]
- 8.Forbes SC, Willcocks RJ, Triplett WT, Rooney WD, Lott DJ, Wang DJ, Pollaro J, Senesac CR, Daniels MJ, Finkel RS, Russman BS, Byrne BJ, Finanger EL, Tennekoon GI, Walter GA, Sweeney HL, Vandenborne K. Magnetic resonance imaging and spectroscopy assessment of lower extremity skeletal muscles in boys with duchenne muscular dystrophy: a multicenter cross sectional study. PLoS One. 2014;9:e106435. doi: 10.1371/journal.pone.0106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambarota G, Cairns BE, Berde CB, Mulkern RV. Osmotic effects on the T2 relaxation decay of in vivo muscle. Magn Reson Med. 2001;46:592–599. doi: 10.1002/mrm.1232. [DOI] [PubMed] [Google Scholar]
- 10.Hogrel JY, Wary C, Moraux A, Azzabou N, Decostre V, Ollivier G, Canal A, Lilien C, Ledoux I, Annoussamy M, Reguiba N, Gidaro T, Le Moing AG, Cardas R, Voit T, Carlier PG, Servais L. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology. 2016;86:1022–1030. doi: 10.1212/WNL.0000000000002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HK, Laor T, Horn PS, Wong B. Quantitative assessment of the T2 relaxation time of the gluteus muscles in children with Duchenne muscular dystrophy: a comparative study before and after steroid treatment. Korean J Radiol. 2010;11:304–311. doi: 10.3348/kjr.2010.11.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynn S, Aartsma-Rus A, Bushby K, Furlong P, Goemans N, De Luca A, Mayhew A, McDonald C, Mercuri E, Muntoni F, Pohlschmidt M, Verschuuren J, Voit T, Vroom E, Wells DJ, Straub V. Measuring clinical effectiveness of medicinal products for the treatment of Duchenne muscular dystrophy. Neuromuscul Disord. 2015;25:96–105. doi: 10.1016/j.nmd.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Mattar FL, Sobreira C. Hand weakness in Duchenne muscular dystrophy and its relation to physical disability. Neuromuscul Disord. 2008;18:193–198. doi: 10.1016/j.nmd.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Mayhew A, Mazzone ES, Eagle M, Duong T, Ash M, Decostre V, Vandenhauwe M, Klingels K, Florence J, Main M, Bianco F, Henrikson E, Servais L, Campion G, Vroom E, Ricotti V, Goemans N, McDonald C, Mercuri E, Group Pot ULW. Development of the Performance of the Upper Limb module for Duchenne muscular dystrophy. Dev Med Child Neurol. 2013;55:1038–1045. doi: 10.1111/dmcn.12213. [DOI] [PubMed] [Google Scholar]
- 15.Nelder JA, Mead R. A simplex method for function minimization. Computer Journal. 1965;7:308–313. [Google Scholar]
- 16.Pane M, Mazzone ES, Fanelli L, De Sanctis R, Bianco F, Sivo S, D'Amico A, Messina S, Battini R, Scutifero M, Petillo R, Frosini S, Scalise R, Vita G, Bruno C, Pedemonte M, Mongini T, Pegoraro E, Brustia F, Gardani A, Berardinelli A, Lanzillotta V, Viggiano E, Cavallaro F, Sframeli M, Bello L, Barp A, Bonfiglio S, Rolle E, Colia G, Catteruccia M, Palermo C, D'Angelo G, Pini A, Iotti E, Gorni K, Baranello G, Morandi L, Bertini E, Politano L, Sormani M, Mercuri E. Reliability of the Performance of Upper Limb assessment in Duchenne muscular dystrophy. Neuromuscul Disord. 2014;24:201–206. doi: 10.1016/j.nmd.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Passamano L, Taglia A, Palladino A, Viggiano E, D'Ambrosio P, Scutifero M, Rosaria Cecio M, Torre V, DE Luca F, Picillo E, Paciello O, Piluso G, Nigro G, Politano L. Improvement of survival in Duchenne Muscular Dystrophy: retrospective analysis of 835 patients. Acta Myol. 2012;31:121–125. [PMC free article] [PubMed] [Google Scholar]
- 18.Ricotti V, Evans MR, Sinclair C, Morrow JM, Janiczek RL, Hanna MG, Yousry T, Muntoni F, Thornton J. Upper limb muscle fat-water quantification in non-ambulant Duchenne muscular dystrophy. Milan, Italy: International Society for Magnetic Resonance in Medicine; 2014. [Google Scholar]
- 19.Triplett WT, Baligand C, Forbes SC, Willcocks RJ, Lott DJ, DeVos S, Pollaro J, Rooney WD, Sweeney HL, Bönnemann CG, Wang DJ, Vandenborne K, Walter GA. Chemical shift-based MRI to measure fat fractions in dystrophic skeletal muscle. Magn Reson Med. 2014;72:8–19. doi: 10.1002/mrm.24917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wary C, Azzabou N, Giraudeau C, Le Louër J, Montus M, Voit T, Servais L, Carlier P. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed. 2015;28:1150–1162. doi: 10.1002/nbm.3352. [DOI] [PubMed] [Google Scholar]
- 21.Willcocks RJ, Arpan IA, Forbes SC, Lott DJ, Senesac CR, Senesac E, Deol J, Triplett WT, Baligand C, Daniels MJ, Sweeney HL, Walter GA, Vandenborne K. Longitudinal measurements of MRI-T2 in boys with Duchenne muscular dystrophy: effects of age and disease progression. Neuromuscul Disord. 2014;24:393–401. doi: 10.1016/j.nmd.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willcocks RJ, Rooney WD, Triplett WT, Forbes SC, Lott DJ, Senesac CR, Daniels MJ, Wang DJ, Harrington AT, Tennekoon GI, Russman BS, Finanger EL, Byrne BJ, Finkel RS, Walter GA, Sweeney HL, Vandenborne K. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large duchenne muscular dystrophy cohort. Ann Neurol. 2016;79:535–547. doi: 10.1002/ana.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wokke BH, Bos C, Reijnierse M, van Rijswijk CS, Eggers H, Webb A, Verschuuren JJ, Kan HE. Comparison of dixon and T1-weighted MR methods to assess the degree of fat infiltration in duchenne muscular dystrophy patients. J Magn Reson Imaging. 2013;38:619–624. doi: 10.1002/jmri.23998. [DOI] [PubMed] [Google Scholar]
- 24.Wren TA, Bluml S, Tseng-Ong L, Gilsanz V. Three-point technique of fat quantification of muscle tissue as a marker of disease progression in Duchenne muscular dystrophy: preliminary study. AJR Am J Roentgenol. 2008;190:W8–W12. doi: 10.2214/AJR.07.2732. [DOI] [PubMed] [Google Scholar]