Abstract
Rationale
The N-methyl-D-aspartate (NMDA) receptor has been recently identified as an important mediator of impulsive choice, as assessed in delay discounting. Although discounting is independently influenced by sensitivity to reinforcer magnitude and delayed reinforcement, few studies have examined how NMDA receptor ligands differentially affect these parameters.
Objectives
The current study examined the effects of various NMDA receptor ligands on sensitivity to reinforcer magnitude and delayed reinforcement in a delay-discounting procedure.
Methods
Following behavioral training, rats received treatments of the following NMDA receptor ligands: the uncompetitive antagonists ketamine (0, 1.0, 5.0, or 10.0 mg/kg; i.p.), MK-801 (0, 0.003, 0.01, or 0.03 mg/kg; s.c.), and memantine (0, 2.5, 5.0, or 10.0 mg/kg; i.p.), the competitive antagonist CGS 19755 (0, 5.0, 10.0, or 20.0 mg/kg; s.c.), the non-competitive NR2B subunit-selective antagonist ifenprodil (0, 1.0, 3.0, or 10.0 mg/kg; i.p), and the partial agonist D-cycloserine (0, 3.25, 15.0, or 30.0 mg/kg; s.c.).
Results
When an exponential model was used to describe discounting, CGS 19755 (5.0 mg/kg) increased impulsive choice without altering sensitivity to reinforcer magnitude. Conversely, ketamine (10.0 mg/kg), memantine (5.0 mg/kg), and ifenprodil (10.0 mg/kg) decreased sensitivity to reinforcer magnitude without altering impulsive choice. MK-801 and D-cycloserine did not alter delay-discounting performance, although two-way ANOVA analyses indicated D-cycloserine (15.0 mg/kg) decreased impulsive choice.
Conclusions
The behavioral changes observed in delay discounting following administration of NMDA receptor antagonists do not always reflect an alteration in impulsive choice. These results emphasize the utility in employing quantitative methods to assess drug effects in delay discounting.
Keywords: impulsive choice, delay discounting, NMDA receptor, sensitivity to reinforcer magnitude, sensitivity to delayed reinforcement, rat
Introduction
Impulsive choice is often conceptualized as the inability to delay gratification and is modeled with delay discounting. In a typical delay-discounting paradigm, subjects are given a choice between two alternatives. One alternative is associated with a small reinforcer delivered immediately, whereas the second alternative is associated with a large reinforcer delivered after a delay. Consistently choosing the small, immediate reinforcer is considered to be a measure of impulsive choice (Ainslie 1975). Increased impulsive choice is observed in individuals with attention-deficit/hyperactivity disorder (ADHD; see Jackson and MacKillop 2016 for a recent meta-analysis), schizophrenia (Ahn et al. 2011; Heerey et al. 2007; Weller et al. 2014), bipolar disorder (Ahn et al. 2011), Parkinson’s disease (Al-Khaled et al. 2015; Evens et al. 2015), pathological gambling (Albein-Urios et al. 2014; Cosenza and Nigro 2015; Petry 2001; Wiehler et al. 2015), and substance use disorders (see Bickel et al. 2014 for a discussion and relevant citations). Determining the neural mechanisms underlying impulsive choice is needed to improve decision making in individuals with disorders characterized by increased sensitivity to delayed reinforcement.
Dysregulation of the glutamatergic system has been identified in individuals with ADHD and substance use disorders (Ben-Shahar et al. 2012; Griffin et al. 2014; Jensen et al. 2009; Miller et al. 2014; Perlov et al. 2007; see Archer and Garcia 2016; Chang et al. 2014; Kalivas 2009; Lesch et al. 2013; Niciu et al. 2014; Pattij and Vanderschuren 2008 for reviews), and recent evidence has identified glutamate N-methyl-D-aspartate (NMDA) receptors as mediators of impulsive choice. Administration of ketamine and memantine increase impulsive choice (Cottone et al. 2013; Floresco et al. 2008), whereas MK-801 and the NR2B-selective antagonist Ro 63-1908 decrease impulsive choice (Higgins et al. 2016; Yates et al. 2015). One important consideration when measuring discounting is that there are two parameters that independently influence behavior: sensitivity to reinforcer magnitude and sensitivity to delayed reinforcement (Ho et al. 1999). Sensitivity to reinforcer magnitude refers to how much an animal prefers the large magnitude reinforcer relative to a small magnitude reinforcer when they are both made available immediately. Sensitivity to delayed reinforcement is typically referred to as impulsive choice. Previous studies have examined how excitotoxic brain lesions alter these parameters in discounting (e.g., Bezzina et al. 2007, 2008, 2009; Kheramin et al. 2002); however, behavioral pharmacology experiments have often ignored how drugs differentially alter sensitivity to reinforcer magnitude/delayed reinforcement. Directly related to glutamate receptors, only one study has examined how NMDA receptor ligands differentially affect sensitivity to reinforcer magnitude and delayed reinforcement. Yates et al. (2015) found that MK-801 decreases sensitivity to delayed reinforcement (i.e., decreases impulsive choice) and increases sensitivity to reinforcer magnitude, whereas ketamine decreases sensitivity to reinforcer magnitude without altering sensitivity to delayed reinforcement.
The primary goal of the current study was to further determine how NMDA receptor ligands affect sensitivity to reinforcer magnitude/delayed reinforcement. Because quantitative analyses have been applied to ketamine and MK-801 (both uncompetitive antagonists), we wanted to extend these analyses to different NMDA receptor ligands. These results will help us determine if ligands that bind to different sites on the NMDA receptor differentially alter sensitivity to reinforcer magnitude and/or delayed reinforcement. Rats were treated with the following drugs: the uncompetitive antagonists ketamine, MK-801, and memantine, the competitive antagonist CGS 19755, the NR2B-selective non-competitive antagonist ifenprodil, and the glycine site partial agonist D-cycloserine (see Monaghan and Jane 2009 for a discussion of the pharmacology of NMDA receptors). Ketamine and MK-801 were included as positive controls, as their effects on sensitivity to reinforcer magnitude/delayed reinforcement have been studied previously (Yates et al. 2015). Memantine was included because it has been shown to increase impulsive choice in an adjusting delay procedure (Cottone et al. 2013), although its effects on sensitivity to reinforcer magnitude have not been examined. Ifenprodil was included because it, unlike ketamine and MK-801, does not produce psychotomimetic effects (Boyce et al. 1999); furthermore, recent evidence has shown that NR2B-selective antagonists decrease impulsive choice (Higgins et al. 2016). CGS 19755 and D-cycloserine were included as negative controls, as administration of either drug does not selectively alter performance in delay discounting (Cottone et al. 2013; van den Bergh et al. 2006), although their effects on sensitivity to reinforcer magnitude have not been tested. A secondary goal of the current study was to determine if the statistical analysis (i.e., ANOVA vs. fitting an exponential function via nonlinear mixed effects modeling) used to assess delay discounting alters interpretation of drug effects on discounting performance.
Methods
Subjects
A total of 48 experimentally naïve Sprague Dawley rats (250-275 g upon arrival to the lab; Harlan Industries, Indianapolis, IN) were used in the current experiment. They were acclimated to an animal housing room and handled for six days before testing began. The housing room was maintained on a 12:12-h cycle (lights on at 630 h), and rats were tested in the light phase (approximately 1100-1500 h). Rats were individually housed in clear polypropylene cages (51 cm long × 26.5 cm wide × 32 cm high) with metal tops containing food and a water bottle. Rats were restricted to approximately 10 g of food each day but had ad libitum access to water. All experimental procedures were carried out according to the Current Guide for the Care and Use of Laboratory Animals (USPHS) under a protocol approved by the Northern Kentucky University Institutional Animal Care and Use Committee.
Drugs
All drugs were purchased from Sigma Aldrich (St. Louis, MO), with the exception of CGS 19755, which was obtained from the NIMH’s Chemical Synthesis and Drug Supply Program. (+)-MK-801 hydrogen maleate (±)-ketamine hydrochloride, memantine hydrochloride, cis-4-[phosphomethyl]-piperidine-2-carboxylic acid (CGS 19755), and D-cycloserine were prepared in sterile 0.9% NaCl (saline). CGS 19755 (20.0 mg/kg) had to be heated and stirred to go into solution. Ifenprodil (+)-tartrate salt was prepared in distilled water. The two highest doses of ifenprodil (3.0 and 10.0 mg/kg) were heated and stirred prior to each injection. Each drug was injected at room temperature in a volume of 1 ml/kg, except CGS 19755, which was administered in a volume of 2 ml/kg. The doses were calculated based on salt weight.
Apparatus
Eight operant conditioning chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) located inside sound attenuating chambers (ENV-018M; MED Associates) were used. The operant chambers used in our laboratory have been described previously (Yates et al. 2016). All responses and scheduled consequences were recorded and controlled by a computer interface, and a computer controlled the experimental session using Med-IV software.
Procedure
Rats went through two days of magazine training and three sessions of lever-press training as described previously (Yates et al. 2015), with one exception. During lever-press training, a random-time 100-s schedule of reinforcement was not used. Rats were given three sessions of magnitude discrimination training as previously described previously (Yates et al. 2015), with the exception that a 20-s limited hold was used as opposed to a 10-s limited hold.
Delay-discounting sessions consisted of five blocks of nine trials. The stimuli used to signal the beginning of each trial differed across blocks of trials (first: house light; second: house light and left stimulus light; third: house light and right stimulus light; fourth: house light and both stimulus lights; fifth: both stimulus lights). The first four trials in a block were forced-choice trials, in which only one lever was pseudo-randomly presented (no more than two consecutive presentations of the same lever). The remaining trials were free-choice trials, in which both levers were extended. Completion of the response requirement (FR 10) on one lever always resulted in immediate delivery of one food pellet, whereas completion of the response requirement (FR 10) on the other lever resulted in delayed delivery of four pellets. We need to note that the first 24 rats were initially trained on an FR 1 schedule as in Yates et al. (2015), but these animals did not discount the large magnitude reinforcer. When we switched to the FR 10 schedule, rats discounted the large magnitude reinforcer (see Online Supplementary Fig. 1). Rats 25-48 were trained on the FR 10 schedule of reinforcement only. The delay to delivery of the large magnitude reinforcer increased across blocks of trials (0, 10, 30, 60, 100 s). Following a response on either lever, the stimuli used to signal the beginning of each trial were extinguished, and each lever was retracted for the remainder of the trial. If a response was not made within 20 s, the trial was scored as an omission, and all stimuli were extinguished for the remainder of the trial. To compensate for the delay to the large magnitude reinforcer, the length of each trial increased across blocks of trials (first: 30 s; second: 40 s; third: 60 s; fourth: 90 s; fifth; 130 s). Each session lasted 52.5 min.
The following drugs were used to determine the contribution of NMDA receptors to delay-discounting performance: the uncompetitive antagonists ketamine (0, 1.0, 5.0, or 10.0 mg/kg; i.p.), MK-801 (0, 0.003, 0.01, or 0.03 mg/kg; s.c.), and memantine (0, 2.5, 5.0, or 10.0 mg/kg; i.p.), the competitive antagonist CGS 19755 (0, 5.0, 10.0, or 20.0 mg/kg; s.c.), the NR2B-selective non-competitive antagonist ifenprodil (0, 1.0, 3.0, or 10.0 mg/kg; i.p.), and the partial agonist D-cycloserine (0, 3.25, 15.0, or 30.0 mg/kg; s.c.). To minimize the number of injections, rats were randomly placed into four groups (n = 12 each group). Group 1 received injections of ketamine, memantine, and ifenprodil. Group 2 received injections of MK-801, D-cycloserine, and ifenprodil. Group 3 received injections of ketamine, memantine, and CGS 19755. Group 4 received injections of MK-801, D-cycloserine, and CGS 19755. Each dose was administered either 15 min (ketamine, MK-801) or 30 min (memantine, CGS 19755, ifenprodil, D-cycloserine) prior to each session. The doses and pretreatment times were chosen based on previous work (Cottone et al. 2013; Ma et al. 2011; Navarrete et al. 2014; van den Bergh et al. 2006; Wooters et al. 2011; Yates et al. 2015). Injections occurred every 2-4 sessions, and the drug and dose order were randomized to control for any possible order effects. Rats were tested in the delay-discounting task between injections days.
Statistical Analyses
Because one rat never discounted the large reinforcer, and one rat did not consistently choose the large reinforcer during the first block of trials, data from these rats were excluded from all analyses. Thirteen baseline periods were recorded to determine if delay discounting systematically changed across the injection phase of the experiment. The final 2-4 sessions before an injection were averaged together, with the exception of the final baseline, which consisted of the three sessions immediately following the final injection. Separate linear trend analyses were used to determine if A and b parameter estimates changed across baselines.
Because omissions violate the assumption of normality (due to most values during baseline being at 0), these data were analyzed with a Friedman test, and Wilcoxon signed-rank post hoc tests (with Bonferroni adjustment) were used when appropriate.
The raw proportion of responses for the large reinforcer was analyzed using a two-way ANOVA, with delay and dose as within-subjects factors. This analysis is commonly used in behavioral pharmacology studies (Baarendse and Vanderschuren 2012; Cardinal et al. 2000; Floresco et al. 2008; Koffarnus et al. 2011; Sukhotina et al. 2008; van Gaalen et al. 2006; Winstanley et al. 2005). If there was a main effect of dose, Bonferroni post hoc tests were used to determine if responses (averaged across each delay) differed across doses. If there was a significant dose × delay interaction, separate one-way ANOVAs were conducted for each delay to determine if responses differed across doses, and Dunnett’s post hoc tests were used when appropriate. Because only two doses of CGS 19755 (0 and 5.0 mg/kg) were included in data analyses (see Results section), the significant dose × delay interaction was probed with paired-samples t tests. When the assumption of sphericity was violated, degrees of freedom were corrected using Greenhouse Geisser estimates of sphericity. If a rat did not respond during one block of trials following drug treatment, this subject’s data for all drug doses were excluded due to the fact that repeated measures ANOVA uses listwise deletion when there are missing data. Additionally, if a rat responded during each block of trials but had more than 12 omissions (half of all free-choice trials), it was excluded from data analysis.
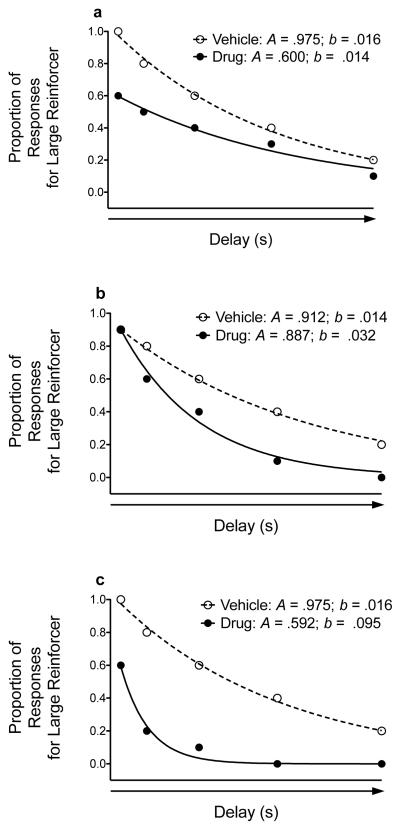
Additionally, we fit an exponential model to the raw data in order to derive two parameter estimates. The exponential model is defined by the equation V = Ae−bD, where V is the subjective value of the reinforcer, A is the intercept and refers to sensitivity to reinforcer magnitude (i.e., how much the animal prefers the large reinforcer relative to the small reinforcer when they are both available immediately), e is Euler’s number, b is the slope of the discounting function (i.e., measure of impulsive choice), and D is the delay to delivery of the large reinforcer. Figure 1 shows how a drug can alter A and b parameter estimates. Although hyperbolic discounting typically provides a better account of discounting relative to the exponential model (see Ainslie and Herrnstein 1981), the exponential model provided a better account of discounting in the current experiment (baseline exponential median R2 = .969; baseline hyperbolic median R2 = .912).
Figure 1.
Hypothetical discounting data showing how a drug can influence sensitivity to reinforcer amount (A parameter; the intercept) and impulsive choice (b parameter; the slope). a) The drug affects the A parameter (by decreasing sensitivity to reinforcer magnitude) without altering the b parameter. b) The drug affects the b parameter (by increasing impulsive choice) without affecting the A parameter. c) The drug affects both the A parameter (by decreasing sensitivity to reinforcer magnitude) and the b parameter (by increasing impulsive choice).
The exponential function was fit to the data with nonlinear mixed effects modeling (NLME; Pinheiro et al. 2007; Young et al. 2009) using the NLME package in the R statistical software package (Pinheiro et al. 2007), with A and b parameters as free parameters. The NLME models defined delay as a fixed, continuous within-subjects variable, dose as a fixed, nominal within-subjects variable, and subject defined as a random factor. Identical NLME models were used to analyze each drug effect on discounting. To determine if rats assigned to each group had similar baseline A and b parameter estimates (i.e., before drug treatment), the NLME model included group as a nominal, between-subjects variable. NLME offers several advantages compared to ANOVA. Specifically, NLME increases statistical power, reduces Type I error rates, and does not delete an entire subject’s data if one dose is excluded (see Young et al. 2009 for a full discussion). If a subject had more than 12 omissions or did not respond during the first block of trials, data for that dose were excluded only (i.e., data for the other doses were kept in the NLME analyses). For example, if a rat had five omissions but did not respond during the first block of trials, data for that dose would be excluded because the A parameter (i.e., intercept) cannot be calculated if the subject did not respond during that block. Statistical significance was defined as p ≤ .05 in all cases, with the exception of the Wilcoxon signed-rank tests.
Results
Table 1 shows A and b parameter estimates averaged across the 13 baseline periods (averaged across 2-4 sessions). Linear trend analyses showed that A parameter estimates (F(1, 45) = 5.269, p = .026) and b parameter estimates (F(1, 45) = 10.458, p = .002) increased across baselines, suggesting that animals became more sensitive to reinforcer magnitude and to delayed reinforcement across the experiment. NLME analysis showed that A parameter estimates (F(3, 177) = 0.574, p = .633) and b parameter estimates (F(3, 177) = 2.25, p = .084) did not differ across the four groups of rats tested (data not shown).
Table 1.
Mean (± SEM) A parameter and b parameter estimates across each baseline period (averaged across 2-4 sessions).
| A parameter | b parameter | |||
|---|---|---|---|---|
|
|
||||
| Baseline | M | SEM | M | SEM |
| 1 | 0.948 | 0.014 | 0.148 | 0.022 |
| 2 | 0.927 | 0.017 | 0.176 | 0.026 |
| 3 | 0.930 | 0.022 | 0.181 | 0.027 |
| 4 | 0.929 | 0.022 | 0.221 | 0.033 |
| 5 | 0.927 | 0.018 | 0.199 | 0.029 |
| 6 | 0.941 | 0.017 | 0.177 | 0.026 |
| 7 | 0.944 | 0.020 | 0.191 | 0.028 |
| 8 | 0.953 | 0.013 | 0.232 | 0.034 |
| 9 | 0.957 | 0.011 | 0.177 | 0.026 |
| 10 | 0.953 | 0.013 | 0.231 | 0.034 |
| 11 | 0.970 | 0.009 | 0.243 | 0.036 |
| 12 | 0.955 | 0.012 | 0.239 | 0.035 |
| 13 | 0.957 | 0.013 | 0.235 | 0.035 |
Table 2 shows omissions following administration of each NMDA receptor ligand. Ketamine (10.0 mg/kg) significantly increased omissions (χ2(3; N = 23) = 20.330, p < .001). The significant increase was due primarily to six animals that had an average of 20 omissions (Range: 11-25). These six animals were removed completely from ANOVA analyses, but their data for other doses were retained in the NLME analyses (see Statistical Analyses section). Memantine (10.0 mg/kg) significantly increased omissions (χ2(3; N = 23) = 53.667, p < .001). Because 20 of the 24 rats treated with the highest dose of memantine (10.0 mg/kg) had 20+ omissions during free-choice trials, this dose was excluded from all subsequent data analyses. Ifenprodil (10.0 mg/kg) significantly increased omissions (χ2(3; N = 24) = 10.022, p = .018). The significant increase in omissions following ifenprodil administration was due primarily to the low number of omissions following vehicle injection (11 of the 12 rats had no omissions, whereas one rat had one omission), in conjunction with two rats having 16 and 21 omissions, respectively, following ifenprodil (10.0 mg/kg). These two rats were excluded from subsequent analyses as described in the Statistical Analyses section. The two highest doses of CGS 19755 (10.0 and 20.0 mg/kg) significantly increased omissions (χ2(3; N = 22) = 62.534, p < .001). Because 16 of the 22 rats treated with CGS 19755 (10.0 mg/kg) had an average of 19 omissions (Range: 12-25), and all rats treated with CGS 19755 (20.0 mg/kg) had an average of approximately 24 omissions (Range: 18-25), these doses were excluded from all subsequent data analyses. One rat had 17 omissions following administration of the lowest dose (5.0 mg/kg) of CGS 19755; therefore, data from this animal were excluded from subsequent analyses. MK-801 (χ2(3; N = 23) = 1.222, p = .748) and D-cycloserine (χ2(3; N = 23) = 2.000, p = .572) did not alter omissions.
Table 2.
Mean (± SEM) omissions following administration of NMDA receptor ligands.
| Drug | Dose | M | SEM |
|---|---|---|---|
| Ketamine | 0.0 mg/kg | 0.217 | 0.153 |
| Ketamine | 1.0 mg/kg | 0.261 | 0.180 |
| Ketamine | 5.0 mg/kg | 0.696 | 0.481 |
| Ketamine | 10.0 mg/kg | 5.522* | 1.920 |
| MK-801 | 0.0 mg/kg | 0.348 | 0.348 |
| MK-801 | 0.003 mg/kg | 0.087 | 0.087 |
| MK-801 | 0.01 mg/kg | 0.043 | 0.044 |
| MK-801 | 0.03 mg/kg | 0.174 | 0.136 |
| Memantine | 0.0 mg/kg | 0.130 | 0.130 |
| Memantine | 2.5 mg/kg | 0.261 | 0.113 |
| Memantine | 5.0 mg/kg | 3.652 | 1.341 |
| Memantine | 10.0 mg/kg | 21.478* | 1.464 |
| Ifenprodil | 0.0 mg/kg | 0.208 | 0.104 |
| Ifenprodil | 1.0 mg/kg | 0.042 | 0.042 |
| Ifenprodil | 3.0 mg/kg | 0.083 | 0.058 |
| Ifenprodil | 10.0 mg/kg | 2.458* | 1.063 |
| CGS 19755 | 0.0 mg/kg | 0.000 | 0.000 |
| CGS 19755 | 5.0 mg/kg | 3.478 | 0.906 |
| CSG 19755 | 10.0 mg/kg | 15.000* | 1.490 |
| CGS 19755 | 20.0 mg/kg | 23.826* | 0.447 |
| D-cycloserine | 0.0 mg/kg | 0.130 | 0.130 |
| D-cycloserine | 3.25 mg/kg | 0.043 | 0.044 |
| D-cycloserine | 15.0 mg/kg | 0.000 | 0.000 |
| D-cycloserine | 30.0 mg/kg | 0.000 | 0.000 |
p < .05, relative to vehicle.
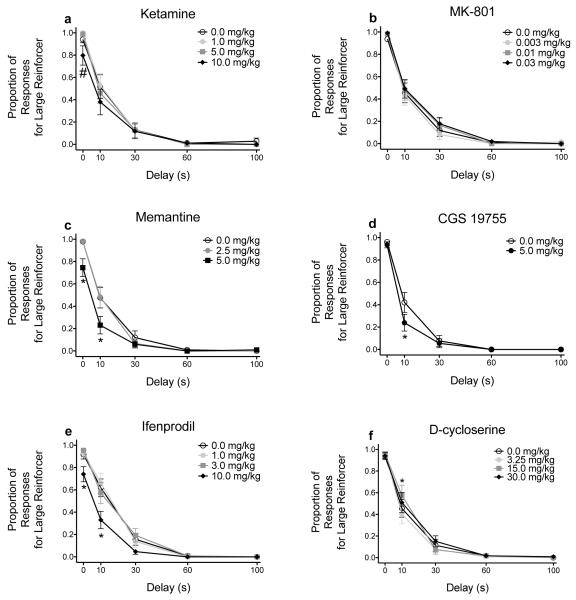
Figure 2 shows the raw proportion of responses for the large magnitude reinforcer across each delay following administration of NMDA receptor ligands. For all drugs, there was a main effect of delay (all F’s ≥ 40.755, all p’s < .001). There was a significant dose × delay interaction following ketamine administration (F(12, 192) = 1.821, p = .047) but no main effect of dose (F(1.583, 25.328) = 3.483, p = .056; Fig. 2a). Separate one-way ANOVAs revealed that ketamine significantly altered responses for the large magnitude reinforcer when its delivery was immediate (F(1.158, 18.532) = 4.188, p = .050), although post hoc tests showed a trend only for the highest dose (10.0 mg/kg) to decrease responding. MK-801 did not alter responding for the large magnitude reinforcer (Dose: F(3, 63) = 1.311, p = .279; Dose × Delay: F(4.496, 94.420) = .703, p = .608; Fig. 2b). Memantine significantly altered responses for the large magnitude reinforcer (Dose: F(1.492, 28.350) = 13.905, p < .001; Dose × Delay: F(2.226, 42.292) = 76.213, p < .001; Fig. 2c). Bonferroni post hoc tests showed that memantine (5.0 mg/kg) decreased responding relative to vehicle, and post hoc tests showed that the decreased responding occurred at 0 s (F(1.142, 21.689) = 7.833, p = .009) and at 10 s (F(2, 38) = 7.299, p = .002). Following administration of the competitive antagonist CGS 19755, there was a significant dose × delay interaction (F(4, 80) = 2.871, p = .028) but no main effect of dose (F(1, 20) = 3.398, p = .080; Fig. 2d). Paired-samples t tests showed that CGS 19755 (5.0 mg/kg) decreased responding relative to vehicle at 10 s (t(20) = 2.260, p .035). The NR2B-selective non-competitive antagonist ifenprodil significantly altered the responses for the large magnitude reinforcer (Dose: F(1.773, 37.225) = 8.338, p = .001; Dose × Delay: F(12, 252) = 3.763, p < .001; Fig. 2e). Bonferroni post hoc tests revealed that ifenprodil (10.0 mg/kg) decreased responding, and one-way ANOVAs showed that the decreased responding occurred at 0 s (F(1.828, 38.390) = 4.734, p = .017) and at 10 s (F(1.775, 37.281) = 6.946, p = .004). There was a significant dose × delay interaction following D-cycloserine administration (F(5.159, 113.509) = 2.695, p = .023) but no main effect of dose (F(3, 66) = 2.169, p = .100; Fig. 2f). One-way ANOVAs showed that D-cycloserine (15.0 mg/kg) increased responding for the large magnitude reinforcer relative to vehicle at 10 s (F(2.254, 49.588) = 5.240, p = .007).
Figure 2.
Mean (± SEM) proportion of responses for the large, delayed reinforcer following administration of ketamine (n = 17 all doses; a), MK-801 (n = 22 all doses; b), memantine (n = 20 all doses; c), CGS 19755 (n = 21 all doses; d), ifenprodil (n = 22 all doses; e), and D-cycloserine (n = 23 all doses; f). *p < .05, relative to vehicle. # indicates a main effect of dose at a specific delay. Note, only data for subjects that responded during each block of trials across each dose are included in this figure because two-way ANOVAs use listwise deletion when there are missing data.
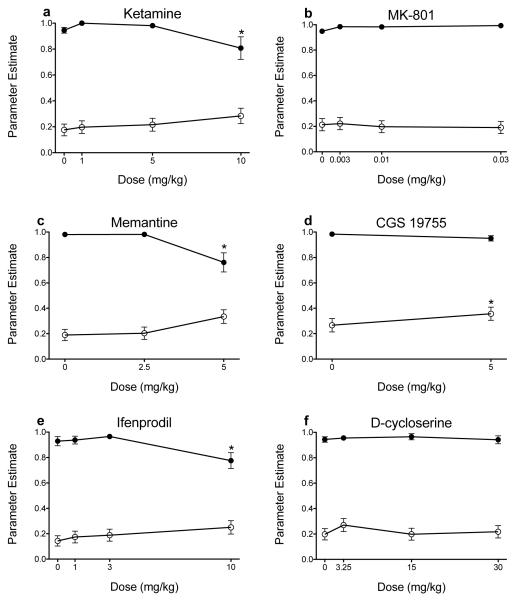
Figure 3 shows A parameter and b parameter estimates following administration of NMDA receptor ligands. The uncompetitive antagonists ketamine and memantine significantly decreased A parameter estimates (Ketamine: F(3, 409) = 3.573, p = .014; Memantine: F(2, 306) = 8.893, p < .001) without altering b parameter estimates (i.e., impulsive choice; Ketamine: F(3, 409) = 0.527, p = .664; Memantine: F(2, 306) = 1.744, p = .177; Figs. 3a and 3c). The uncompetitive antagonist MK-801 did not affect A parameter estimates (F(3, 429) = .314, p = .815) or b parameter estimates (F(3, 429) = 1.329, p = .265; Fig. 3b). The competitive antagonist CGS 19755 significantly increased impulsive choice (F(1, 198) = 7.036, p = .009) without altering A parameter estimates (F(1, 198) = .038, p = .846; Fig. 3d). Ifenprodil significantly decreased sensitivity to reinforcer magnitude (F(3, 439) = 4.917, p = .002) without altering sensitivity to delayed reinforcement (F(3, 439) = 2.136, p = .095; Fig. 3e). D-cycloserine did not significantly alter A parameter estimates (F(3, 430) = 0.288, p = .834) or b parameter estimates (F(3, 430) = 1.597, p = .189; Fig. 3f).
Figure 3.
Mean (± SEM) A parameter (closed circles) and b parameter (open circles) estimates following administration of ketamine (n = 17-23 per dose; a), MK-801 (n = 23 all doses; b), memantine (n = 20-23 per dose; c), CGS 19755 (n = 22 all doses; d), ifenprodil (n = 22-24 per dose; e), and D-cycloserine (n = 23 all doses; f). *p < .05, relative to vehicle. Note, if a subject’s data were excluded from one dose, data for the other doses were included in this figure because NLME analyses do not use listwise deletion when there are missing data.
Discussion
In the current experiment, impulsive choice was measured with a behavioral task employing a concurrent schedule of reinforcement (e.g., Evenden and Ryan 1996), which has been used consistently during the past 20 years (e.g., Baarendse and Vanderschuren 2012; Cardinal et al. 2000; Floresco et al. 2008; Fox et al. 2008; Higgins et al. 2016; Isherwood et al. 2015; Koffarnus et al. 2011; Liu et al. 2004; Slezak and Anderson 2009; Simon et al. 2007; Stanis et al. 2008; Sukhotina et al. 2008; van Gaalen et al. 2006; Winstanley et al. 2005, 2007; Yates et al. 2015). Because delay discounting is influenced by both reinforcer magnitude and delay (Ho et al. 1999), the exponential discounting function was used to determine how NMDA receptor ligands affect sensitivity to these parameters. When traditional analyses (two-way ANOVA) were used to characterize discounting, results indicated that ketamine, memantine (5.0 mg/kg), CGS 19755 (5.0 mg/kg), and ifenprodil (10.0 mg/kg) increased impulsive choice, whereas D-cycloserine (15.0 mg/kg) decreased impulsive choice. However, when parameter estimates derived from the exponential model were analyzed with NLME, results indicated that ketamine (10.0 mg/kg), memantine (5.0 mg/kg) and ifenprodil (10.0 mg/kg) significantly decreased sensitivity to reinforcer magnitude without altering impulsive choice. These results provide additional evidence that NMDA receptor ligands mediate delay-discounting performance, although competitive antagonism appears to selectively alter impulsive choice, whereas uncompetitive antagonism decreases sensitivity to reinforcer magnitude only.
In the current study, administration of ketamine and memantine did not alter impulsive choice (b parameter estimates) in the delay-discounting task. Floresco et al. (2008) previously reported that ketamine (5.0 mg/kg) increases impulsive choice in a procedure similar to the one used in the current experiment. Although both studies utilized the discounting procedure developed by Evenden and Ryan (1996), Floresco et al. (2008) used shorter delays (0.4-6.5 s) relative to the current study (0-100 s). The increased discounting observed following ketamine administration in the Floresco et al. (2008) study relative to the current results may be due to baseline differences in impulsive choice. Indeed, baseline differences have been observed in a probability-discounting procedure following ketamine administration; animals with a low baseline level of discounting respond less for the probabilistic reinforcer following ketamine (10.0 mg/kg) administration, whereas ketamine does not affect animals with a high baseline level of discounting (Yates et al. 2016). To test this hypothesis, we reanalyzed the raw data and the parameter estimates taking into account baseline differences in impulsive choice. The results of these additional analyses revealed no interactions between ketamine dose and baseline level of impulsivity (data not shown). Another possible explanation for the differential outcomes across studies is the analysis used in each experiment. Floresco et al. (2008) used a two-way ANOVA to analyze the raw responses for the large magnitude reinforcer and found a main effect of dose, which was interpreted as an increase in impulsivity. When we used a two-way ANOVA, we found an interaction between dose and delay, and post hoc tests revealed that ketamine altered responses at the 0-s delay, corroborating previous results that ketamine increases impulsive choice (Cottone et al. 2013; Floresco et al. 2008). However, the b parameter estimate did not change as a function of dose, indicating that the slope of the discounting function remained unchanged following ketamine administration. These results highlight the importance of examining the behavioral mechanisms being altered in delay discounting (i.e., sensitivity to reinforcer magnitude and sensitivity to delayed reinforcement) following pharmacological manipulations.
Cottone et al. (2013) have previously shown that memantine, as well as ketamine, increases impulsive choice in a modified adjusting delay procedure. However, when we applied the exponential model to our data, we observed a decrease in sensitivity to reinforcer magnitude only. Methodological differences could account for the discrepant results of the current experiment and the results obtained by Cottone et al. (2013) The current study used a discounting procedure in which the delay to the large reinforcer increases across blocks of trials (e.g., Evenden and Ryan 1996), whereas Cottone et al. (2013) used an adjusting delay procedure, in which the delay to the large reinforcer titrates according to an animal’s responses (e.g., Perry et al. 2005). One important consideration is that the adjusting delay procedure uses a single data point as the dependent variable: the mean adjusting delay (MAD). By generating a single data point, sensitivity to reinforcer magnitude and sensitivity to delayed reinforcement are confounded; therefore, one cannot determine the behavioral mechanisms underlying MAD scores. The change in MAD scores observed in the Cottone et al. (2013) experiment could reflect a change in sensitivity to reinforcer magnitude as opposed to a change in impulsive choice per se. To illustrate the potential interpretational caveats of using a single data point to interpret the effects of drugs on discounting, we reanalyzed the raw proportion of responses by calculating the area under the curve (AUC; see Myerson et al. 2001 for the calculations involved in AUC). AUC values range from 0-1, with values closer to 0 indicating high impulsivity. We found that memantine (5.0 mg/kg), as well as ifenprodil, significantly decreased AUCs relative to vehicle (memantine: .089 ± .019 vs. .154 ± .028; ifenprodil: .103 ± .017 vs. .181 ± .023), indicating that these drugs increased impulsive choice.
One surprising result is that MK-801 did not affect sensitivity to reinforcer magnitude or sensitivity to delayed reinforcement. Previous studies using a similar procedure have reported a decrease in impulsive choice following MK-801 administration (Higgins et al. 2016; Yates et al. 2015), as well as an increase in sensitivity to reinforcer magnitude (Yates et al. 2015). There are several procedural differences that make direct comparisons between Yates et al. (2015) and the current study somewhat difficult. First, Yates et al. (2015) used higher doses of MK-801 (0.01-0.3 mg/kg) relative to the current study (0.003-0.03 mg/kg). In the current study, we omitted the highest doses used in the Yates et al. (2015) study because they significantly suppressed behavior in rats (i.e., caused a significant increase in omissions). Additionally, the delays to delivery of the large magnitude reinforcer differed across studies (0-50 s vs. 0-100 s). Another important consideration is that Yates et al. (2015), as well as Higgins et al. (2016) used an FR 1 schedule, whereas the current study incorporated an FR 10. Considering several variables are known to modulate the effects of drugs on discounting, such as the use of stimuli during the delay to reinforcement (Cardinal et al. 2000; Zeeb et al. 2010), baseline differences in impulsivity (Adriani et al. 2004; Hand et al. 2009; Wooters and Bardo 2011; Zeeb et al. 2010), and the order in which delays/probabilities are presented (Maguire et al. 2014; St. Onge et al. 2010; Tanno et al. 2014; Yates et al. 2016), the response requirement may be another variable that alters how drugs affect sensitivity to reinforcer magnitude and/or sensitivity to delayed reinforcement. Indeed, there is some evidence to this, as increasing the FR requirement promotes responding for the large magnitude reinforcer relative to the small magnitude reinforcer (Huskinson and Anderson 2013). Additionally, ketamine decreases sensitivity to reinforcer magnitude in probability discounting (a task similar to the one used in the current experiment) when an FR 1 schedule is used (Yates et al. 2015) but has no effect when an FR 10 schedule is used (Yates et al. 2016).
The inability to replicate the effects of MK-801 on sensitivity to reinforcer magnitude may be explained by the ceiling effect observed with A parameter estimates. Because A parameter estimates at the end of baseline training are near the maximum value of 1.0, we are unable to see an increase in sensitivity to reinforcer magnitude following drug administration. This limitation does not necessarily reflect a weakness in using quantitative analyses to assess discounting. Instead, this experiment shows a limitation of using the Evenden and Ryan (1996) procedure to measure discounting. In the current procedure, rats consistently chose the large magnitude reinforcer when its delivery is immediate, which results in maximal responding for this alternative at the 0-s delay. Because responses at the 0-s delay determine the intercept (A parameter) of the exponential function, this accounts for the ceiling effect observed with this parameter. One method that can circumvent this limitation is the used of a concurrent-chains procedure, which ensures subjects cannot exclusively respond for one alternative (see Aparicio et al. 2015; Oliveira et al. 2014 for a detailed description of the concurrent-chains procedure).
In the current study, CGS 19755 (10.0 and 20.0 mg/kg) suppressed behavior, as evidenced by a significant increase in omissions. Although Cottone et al. (2013) reported an increase in impulsive choice following CGS 19755 (20.0 mg/kg) administration, the same dose caused a significant increase in response latency and decreased the number of trials completed, making interpretations of the data somewhat difficult. In the current experiment, rats had to complete the response requirement within 20 s, or the trial was scored as an omission. Because CGS 19755 increases the latency to respond (Cottone et al. 2013), it was not surprising that omissions increased following administration of the two highest doses. Although the highest doses increased omissions, CGS 19755 (5.0 mg/kg) significantly increased impulsive choice without altering omissions or sensitivity to reinforcer magnitude. These results provide some evidence that competitive and uncompetitive antagonists differentially mediate delay-discounting performance, as CGS 19755 increases sensitivity to delayed reinforcement, whereas ketamine and memantine decrease sensitivity to reinforcer magnitude only.
Similar to the findings with memantine, ifenprodil decreased responding for the large magnitude reinforcer at the 0-s and 10-s delays. Results from the ANOVA analysis suggest that ifenprodil increased impulsive choice, whereas results from the exponential model revealed a significant change in the A parameter estimate only. The finding that ifenprodil decreased A parameter estimates without altering impulsive choice is surprising considering the NR2B-selective antagonist Ro 63-1908 decreases impulsive choice in a delay-discounting procedure (Higgins et al. 2016). One limitation to the current study is that ifenprodil is known to act on various receptors, including α1 adrenergic receptors, 5-HT1A receptors, and sigma receptors (Chenard et al. 1991). The current results do not appear be mediated by ifenprodil’s actions on 5-HT1A or α1 adrenergic receptors, as blocking either receptor does not significantly alter delay discounting (Evenden and Ryan 1999; Schwager et al. 2014). To date, we are unaware of any studies examining the effect of sigma receptor ligands on discounting, so we cannot rule out the possibility that the results obtained with ifenprodil are mediated by these receptors.
D-cycloserine did not alter sensitivity to reinforcer magnitude, but its effects on impulsive choice were dependent on the analysis used. When a two-way ANOVA was applied to the data, D-cycloserine (15.0 mg/kg) increased preference for the large reinforcer at the 10-s delay. However, when the exponential model was used, D-cycloserine did not alter A or b parameter estimates. The results of the exponential analysis are consistent with a previous report indicating no effect of D-cycloserine on delay discounting (van den Bergh et al. 2006). The discrepancy between analyses may be explained by the finding that ANOVA has a higher Type I error rate relative to NLME (Young et al. 2009); therefore, the significant effect observed with D-cycloserine following ANOVA analysis may reflect a spurious effect. Despite this discrepancy, considering D-cycloserine is a partial agonist at the glycine site of the NR1 subunit (Henderson et al. 1990) and glycine and glutamate are both required to activate the NMDA receptor (Johnson and Ascher 1987), examining how glycine site antagonists alter delay discounting is a potential future direction that will help further our understanding of the contribution of NMDA receptors to impulsive choice.
One important question that needs to be addressed is what a change in sensitivity to reinforcer magnitude means. The first interpretation is that the decrease could reflect impairment in discrimination between the small and large magnitude reinforcers. This interpretation is somewhat plausible as ketamine and memantine impair discrimination of two visual stimuli (Talpos et al. 2012; Tang and Franklin 1983; Ward et al. 2013). Ketamine also impairs acquisition of visuo-auditory conditional discrimination (Dix et al. 2010) and spatial learning (Alessandri et al. 1989; Lalonde and Joyal 1993), spatial memory (Alessandri et al. 1989; Venâncio et al. 2011), and attentional set shifting (Kos et al. 2011). However, MK-801, which has been shown to disrupt learning processes (Harder et al. 1998; Li et al. 2011; Rapanelli et al. 2013; van der Staay et al. 2011), does not decrease sensitivity to reinforcer magnitude. Furthermore, ifenprodil generally does not disrupt learning in animals (Doyle et al. 1998; Fraser et al. 1996; Mikolajczak et al. 2002; Parada et al. 1992) and lacks the psychotomimetic-like effects observed with NMDA receptor channel blockers (Boyce et al. 1999), but it decreased sensitivity to reinforcer magnitude in the current experiment. Therefore, the decrease in A parameter estimates does not appear to be due exclusively to an impairment in discrimination.
Another interpretation is that the decrease in sensitivity to reinforcer magnitude could be due to satiation. Memantine decreases consumption of palatable food (Popik et al. 2011; Smith et al. 2015), and ifenprodil decreases eating behavior (Khan et al. 1999). Additionally, ketamine (10.0 mg/kg), memantine (5.0 mg/kg), and ifenprodil (10.0 mg/kg) decreased the number of pellets earned during the session (ketamine: 1.826 ± .056 vs. 1.549 ± .120 pellets/trial; memantine: 1.804 ± .064 vs. 1.363 ± .081 pellets/trial; ifenprodil: 1.846 ± .053 vs. 1.457 ± .092 pellets/trial). However, satiation may not be a complete account for the decrease in A parameter estimates following ketamine, memantine, and ifenprodil administration. Ketamine, which increases consumption of palatable food (Garcia et al. 2009), decreased sensitivity to reinforcer magnitude in the current experiment. Additionally, even if a drug selectively increases impulsive choice, the animal is going to earn less food relative to vehicle conditions because they consistently choose the small magnitude reinforcer. For example, in the current study, CGS 19755 (5.0 mg/kg) increased impulsive choice without altering sensitivity to reinforcer magnitude, and it significantly decreased the number of pellets earned (1.502 ± .066 pellets/trial) relative to vehicle (1.739 ± .072 pellets/trial). One way to avoid this potential confound in future studies is to use the aforementioned concurrent-chains procedure (Aparicio et al. 2015; Oliveira et al. 2014), which ensures that each rat earns the same amount of food regardless of their preference for the small magnitude or large magnitude reinforcer. Another way to prevent this confound is to use isocaloric reinforcers, such as saccharin (e.g., Blasio et al. 2012).
In conclusion, the current results are important because we show that uncompetitive and competitive NMDA receptor antagonists differentially alter discounting performance. Specifically, when quantitative analyses are applied to the data, the uncompetitive antagonists ketamine and memantine decrease sensitivity to reinforcer magnitude, whereas CGS 19755 increased impulsive choice without altering sensitivity to reinforcer magnitude. These results also show that quantitative analyses can help increase our understanding of how certain drugs affect discounting. Although the results are promising, future studies should consider using a procedure that does not produce a ceiling effect with A parameter estimates (e.g., the concurrent-chains procedure), which will better enable one to observe an increase in sensitivity to reinforcer magnitude following drug administration.
Supplementary Material
Acknowledgements
We would like to thank Cliff Brown, Kerry Breitenstein, Anthony Johnson, and Sara Sharpe for assistance in data collection. We also would like to thank Dr. Mark Bardgett for reviewing a draft of the manuscript and providing feedback. Finally, we thank the NIMH’s Chemical Synthesis and Drug Supply Program for generously providing CGS 19755.
The current study was supported by NIH grant P20GM103436, as well as a Northern Kentucky University Faculty Project Grant.
References
- Adriani W, Rea M, Baviera M, Invernizzi W, Carli M, Ghirardi O, Caprioli A, Laviola G. Acetyl-L-carnitine reduces impulsive behaviour in adolescent rats. Psychopharmacology. 2004;176:296–304. doi: 10.1007/s00213-004-1892-9. [DOI] [PubMed] [Google Scholar]
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O’Donnell BF. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G, Herrnstein RJ. Preference reversal and delayed reinforcement. Anim Learn Behav. 1981;9:476–482. [Google Scholar]
- Albein-Urios N, Martinez-González JM, Lozano O, Verdejo-Garcia A. Monetary delay discounting in gambling and cocaine dependence with personality comorbidities. Addict Behav. 2014;39:1658–1662. doi: 10.1016/j.addbeh.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Bättig K, Welzl H. Effects of ketamine on tunnel maze and water maze performance in the rat. Behav Neural Biol. 1989;52:194–212. doi: 10.1016/s0163-1047(89)90313-0. [DOI] [PubMed] [Google Scholar]
- Al-Khaled M, Heldmann M, Bolstorff I, Hagenah J, Münte TF. Intertemporal choice in Parkinson’s disease and restless legs syndrome. Parkinsonism Relat Disord. 2015;21:1330–1335. doi: 10.1016/j.parkreldis.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Aparicio CF, Elcoro M, Alonso-Alvarez B. A long-term study of the impulsive choices of Lewis and Fischer 344 rats. Learn Behav. 2015;43:251–271. doi: 10.3758/s13420-015-0177-y. [DOI] [PubMed] [Google Scholar]
- Archer T, Garcia D. Attention-deficit/hyperactivity disorder: focus upon aberrant N-methyl-D-aspartate receptors systems. Curr Top Behav Neurosci. 2016;29:295–311. doi: 10.1007/7854_2015_415. [DOI] [PubMed] [Google Scholar]
- Baarendse PJJ, Vanderschuren LJMJ. Dissociable effects of monoamine reuptake inhibitors on distinct forms of impulsive behavior in rats. Psychopharmacology. 2012;219:313–326. doi: 10.1007/s00213-011-2576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar OM, Szumlinksi KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Hampson CL, Bradshaw CM, Szabadi E, Anderson IM, Deakin JFW. Effect of disconnecting the orbital prefrontal cortex from the nucleus accumbens core on inter-temporal choice behaviour: a quantitative analysis. Behav Brain Res. 2008;191:272–279. doi: 10.1016/j.bbr.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Body S, Cheung THC, Body S, Deakin JFW, Anderson IM, Bradshaw CM, Szabadi E. Quantitative analysis of the effect of lesions of the subthalamic nucleus on intemporal choice: further evidence for enhancement of the incentive value of food reinforcers. Behav Pharmacol. 2009;20:437–446. doi: 10.1097/FBP.0b013e3283305e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Cheung THC, Asgari K, Hampson CL, Body S, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of quinolinic acid-induced lesions of the nucleus accumbens core on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2007;195:71–84. doi: 10.1007/s00213-007-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MK, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76(Pt. B):518–527. doi: 10.1016/j.neuropharm.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasio A, Narayan AR, Kaminski BJ, Steardo L, Sabino V, Cottone P. A modified adjusting delay task to assess impulsive choice between isocaloric reinforcers in non-deprived male rats: effects of 5-HT2A/C and 5-HT1A receptor agonists. Psychopharmacology. 2012;219:377–386. doi: 10.1007/s00213-011-2517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S, Wyatt A, Webb JK, O’Donnell R, Mason G, Rigby M, Sirinathsinghji D, Hill RG, Rupniak NM. Selective NMDA NR2B antagonists induce antinociception without motor dysfunction: correlation with restricted locallisation of NR2B subunit in dorsal horn. Neuropharmacology. 1999;38:611–623. doi: 10.1016/s0028-3908(98)00218-4. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Chang JP, Lane HY, Tsai GE. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr Pharm Des. 2014;20:5180–5185. doi: 10.2174/1381612819666140110115227. [DOI] [PubMed] [Google Scholar]
- Chenard BL, Shalaby IA, Koe BK, Rounau RT, Butler TW, Prochiniak MA, Schmidt AW, Fox CB. Separation of α1 adrenergic and N-methyl-D-aspartate antagonist activity in a series of ifenprodil compounds. J Med Chem. 1991;34:3085–3090. doi: 10.1021/jm00114a018. [DOI] [PubMed] [Google Scholar]
- Cosenza M, Nigro G. Wagering the future: cognitive distortions, impulsivity, delay discounting, and time perspective in adolescent gambling. J Adolesc. 2015;45:56–66. doi: 10.1016/j.adolescence.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Cottone P, Iemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226:127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix S, Gilmour G, Potts S, Smith JW, Tricklebank M. A within-subject cognitive battery in the rat: differential effects of NMDA receptor antagonists. Psychopharmacology. 2010;212:227–242. doi: 10.1007/s00213-010-1945-1. [DOI] [PubMed] [Google Scholar]
- Doyle KM, Feerick S, Kirkby DL, Eddleston A, Higgins GA. Comparison of various N-methyl-D-aspartate receptor antagonists in a model of short-term memory and on overt behaviour. Behav Pharmacol. 1998;9:671–681. doi: 10.1097/00008877-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotoninergic drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Evens R, Stankevich Y, Dshemuchadse M, Storch A, Wolz M, Reichmann H, Schlaepfer TE, Goschke T, Lueken U. The impact of Parkinson’s disease and subthalamic deep brain stimulation on reward processing. Neuropsychologia. 2015;75:11–19. doi: 10.1016/j.neuropsychologia.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Fox AT, Hand DJ, Reilly MP. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behav Brain Res. 2008;187:146–152. doi: 10.1016/j.bbr.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Fraser CM, Cooke MJ, Fisher A, Thompson ID, Stone TW. Interactions between ifenprodil and dizocilpine on mouse behaviour in models of anxiety and working memory. Eur Neuropsychopharmacol. 1996;6:311–316. doi: 10.1016/s0924-977x(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, Gavioli EC, Quevedo J. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Haun HL, Hazelbaker CL, Ramachandra VS, Becker HC. Increased extracellular glutamate in the nucleus accumbens promotes excessive ethanol drinking in ethanol dependent mice. Neuropsychopharmacology. 2014;39:707–717. doi: 10.1038/npp.2013.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand DJ, Fox AT, Reilly MP. Differential effects of d-amphetamine on impulsive choice in spontaneously hypertensive and Wistar-Kyoto rats. Behav Pharmacol. 2009;20:549–553. doi: 10.1097/FBP.0b013e3283305ee1. [DOI] [PubMed] [Google Scholar]
- Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br J Pharmacol. 1998;125:1013–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G, Johnson JW, Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, MacMillan C, Sevo J, Zeeb FD, Thevarkunnel S. Enhanced attention and impulsive action following NMDA receptor GluN2B-selective antagonist pretreatment. Behav Brain Res. 2016;311:1–14. doi: 10.1016/j.bbr.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Ho M-Y, Mobini S, Chian T-J, Bradshaw CM, Szabadi E. Theory and method in the quantitative analysis of “impulsive choice” behaviour: implications for psychopharmacology. Psychopharmacology. 1999;146:362–372. doi: 10.1007/pl00005482. [DOI] [PubMed] [Google Scholar]
- Huskinson SL, Anderson KG. Effects of different fixed-ratio requirements on delay discounting in rats. Behav Process. 2013;100:18–22. doi: 10.1016/j.beproc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Isherwood SN, Pekcec A, Nicholson JR, Robbins TW, Dalley JW. Dissociable effects of mGluR5 allosteric modulation on distinct forms of impulsivity in rats: interaction with NMDA receptor antagonism. Psychopharmacology. 2015;232:3327–3344. doi: 10.1007/s00213-015-3984-0. [DOI] [PubMed] [Google Scholar]
- Jackson JN, MacKillop J. Attention-deficit/hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:316–325. doi: 10.1016/j.bpsc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen V, Rinholm JE, Johansen TJ, Medin T, Storm-Mathisen J, Sagvolden T, Hvalby O, Bergersen LH. N-methyl-D-aspartate receptor subunit dysfunction at hippocampal glutamatergic synapses in an animal model of attention-deficit/hyperactivity disorder. Neuroscience. 2009;158:353–364. doi: 10.1016/j.neuroscience.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Khan AM, Currás MC, Dao J, Jamal FA, Turkowski CA, Goel RK, Gillard ER, Wolfsohn SD, Stanley BG. Lateral hypothalamic NMDA receptor subunits NR2A and/or NR2B mediate eating: immunochemical/behavioral evidence. Am J Physiol. 1999;276:R880–R891. doi: 10.1152/ajpregu.1999.276.3.R880. [DOI] [PubMed] [Google Scholar]
- Kheramin S, Body S, Mobini S, Ho M-Y, Velázquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NDMA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology. 2011;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Joyal CC. Effects of ketamine and L-glutamic acid diethyl ester on spatial and nonspatial learning tasks in rats. Pharmacol Biochem Behav. 1993;44:539–545. doi: 10.1016/0091-3057(93)90164-o. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Merker S, Reif A, Novak M. Dances with black widow spiders: dysregulation of glutamate signalling enters center stage in ADHD. Eur Neuropharmacol. 2013;23:479–491. doi: 10.1016/j.euroneuro.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Li JT, Su YA, Guo CM, Feng Y, Yang Y, Huang RH, Si TM. Persisting cognitive deficits induced by low-dose, subchronic treatment with MK-801 in adolescent rats. Eur J Pharmacol. 2011;652:65–72. doi: 10.1016/j.ejphar.2010.10.074. [DOI] [PubMed] [Google Scholar]
- Liu YP, Wilkinson LS, Robbins TW. Effects of acute and chronic buspirone on impulsive choice and efflux of 5-HT and dopamine in hippocampus, nucleus accumbens and prefrontal cortex. Psychopharmacology. 2004;173:175–185. doi: 10.1007/s00213-003-1726-1. [DOI] [PubMed] [Google Scholar]
- Ma Y-Y, Yu P, Guo C-Y, Cui C-L. Effects of ifenprodil on morphine-induced conditioned place preference and spatial learning and memory in rats. Neurochem Res. 2011;36:383–391. doi: 10.1007/s11064-010-0342-9. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend on the manner in which delay is varied. Neuropharmacol. 2014;87:173–179. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczak P, Okulicz-Kozaryn I, Polanska A, Szczawinska K, Bobkiewicz-Kozlowska T. Effect of multiple ifenprodil or spermidine treatment on social recognition in rats. J Basic Clin Physiol Pharmacol. 2002;13:61–67. doi: 10.1515/jbcpp.2002.13.1.61. [DOI] [PubMed] [Google Scholar]
- Miller EM, Pomerleau F, Huetti P, Gerhardt GA, Glaser PE. Aberrant glutamate signaling in the prefrontal cortex and striatum of the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Psychopharmacology. 2014;231:3019–3029. doi: 10.1007/s00213-014-3479-4. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Jane DE. Pharmacology of NMDA receptors. In: Van Dongen AM, editor. Biology of the NMDA receptor. CRC Press; Boca Raton, FL: 2009. pp. 257–281. [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete A, Flores-Machorro FX, Téllez-Ballesteros RI, Alfaro-Romero A, Balderas JL, Reyes A. Study on action mechanism of 1-(4-methoxy-2-methylphenyl)piperazine (MMPP) in acquisition, formation, and consolidation of memory in mice. Drug Dev Res. 2014;75:59–67. doi: 10.1002/ddr.21094. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Sanacora G, Zarate CA., Jr Glial abnormalities in substance use disorders and depression: does shared glutamatergic dysfunction contribute to comorbidity? World J Biol Psychiatry. 2014;15:2–16. doi: 10.3109/15622975.2013.829585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Green L, Myerson J. Pigeons’ delay discounting functions established using a concurrent-chains procedure. J Exp Anal Behav. 2014;102:151–161. doi: 10.1002/jeab.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada J, Czuczwar SJ, Turski WA. NBQX does not affect learning and memory tasks in mice: a comparison with D-CPPene and ifenprodil. Brain Res Cogn Brain Res. 1992;1:67–71. doi: 10.1016/0926-6410(92)90006-d. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Perlov E, Phillipsen A, Hesslinger B, Buechert M, Ahrendts J, Feige B, Bubl E, Hennig J, Ebert D, Tebartz van Elst L. Reduced cingulate glutamate/glutamine-to-creatine ratios in adult patients with attention deficit/hyperactivity disorder – a magnet resonance spectroscopy study. J Psychiatry Res. 2007;41:934–941. doi: 10.1016/j.jpsychires.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. Linear and nonlinear mixed effects models. R Foundation for Statistical Computing; Vienna, Austria: 2007. pp. 1–89. [Google Scholar]
- Popik P, Kos T, Zhang Y, Bisaga A. Memantine reduces consumption of highly palatable food in a rat model of binge eating. Amino Acids. 2011;40:477–485. doi: 10.1007/s00726-010-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapanelli M, Frick LR, Bernardez-Vidal M, Zanutto BS. Different MK-801 administration schedules induce mild to severe learning impairments in an operant conditioning task: role of buspirone and risperidone in ameliorating these cognitive deficits. Behav Brain Res. 2013;257:156–165. doi: 10.1016/j.bbr.2013.09.043. [DOI] [PubMed] [Google Scholar]
- Schwager AL, Haack AK, Taha SA. Impaired flexibility in decision making in rats after administration of the pharmacological stressor yohimbine. Psychopharmacology. 2014;231:3941–3952. doi: 10.1007/s00213-014-3529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–549. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and d-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–436. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- Smith KL, Rao RR, Velázquez-Sánchez C, Valenza M, Giuliano C, Everitt BJ, Sabino V, Cottone P. The uncompetitive N-methyl-D-asparate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: role of the nucleus accumbens shell. Neuropsychopharmacology. 2015;40:1163–1171. doi: 10.1038/npp.2014.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Dep. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Onge JR, Chiu YC, Florescro SB. Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology. 2010;211:209–21. doi: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- Sukhotina IA, Dravolina OA, Novitskaya Y, Zvartau EE, Danysz W, Bespalov AY. Effects of mGlu1 receptor blockade on working memory, time estimation, and impulsivity in rats. Psychopharmacology. 2008;196:211–220. doi: 10.1007/s00213-007-0953-2. [DOI] [PubMed] [Google Scholar]
- Talpos JC, Fletcher AC, Circelli C, Tricklebank MD, Dix SL. The pharmacological sensitivity of a touchscreen-based visual discrimination task in the rat using simple and perceptually challenging stimuli. Psychopharmacology. 2012;221:437–449. doi: 10.1007/s00213-011-2590-z. [DOI] [PubMed] [Google Scholar]
- Tang AH, Franklin SR. Disruption of brightness discrimination in a shock avoidance task by phencyclidine and its antagonism in rats. J Pharmacol Exp Ther. 1983;225:503–508. [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Hensen C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: Interactions with order of delay presentation. Psychopharmacology. 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh FS, Bloemarts E, Groenink L, Olivier B, Oosting RS. Delay aversion: effects of 7-OH DPAT, 5-HT1A/1B-receptor stimulation and D-cycloserine. Pharmacol Biochem Behav. 2006;85:736–743. doi: 10.1016/j.pbb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Venâncio C, Magalhães A, Antunes L, Summavielle T. Impaired spatial memory after ketamine administration in chronic low doses. Curr Neuropharmacol. 2011;9:251–255. doi: 10.2174/157015911795016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KC, Khattak HZ, Richardson L, Lee JL, Vreugdenhil M. NMDA receptor antagonists distort visual grouping in rats performing a modified two-choice visual discrimination task. Psychopharmacology. 2013;229:627–637. doi: 10.1007/s00213-013-3123-8. [DOI] [PubMed] [Google Scholar]
- Weller RE, Avsar KB, Cox JE, Reid MA, White DM, Lahti AC. Delay discounting and task performance consistency in patients with schizophrenia. Psychiatry Res. 2014;215:286–293. doi: 10.1016/j.psychres.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiehler A, Bromberg U, Peters J. The role of prospection in steep temporal reward discounting in gambling addiction. Front Psychiatry. 2015;6:112. doi: 10.3389/fpsyt.2015.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DEH, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. ΔFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. Methylphenidate and fluphenazine, but not amphetamine, differentially affect impulsive choice in Spontaneously Hypertensive, Wistar-Kyoto and Spargue-Dawley rats. Brain Res. 2011;1396:45–53. doi: 10.1016/j.brainres.2011.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Discriminative stimulus effects of NMDA, AMPA, and mGluR5 glutamate receptor ligands in methamphetamine-trained rats. Behav Pharmacol. 2011;22:516–524. doi: 10.1097/FBP.0b013e328349aafa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Batten SR, Bardo MT, Beckmann JS. Role of ionotropic glutamate receptors in delay and probability discounting in the rat. Psychopharmacology. 2015;232:1187–1196. doi: 10.1007/s00213-014-3747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates JR, Breitenstein KA, Gunkel BT, Hughes MN, Johnson AB, Rogers KK, Sharpe SM. Effects of NMDA receptor antagonists on probability discounting depend on the order of probability presentation. Pharmacol Biochem Behav. 2016;150:151–31. doi: 10.1016/j.pbb.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: advantages and cautionary notes. Learn Motiv. 2009;40:160–177. [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology. 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.