Abstract
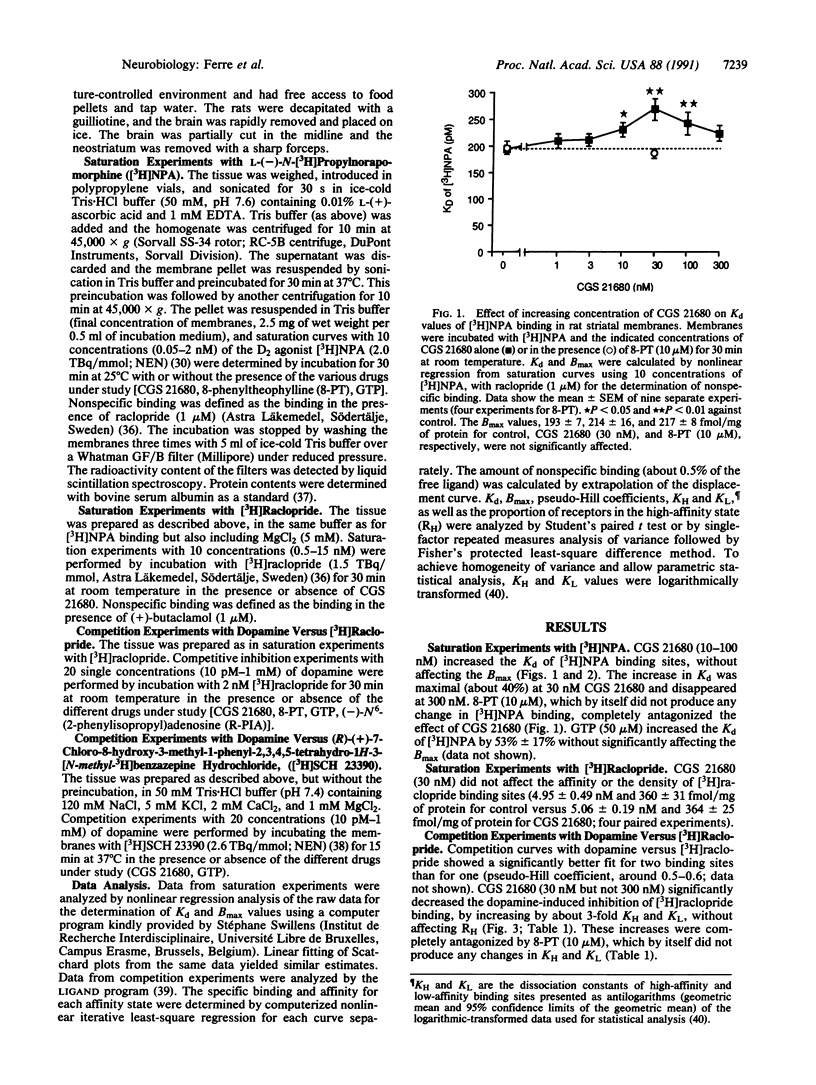
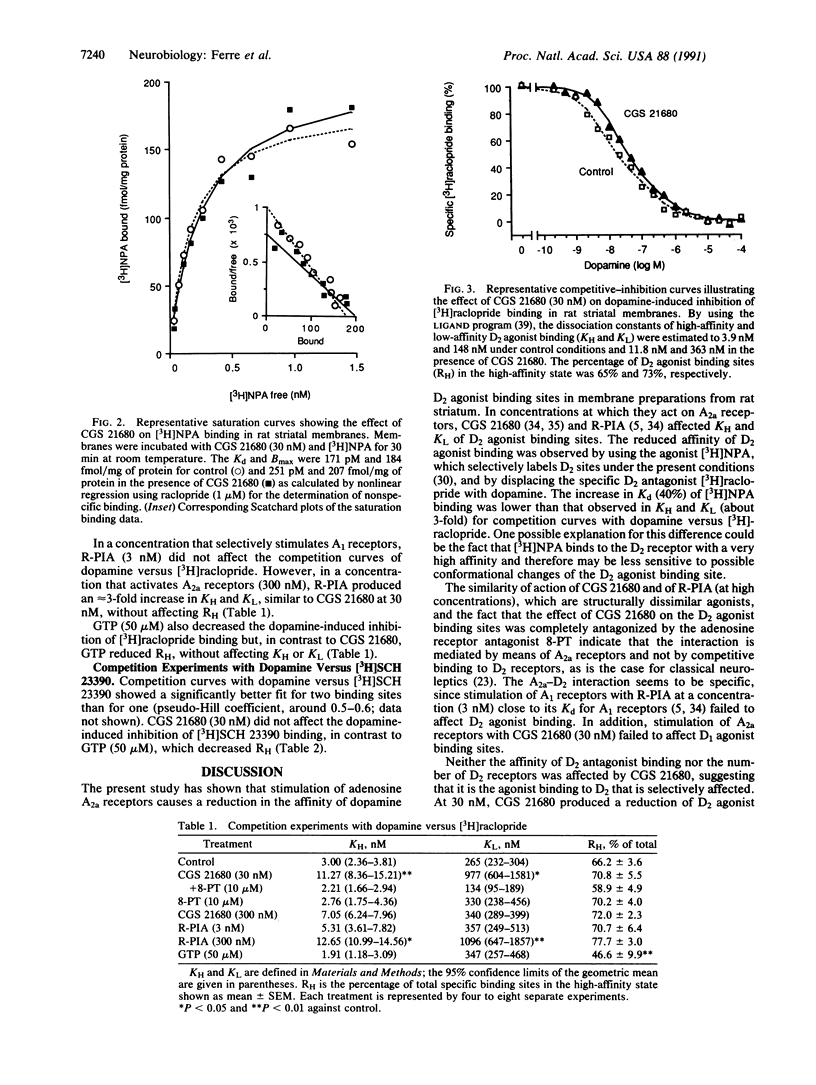
Since high-affinity adenosine A2 receptors (A2a) are localized exclusively in dopamine-rich regions in the central nervous system and mediate inhibition of locomotor activity, we have examined the effect of A2a receptor activation on D1 and D2 receptor binding in membrane preparations of the rat striatum. The A2a agonist 2-[p-(2-carboxyethyl)phenethylamino]-5'- N-ethylcarboxamidoadenosine (CGS 21680) increased the Kd of the dopamine D2 agonist L-(-)-N-[3H]propylnorapomorphine without affecting the Bmax. The increase in Kd was maximal (40%) at 30 nM CGS 21680. CGS 21680 (30 nM) decreased the dopamine-induced inhibition of [3H]raclopride (a D2 antagonist) binding due to an increase (about 3-fold) in KH and KL, the dissociation constants of high- and low-affinity binding sites. The effects of CGS 21680 were antagonized by the adenosine antagonist 8-phenyltheophylline (10 microM). (-)-N6-(2-Phenylisopropyl)adenosine produced an effect similar to that of CGS 21680, provided the concentration used was high enough to stimulate A2a receptors (300 nM). GTP (50 microM) also decreased the dopamine-induced inhibition of [3H]raclopride binding but, in contrast to CGS 21680, GTP decreased the proportion of D2 receptors in the high-affinity state. CGS 21680 (30 nM) did not affect the Kd or Bmax of [3H]raclopride and failed to affect ligand binding to D1 receptors. Thus, stimulation of A2a receptors potently reduces the affinity of D2 agonist binding sites within the plasma membrane of striatal neurons. This A2a-D2 interaction may underlie the neuroleptic-like actions of adenosine agonists and the enhancing effects of adenosine antagonists, such as caffeine, on locomotor activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnati L. F., Celani M. F., Fuxe K. Cholecystokinin peptides in vitro modulate the characteristics of the striatal 3H-N-propylnorapomorphine sites. Acta Physiol Scand. 1983 May;118(1):79–81. doi: 10.1111/j.1748-1716.1983.tb07244.x. [DOI] [PubMed] [Google Scholar]
- Agnati L. F., Fuxe K., Benfenati F., Battistini N. Neurotensin in vitro markedly reduces the affinity in subcortical limbic 3H-N-propylnorapomorphine binding sites. Acta Physiol Scand. 1983 Dec;119(4):459–461. doi: 10.1111/j.1748-1716.1983.tb07350.x. [DOI] [PubMed] [Google Scholar]
- Alexander S. P., Reddington M. The cellular localization of adenosine receptors in rat neostriatum. Neuroscience. 1989;28(3):645–651. doi: 10.1016/0306-4522(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Booth R. G., Baldessarini R. J. Adenosine A2 stimulation of tyrosine hydroxylase in rat striatal minces is reversed by dopamine D2 autoreceptor activation. Eur J Pharmacol. 1990 Aug 28;185(2-3):217–221. doi: 10.1016/0014-2999(90)90643-k. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Creese I., Sibley D. R., Hamblin M. W., Leff S. E. The classification of dopamine receptors: relationship to radioligand binding. Annu Rev Neurosci. 1983;6:43–71. doi: 10.1146/annurev.ne.06.030183.000355. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V. The physiological role of adenosine in the central nervous system. Int Rev Neurobiol. 1985;27:63–139. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Durcan M. J., Morgan P. F. Evidence for adenosine A2 receptor involvement in the hypomobility effects of adenosine analogues in mice. Eur J Pharmacol. 1989 Sep 22;168(3):285–290. doi: 10.1016/0014-2999(89)90789-9. [DOI] [PubMed] [Google Scholar]
- Ferré S., Herrera-Marschitz M., Grabowska-Andén M., Casas M., Ungerstedt U., Andén N. E. Postsynaptic dopamine/adenosine interaction: II. Postsynaptic dopamine agonism and adenosine antagonism of methylxanthines in short-term reserpinized mice. Eur J Pharmacol. 1991 Jan 3;192(1):31–37. doi: 10.1016/0014-2999(91)90065-x. [DOI] [PubMed] [Google Scholar]
- Ferré S., Herrera-Marschitz M., Grabowska-Andén M., Ungerstedt U., Casas M., Andén N. E. Postsynaptic dopamine/adenosine interaction: I. Adenosine analogues inhibit dopamine D2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol. 1991 Jan 3;192(1):25–30. doi: 10.1016/0014-2999(91)90064-w. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Fuxe K., Agnati L. Effect of some phosphodiesterase inhibitors on central dopamine mechanisms. Eur J Pharmacol. 1976 Jul;38(1):31–38. doi: 10.1016/0014-2999(76)90198-9. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Celani M. F., Martire M., Zini I., Zoli M., Agnati L. F. l-Glutamate reduces the affinity of [3H]N-propylnorapomorphine binding sites in striatal membranes. Eur J Pharmacol. 1984 Apr 13;100(1):127–130. doi: 10.1016/0014-2999(84)90326-1. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Ungerstedt U. Action of caffeine and theophyllamine on supersensitive dopamine receptors: considerable enhancement of receptor response to treatment with DOPA and dopamine receptor agonists. Med Biol. 1974 Feb;52(1):48–54. [PubMed] [Google Scholar]
- Green R. D., Proudfit H. K., Yeung S. M. Modulation of striatal dopaminergic function by local injection of 5'-N-ethylcarboxamide adenosine. Science. 1982 Oct 1;218(4567):58–61. doi: 10.1126/science.7123218. [DOI] [PubMed] [Google Scholar]
- Heffner T. G., Wiley J. N., Williams A. E., Bruns R. F., Coughenour L. L., Downs D. A. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacology (Berl) 1989;98(1):31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Hyttel J. SCH 23390 - the first selective dopamine D-1 antagonist. Eur J Pharmacol. 1983 Jul 15;91(1):153–154. doi: 10.1016/0014-2999(83)90381-3. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F., Schulz R., Hutchison A. J., Do U. H., Sills M. A., Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989 Dec;251(3):888–893. [PubMed] [Google Scholar]
- Jarvis M. F., Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989 Sep 13;168(2):243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Köhler C., Hall H., Ogren S. O., Gawell L. Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem Pharmacol. 1985 Jul 1;34(13):2251–2259. doi: 10.1016/0006-2952(85)90778-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lupica C. R., Cass W. A., Zahniser N. R., Dunwiddie T. V. Effects of the selective adenosine A2 receptor agonist CGS 21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hippocampus and striatum. J Pharmacol Exp Ther. 1990 Mar;252(3):1134–1141. [PubMed] [Google Scholar]
- Maenhaut C., Van Sande J., Libert F., Abramowicz M., Parmentier M., Vanderhaegen J. J., Dumont J. E., Vassart G., Schiffmann S. RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1169–1178. doi: 10.1016/s0006-291x(05)80909-x. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Onali P., Olianas M. C., Bunse B. Evidence that adenosine A2 and dopamine autoreceptors antagonistically regulate tyrosine hydroxylase activity in rat striatal synaptosomes. Brain Res. 1988 Jul 26;456(2):302–309. doi: 10.1016/0006-8993(88)90232-6. [DOI] [PubMed] [Google Scholar]
- Onali P., Olianas M. C., Gessa G. L. Characterization of dopamine receptors mediating inhibition of adenylate cyclase activity in rat striatum. Mol Pharmacol. 1985 Aug;28(2):138–145. [PubMed] [Google Scholar]
- Parkinson F. E., Fredholm B. B. Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):85–89. doi: 10.1007/BF00178977. [DOI] [PubMed] [Google Scholar]
- Prémont J., Perez M., Blanc G., Tassin J. P., Thierry A. M., Hervé D., Bockaert J. Adenosine-sensitive adenylate cyclase in rat brain homogenates: kinetic characteristics, specificity, topographical, subcellular and cellular distribution. Mol Pharmacol. 1979 Nov;16(3):790–804. [PubMed] [Google Scholar]
- Schiffmann S. N., Libert F., Vassart G., Dumont J. E., Vanderhaeghen J. J. A cloned G protein-coupled protein with a distribution restricted to striatal medium-sized neurons. Possible relationship with D1 dopamine receptor. Brain Res. 1990 Jun 11;519(1-2):333–337. doi: 10.1016/0006-8993(90)90097-u. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine receptors and the dopamine hypothesis of schizophrenia. Synapse. 1987;1(2):133–152. doi: 10.1002/syn.890010203. [DOI] [PubMed] [Google Scholar]
- Seeman P., Niznik H. B., Guan H. C., Booth G., Ulpian C. Link between D1 and D2 dopamine receptors is reduced in schizophrenia and Huntington diseased brain. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10156–10160. doi: 10.1073/pnas.86.24.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H. Adenosine as a neuromodulator. Annu Rev Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Katims J. J., Annau Z., Bruns R. F., Daly J. W. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981 May;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck B. Sensitization by caffeine of central catecholamine receptors. J Neural Transm. 1973;34(1):61–72. doi: 10.1007/BF01244827. [DOI] [PubMed] [Google Scholar]
- Zahniser N. R., Molinoff P. B. Effect of guanine nucleotides on striatal dopamine receptors. Nature. 1978 Oct 5;275(5679):453–455. doi: 10.1038/275453a0. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- von Euler G., Fuxe K., Benfenati F., Hansson T., Agnati L. F., Gustafsson J. A. Neurotensin modulates the binding characteristics of dopamine D2 receptors in rat striatal membranes also following treatment with toluene. Acta Physiol Scand. 1989 Apr;135(4):443–448. doi: 10.1111/j.1748-1716.1989.tb08602.x. [DOI] [PubMed] [Google Scholar]
- von Euler G., Meister B., Hökfelt T., Eneroth P., Fuxe K. Intraventricular injection of neurotensin reduces dopamine D2 agonist binding in rat forebrain and intermediate lobe of the pituitary gland. Relationship to serum hormone levels and nerve terminal coexistence. Brain Res. 1990 Oct 29;531(1-2):253–262. doi: 10.1016/0006-8993(90)90781-6. [DOI] [PubMed] [Google Scholar]
- von Euler G., van der Ploeg I., Fredholm B. B., Fuxe K. Neurotensin decreases the affinity of dopamine D2 agonist binding by a G protein-independent mechanism. J Neurochem. 1991 Jan;56(1):178–183. doi: 10.1111/j.1471-4159.1991.tb02578.x. [DOI] [PubMed] [Google Scholar]



