Abstract
Mercury exists in the environment in various forms, all of which pose a risk to human health. Despite guidelines regulating the industrial release of mercury into the environment, humans continue to be exposed regularly to various forms of this metal via inhalation or ingestion. Following exposure, mercuric ions are taken up by and accumulate in numerous organs, including brain, intestine, kidney, liver, and placenta. In order to understand the toxicological effects of exposure to mercury, a thorough understanding of the mechanisms that facilitate entry of mercuric ions into target cells must first be obtained. A number of mechanisms for the transport of mercuric ions into target cells and organs have been proposed in recent years. However, the ability of these mechanisms to transport mercuric ions and the regulatory features of these carriers have not been characterized completely. The purpose of this review is to summarize the current findings related to the mechanisms that may be involved in the transport of inorganic and organic forms of mercury in target tissues and organs. This review will describe mechanisms known to be involved in the transport of mercury and will also propose additional mechanisms that may potentially be involved in the transport of mercuric ions into target cells.
Keywords: Inorganic mercury, Methylmercury, Transport, Kidney, Brain
Introduction
Since the beginning of the industrial revolution, the global human population has been faced with the problem of environmental pollution. As the human population has grown, the level of environmental pollution has increased, making it a serious, world-wide health problem (SOER 2015). Humans are exposed regularly to environmental pollutants in air, soil, water, and/or food. While air pollution represents a major route of human exposure to toxicants [because exposure to this form of pollution is nearly unavoidable (WHO 2016)], oral exposure via ingestion of contaminated water and/or food also represents an important route of human exposure to toxicants. As one would expect, exposure to toxicants via multiple routes enhances one’s total toxicant burden, which can negatively affect health outcomes in certain populations.
Mercury (Hg) is a ubiquitous metal pollutant that can be found in air, soil, and water. It exists in metallic, inorganic, and/or organic forms in the environment, and its presence in the world-wide environment is becoming increasingly problematic. While a fraction of Hg enters the environment through natural processes such as volcanic activity and the breakdown of minerals in rocks and soils, the majority of Hg is released into the environment through industrial processes such as mining and the burning of fossil fuels (ATSDR 1999). It has been estimated that the combustion of fossil fuels (primarily coal), biofuels, and wastes accounts for the greatest portion (approximately 35–77 %) of anthropogenic Hg emissions (Ambrose et al. 2015). Approximately half of these emissions can be attributed to coal-fired power plants (Ambrose et al. 2015; Holzman 2010), which represent the largest source of environmental Hg in the USA (USEPA 1997, 1998). Although the regulations regarding Hg emissions have become more stringent in recent years, the regulatory mandates are in need of improvement (Rallo et al. 2012). Elemental Hg released from power plants can remain in the atmosphere for up to 2 years and can be transported to a different hemisphere before settling in soil and water (Holzman 2010). It is important to note that China is the largest emitter of Hg in the world (Liang et al. 2015) and this Hg can easily spread to multiple continents. Given the ability of Hg to travel in the atmosphere and the lack of strict regulatory guidelines in many countries, the deposition of Hg in the environment continues to be a major problem.
Although exposure to Hg through environmental sources may affect a large percentage of the population, this route of exposure does not often lead to toxicological consequences in humans. In contrast, human exposure via dental amalgams, medicinal therapies, and/or dietary sources has been shown to lead to toxicological consequences (ATSDR 1999; Clarkson and Magos 2006; Risher and De Rosa 2007; Zalups 2000). Of the different routes of exposure, humans are exposed to the greatest levels of Hg via ingestion of food contaminated with organic forms of Hg (i.e., methylmercury; CH3Hg+). High levels of CH3Hg+ are found often in the muscle of large predatory freshwater and saltwater fish, such as northern pike, salmon, swordfish, tuna, and shark. Accumulation of Hg in tissues of these fish is related primarily to their ingestion of other contaminated fish, their longevity in contaminated waters, and their inability to efficiently eliminate Hg (Ward et al. 2010). Following the ingestion of contaminated food, CH3Hg+ is absorbed readily by the gastrointestinal tract and subsequently enters systemic circulation, which facilitates the delivery of mercuric ions to target organs (ATSDR 1999).
Occupational exposure also represents a major route of exposure to Hg. In particular, artisanal and small-scale gold mining (ASGM) are major sources of occupational exposure to Hg. Even though these activities have been banned in many countries, millions of people continue to use these methods to extract gold from ore. Indeed, recent estimates suggest that over 16 million artisanal miners remain active, particularly in South America (Seccatore et al. 2014). The air around amalgam burning sites often has very high levels of mercury vapor and usually exceeds the World Health Organization (WHO) limits for exposure (Gibb and O’Leary 2014; Nakazawa et al. 2016). Furthermore, the concentration of Hg in the urine of adults often exceeds the recommended limits, suggesting that exposure to Hg via mining activities increases the probability of developing acute and/or chronic pathologies (Kristensen et al. 2014). Children are also at risk of exposure to toxic levels of Hg due to participation in amalgam burning or by being in the proximity of the amalgam burning sites. Some of these children have been shown to exhibit long-term neurological deficits (Ohlander et al. 2016), suggesting that they have been exposed to toxic levels of Hg.
Exposure to the various forms of Hg can lead to detrimental effects in many organ systems. These include, but are not limited to, the cardiovascular (Carmignani et al. 1992; Soni et al. 1992; Wakita 1987; Wang et al. 2000; Warkany and Hubbard 1953), gastrointestinal (Afonso and De Alvarez 1960; Bluhm et al. 1992; Lundgren and Swensson 1949; Murphy et al. 1979), neurological (Jaffe et al. 1983; Lin and Lim 1993), hepatobiliary (Jaffe et al. 1983; Murphy et al. 1979; Samuels et al. 1982), and renal (Murphy et al. 1979; Rowens et al. 1991; Samuels et al. 1982) systems.
When evaluating the handling of mercuric ions in biological systems, one must consider the chemical characteristics of mercuric ions. Mercuric ions have a high affinity for nucleophilic functional groups, particularly sulfhydryl (thiol; -S) groups. Given the tendency of mercuric ions to form strong bonds with thiols, one can assume that all mercuric ions within biological systems are bound to thiol-containing molecules, such as glutathione (GSH), cysteine (Cys), homocysteine (Hcy), N-acetylcysteine (NAC), and albumin. Mercuric ions do not likely exist as inorganic salts, or in an unbound, “free” ionic state (Hughes 1957).
Inorganic mercury (Hg2+) forms linear II, coordinate covalent complexes with low molecular weight thiol-containing molecules (Fuhr and Rabenstein 1973; Rubino et al. 2004), while organic forms of mercury, such as methylmercury (CH3Hg+), bind to thiol-containing molecules to form linear I, coordinate covalent complexes (Fig. 1). Mercuric conjugates of thiol-containing molecules appear to be thermodynamically stable in an aqueous environment throughout a pH range of 1–14 (Fuhr and Rabenstein 1973). The affinity constant for mercuric ions bound to thiolate anions is approximately 1015–1020. Although the bond between a mercuric ion and a thiol-containing molecule appears to be stable in simple aqueous solutions, it is likely that this interaction is much more complex within living organisms (Fuhr and Rabenstein 1973). Various factors such as thiol competition and exchange may lead to an unstable bond between mercuric ions and certain thiol-containing molecules. For example, shortly after exposure to Hg2+, most of the mercuric ions present in plasma are likely bound to thiol-containing proteins such as albumin (Cember et al. 1968; Friedman 1957; Lau and Sarkar 1979; Mussini 1958). Interestingly, the amount of Hg in blood is reduced rapidly following exposure to Hg, suggesting that mercuric ions are being taken up by target cells. Yet, Hg–albumin complexes do not appear to be taken up readily by target cells such as proximal tubular cells (Zalups and Barfuss 1993). Therefore, it appears that Hg bound to plasma proteins (e.g., Hg–albumin) dissociate in the presence of other thiols, permitting the mercuric ions to bind to the most abundant non-protein thiol, which may form a transportable mercuric complex that can be taken up more readily into target cells.
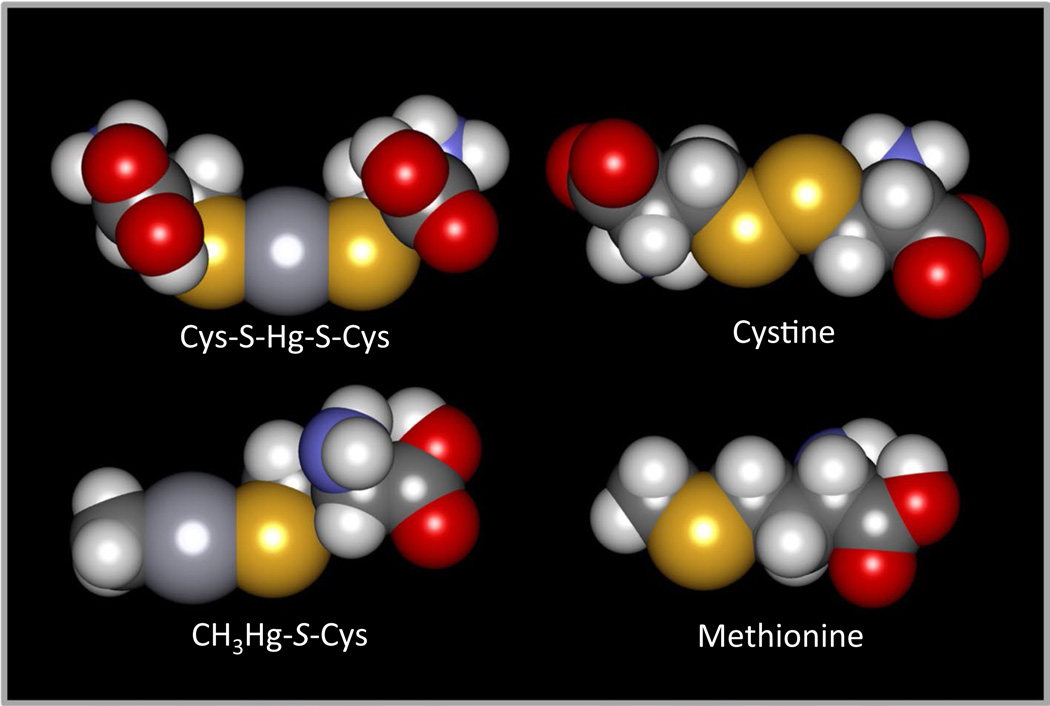
Fig. 1.
Space-fill models of Cys S-conjugates of Hg2+ and CH3Hg+ compared with the amino acids, cystine, and methionine. The Cys S-conjugate of Hg2+ (Cys-S-Hg-S-Cys) is similar in shape and size to cystine, while the Cys S-conjugate of CH3Hg+ (CH3Hg+-SCys) is similar to methionine. Owing to these similarities, it has been hypothesized that Cys- S-Hg-S-Cys and Cys-S-CH3Hg+ are transportable substrates of carrier proteins that mediate the transport of cystine and methionine, respectively
Transport of inorganic mercury
Intestinal transport of inorganic mercury
Understanding the mechanisms responsible for the intestinal transport of inorganic mercury (Hg2+) is important considering that exposure to this metal may occur via consumption of food and/or liquids contaminated with Hg2+. It has been suggested that the transport of Hg2+ across the plasma membranes of enterocytes utilizes both passive and active mechanisms (Andres et al. 2002; Hoyle and Handy 2005; Laporte et al. 2002). In addition, the method by which Hg2+ is transported across intestinal enterocytes is thought to be dependent upon the species of Hg2+ presented to enterocytes (Foulkes 2000). It is important to note that the species of Hg2+ present in the lumen of the intestine is highly dependent upon the components of ingested food in that ingested food often has a high concentration of thiol-containing molecules, such as amino acids and peptides, which can bind to Hg2+. Thiol S-conjugates of Hg2+ formed in the lumen of the gastrointestinal tract may be similar in shape and size to certain endogenous molecules (e.g., amino acids and/or polypeptides) that are absorbed along the small intestine. Owing to these similarities and because amino acid and peptide transporters are prevalent in enterocytes (Dave et al. 2004; Ganapathy et al. 2001), it is possible that thiol S-conjugates of Hg2+ are taken up into enterocytes by one or more of amino acid and/or peptide transporters. The duodenum, which is a major site of amino acid absorption, also appears to be the initial site of Hg2+ absorption (Endo et al. 1984). This observation supports the theory that amino acid and/or peptide transporters may be involved in the intestinal uptake of mercuric species. The identities of specific amino acid/peptide transporters that are involved in the intestinal uptake of Hg2+ have not yet been reported.
A fraction of ingested Hg2+ may be taken up following ligand exchange whereby the mercuric ion dissociates from the thiol to which it is bound and is transported subsequently into cells via one or more ion transporters. The divalent metal transporter 1 (DMT1; SLC11A2), which is localized in the apical membrane of enterocytes (Canonne-Hergaux et al. 1999), may play a role in the uptake of mercuric ions. In vivo studies in mice showed that decreased expression of intestinal DMT1 corresponded to a decrease in the intestinal accumulation of Hg2+. These data suggest that DMT1 may play a role in the intestinal uptake of Hg2+ (Ilback et al. 2008). Recently, more direct studies in yeast and cultured enterocytes (Caco-2 cells) indicate that DMT1 is capable of mediating the cellular uptake of mercuric ions (Vazquez et al. 2015).
The intestine also appears to play an important role in the net fecal elimination of Hg2+. Mercuric ions in blood may cross enterocytes and enter the intestinal lumen by paracellular and/or transcellular mechanisms (Hoyle and Handy 2005; Sugawara et al. 1998; Zalups et al. 1999). Data from in vivo studies in rats with ligated bile ducts indicate that a fraction of the Hg2+ that is excreted in the feces is due to secretion of Hg2+ from blood into the intestinal lumen (Zalups 1998). This secretion likely involves the transport of thiol S-conjugates of Hg2+, which may occur via amino acid transporters, multidrug resistance-associated proteins (MRPs), and organic anion transporters (OATs). Many amino acid transporters are counter-exchangers, and thus, they may be able to mediate the export of mercuric complexes in exchange for amino acids at the luminal and basolateral membranes. MRPs have been characterized as export proteins, and thus, they may be involved in the export of Hg2+ across the basolateral and luminal membrane of enterocytes. At the basolateral membrane, MRP3 (ABCC3) mediates the uptake of compounds from the interstitial space into enterocytes and, therefore, this carrier may also be involved in the basolateral uptake of Hg2+ into enterocytes (Prime-Chapman et al. 2004; Rost et al. 2002; Shoji et al. 2004; Yokooji et al. 2007). MRP4 is also localized in the basolateral membrane of enterocytes (Ming and Thakker 2010); however, in vitro studies have suggested that mercuric species are not substrates of MRP4 (Bridges et al. 2013). The export of mercuric species from within enterocytes, across the apical membrane, and into the intestinal lumen may be mediated by MRP2 (ABCC2). MRP2 has been shown to be involved in the intestinal secretion of organic anions (Itagaki et al. 2008) and has been implicated previously in the transport of mercuric ions (Bridges et al. 2008a, b, 2011). Owing to the broad substrate specificity of MRP2 and MRP3, it is possible that they may be involved in the basolateral to apical movement of thiol S-conjugates of Hg2+ across enterocytes. Other members of the MRP family, including MRP5 (ABCC5), MRP6 (ABCC6), and MRP7 (ABCC7), have been identified in enterocytes (Maher et al. 2005); however, the membrane localization of these proteins and their ability to transport mercuric species are unknown currently. OATs have been implicated in the transport of mercuric species in other organs and target cells; therefore, it is possible that one or more OATs may be involved in the intestinal secretion of certain mercuric species. OAT2 (SLC22A7) and OAT10 (SLC22A13) have been identified in the intestine although the ability of either carrier to mediate the transport of mercuric species is unknown at present.
Renal transport of inorganic mercury
Following exposure to elemental or inorganic forms of Hg, the majority of mercuric ions accumulate in the kidneys (Ashe et al. 1953; Clarkson and Magos 1966; Friberg 1959; Friberg et al. 1957; Hahn et al. 1990). In fact, the uptake and accumulation of Hg2+ in the kidneys occurs rapidly with as much as 40 % of a non-nephrotoxic dose (0.5 µmol kg−1) of Hg2+ present in the kidneys of rats within 1–3 h after exposure (Zalups 1993a). Hg2+ accumulates primarily in the segments of the nephron that are located in the cortex and outer stripe of the outer medulla (Berlin and Gibson 1963; Berlin and Ullberg 1963; Taugner 1966; Taugner et al. 1966). Histochemical and autoradiographic studies using mice and rats indicate that the accumulation of Hg2+ in the renal cortex and outer stripe of the outer medulla occurs almost exclusively along the pars convoluta and the pars recta of the proximal tubule (Hultman et al. 1985, 1992; Hultman and Enestrom 1986, 1992; Magos et al. 1985; Rodier et al. 1988; Taugner et al. 1966; Zalups 1991). Although the proximal tubule is the primary site of accumulation for mercuric ions, the possibility remains that other renal tubular segments may also take up, accumulate, and export mercuric ions.
The proximal tubular uptake of Hg2+ has been shown to utilize mechanisms localized in the luminal and basolateral plasma membranes of proximal tubular cells (Zalups and Minor 1995). At the luminal membrane, a Cys S-conjugate of Hg2+ (Cys-S-Hg-S-Cys) appears to be the primary species of Hg2+ transported into cells. It appears that this conjugate is formed from GSH S-conjugates of Hg2+ (G-S-Hg-S-G), which are filtered freely at the glomerulus and may also be secreted from proximal tubular cells themselves. Once G-S-Hg-S-G enters the tubular lumen, it is thought to be degraded rapidly and sequentially by γ-glutamyltransferase and cysteinylglycinase to yield either Cys-S-Hg-S-Cys or a mixed S-conjugate of Hg2+ (Berndt et al. 1985; de Ceaurriz et al. 1994; Tanaka-Kagawa et al. 1993; Tanaka et al. 1990; Zalups 1995). In vitro studies in which isolated proximal tubules were perfused with various conjugates of Hg2+ also indicate that Cys-S-Hg-S-Cys is readily taken up at the luminal membrane of proximal tubular cells (Cannon et al. 2000, 2001).
Subsequent experiments in isolated, perfused proximal tubules showed that the luminal uptake of Cys-S-Hg-S-Cys involves at least one Na+-dependent and one Na+-independent amino acid carrier (Cannon et al. 2001). Given the similarities in size and shape between Cys-S-Hg-S-Cys and the amino acid cystine (Fig. 1), it was proposed that the luminal uptake of Cys-S-Hg-S-Cys into proximal tubular cells may involve one or more cystine transporters. Indeed, recent in vitro studies have shown that System b0,+ is involved in the Na+-independent transport of Cys-S-Hg-S-Cys into proximal tubular cells (Bridges et al. 2004). System b0,+ is a heterodimeric transporter, comprised of b0,+AT (SLC7A9) and rBAT (SLC3A1), that mediates the luminal uptake of cystine and neutral and basic amino acids into proximal tubular cells (Palacin et al. 1998, 2001). While additional studies have shown that Hcy S-conjugates of Hg2+ (Hcy-S-Hg-S-Hcy) are also transported by System b0,+ (Bridges and Zalups 2004), it appears that that mercuric conjugates of GSH (G-S-Hg-S-G), N-acetylcysteine (NAC-S-Hg-S-NAC), and cysteinylglycine (CysGly; CysGly-S-Hg-S-CysGly) are not substrates of this carrier (Bridges et al. 2004). Together, these data indicate that Cys-S-Hg-S-Cys and Hcy-S-Hg- S-Hcy gain access to proximal tubular cells at the luminal plasma membrane via System b0,+ (Fig. 2).
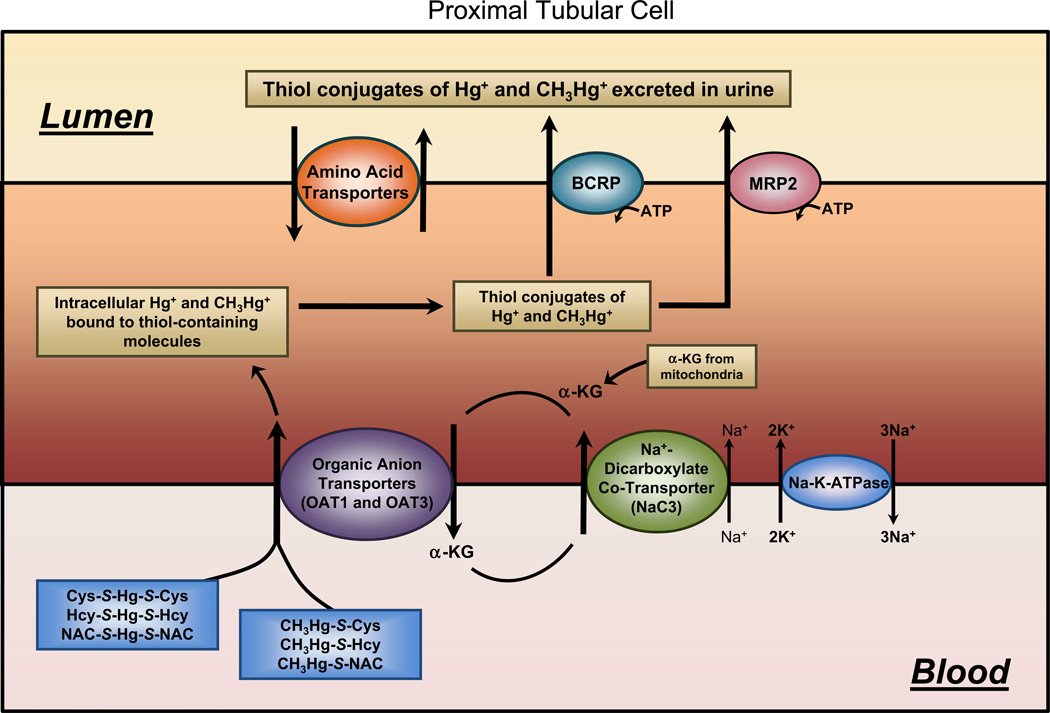
Fig. 2.
Diagram of a proximal tubular cell outlining the mechanisms involved in the uptake and export of Hg2+ and CH3Hg+. At the luminal plasma membrane of proximal tubular cells, it appears that amino acid transporters are the primary mechanisms by which mercuric ions are taken up into cells. At the basolateral membrane, organic anion transporters (OAT) appear to be primarily involved in this uptake. Mercuric species may be secreted from proximal tubular cells into the tubular lumen via the actions of the breast cancer resistance protein (BCRP) or the multidrug resistance-associated protein (MRP2) located on the luminal plasma membrane
Recently, a novel aspartate/glutamate transporter (AGT1; SLC7A13) has been identified in the luminal membrane of the epithelial cells lining the S3 segments of proximal tubules (Nagamori et al. 2016). This transporter has been shown to mediate the luminal uptake of cystine and it appears to co-localize with rBAT. The ability of AGT1 to transport mercuric species has not been shown, but we propose that this carrier may be involved in some aspect of the uptake of mercuric ions along the S3 segment of proximal tubules.
Another transporter that may be involved in the luminal uptake of mercuric species is OAT5 (SLC22A10). This carrier is localized in the apical plasma membrane of proximal tubular cells and has been shown to mediate the cellular uptake of a variety of organic anions from the tubular lumen (Anzai et al. 2005). To date, there are no data implicating OAT5 in the luminal uptake of mercuric species.
The basolateral uptake of Hg2+, whereby mercuric species are taken up from peritubular blood into proximal tubular cells, represents approximately 40–60 % of the uptake of Hg2+ by proximal tubules (Zalups and Minor 1995). Pretreatment of rats with para-aminohippurate (PAH), which specifically inhibits members of the organic anion transporter (OAT) family (Hosoyamada et al. 1999; Sekine et al. 1997, 2006), significantly reduced the renal uptake of Hg2+. Therefore, it appears that one or more OATs are involved in the basolateral uptake of Hg2+. OAT1 (SLC22A6) and OAT3 (SLC22A8) have both been localized in the basolateral plasma membrane of proximal tubular epithelial cells (Breljak et al. 2016; Kojima et al. 2002; Motohashi et al. 2002), and thus, they are likely candidates for the uptake of mercuric species at the basolateral membrane of these cells. Indeed, findings from MDCK II cells stably transfected with OAT1 showed that Cys and Hcy S-conjugates of Hg2+ are transportable substrates of this carrier (Aslamkhan et al. 2003; Zalups and Ahmad 2004; Zalups et al. 2004). In addition, experiments using Xenopus laevis oocytes demonstrated that OAT3 plays a role in the uptake of Cys-S-Hg-S-Cys (Aslamkhan et al. 2003; Zalups et al. 2004). Taken together, these data indicate that OAT1 and OAT3 are likely involved in the basolateral uptake of select mercuric species.
Once mercuric ions gain access to the intracellular compartment of cells, they bind to protein and non-protein thiols such as GSH and metallothionein (MT). The binding of Hg to larger thiol-containing molecules such as MT likely yields a non-transportable form of Hg and thus facilitates retention of mercuric ions within the cell. Exposure to Hg2+ has been shown to increase the mRNA expression of MT, which in turn leads to enhanced formation of Hg–MT complexes that are retained within renal epithelial cells (Zalups and Cherian 1992; Zalups et al. 1993; Zalups and Koropatnick 2000).
A small fraction of mercuric ions are transported out of proximal tubular cells without the use of a chelating agent (Bridges et al. 2011). However, the most efficient means of extracting mercuric ions from within target cells is through the use of a dithiol, metal chelator/complexing agent, such as 2,3-dimercapto-1-propanesulfonic acid (DMPS) or dimercaptosuccinic acid (DMSA; Fig. 2) (Aposhian 1983; Planas-Bohne 1981; Zalups and Bridges 2012). The ability of these two compounds to extract mercuric ions from target cells has been well characterized. The DMPS-mediated extraction of Hg2+ has been shown to occur via a direct secretory process whereby mercuric ions move directly into the tubular lumen for subsequent elimination in urine (Diamond et al. 1988). DMPS is taken up at the basolateral membrane of proximal tubular cells via OAT1, OAT3, and the sodium-dependent dicarboxylate transporter (NaC1; SCL13A2) (Bahn et al. 2002; Burckhardt et al. 2002; Islinger et al. 2001; Rodiger et al. 2010), while DMSA has been shown to be taken up by NaC1 (Burckhardt et al. 2002). It has been hypothesized that once internalized, DMPS and DMSA form complexes with intracellular Hg2+ (Zalups and Bridges 2012). These complexes appear to be transported across the luminal plasma membrane by MRP2, which is localized in the luminal plasma membrane of proximal tubular cells (Schaub et al. 1997, 1999). In vitro studies using inside-out membrane vesicles prepared from Sf9 cells transfected with human MRP2 provide direct evidence implicating MRP2 in the transport of both DMPS and DMSA-S-conjugates of Hg2+ (Bridges et al. 2008a, b). Studies in Mrp2-deficient (TR−) rats exposed to HgCl2 and treated subsequently with DMPS or DMSA (Bridges et al. 2008a, b) also provided strong in vivo evidence supporting a role for MRP2 in the secretion of mercuric ions. MRP4 (ABCC4) is also localized in the apical membrane of proximal tubular cells, and although it has a substrate specificity that is similar to that of MRP2, it does not appear to be involved in the transport of DMPS S-conjugates of Hg2+ (Bridges et al. 2013).
Secretion of mercuric ions at the luminal plasma membrane of proximal tubules may also occur via the breast cancer resistance protein (BCRP; ABCG2; Fig. 2). This carrier is localized in the apical plasma membrane of proximal tubular cells, and it has been shown to transport a wide variety of compounds (Leslie et al. 2005; Vlaming et al. 2009). A recent study using inside-out membrane vesicles from Sf9 cells overexpressing human BCRP provided direct evidence demonstrating that Cys and DMPS S-conjugates of Hg2+ are transportable substrates of BCRP (Bridges et al. 2015). In the same study, the disposition of mercuric ions in Bcrp-knockout rats was examined and the findings from these experiments demonstrated further the ability of Bcrp to transport mercuric ions.
Hepatic transport of Hg2+
Numerous in vivo studies in rats have shown that mercuric ions accumulate in hepatocytes following exposure to HgCl2 (Bridges et al. 2008a, b, 2011, 2014, 2015; Oliveira et al. 2016, 2015; Zalups 1993a, b). When mercuric ions are presented to the sinusoidal membrane of hepatocytes, they are most likely bound to non-protein thiols and plasma proteins, such as albumin, ferritin, and γ-globulins. Since these mercuric complexes may be quite large, there are only a few mechanisms that may be involved in the uptake of Hg2+ across the sinusoidal membrane of hepatocytes.
One of these mechanisms is endocytosis. Phagocytosis, pinocytosis, and receptor-mediated endocytosis have been shown to be involved in the uptake of molecules from sinusoidal blood into hepatocytes (Marbet et al. 2006; Rahner et al. 2000). Mercuric ions may be taken up into hepatocytes via an endocytic process mediated by albumin and/ or one of the iron-binding proteins. Thus, endocytosis of Hg–ferritin or Hg–albumin complexes may also serve as a route of entry of Hg2+ into hepatocytes. Indeed, it has been suggested that ferritin may serve as a detoxifying protein due its ability to bind the cationic forms of a number of elements (Joshi et al. 1989).
Another method by which Hg2+ may cross the sinusoidal membrane of hepatocytes may involve specific mechanisms of active transport in the plasma membrane. Various species of Hg2+ may enter hepatocytes at the sinusoidal membrane via transporters that mediate the uptake of endogenous compounds that are similar structurally to certain mercuric species. OAT2 (SLC22A7) has been localized in the sinusoidal membrane of hepatocytes (Simonson et al. 1994), and it is involved in the uptake of organic anions into hepatocytes. As a member of the OAT family, OAT2 shares some similarities with OAT1 and OAT3, which have been shown to transport Hg2+. However, there are also significant differences between these two carriers and OAT2 (Henjakovic et al. 2015), which may mean that OAT2 is not involved in the uptake of Hg2+ into hepatocytes. In addition, OATP1B1 (SLCO1B1), OATP1B3 (SLCO1B3), and OATP2B1 (SLCO2B1) have been identified in the sinusoidal membrane of hepatocytes (Pfeifer et al. 2014) where they mediate the hepatic uptake of organic anions. A role for these carriers in the uptake of mercuric species has not been reported. Also, numerous amino acid transporters have been identified in the sinusoidal membrane of hepatocytes (Bode 2001; Kilberg 1982; Wagner et al. 2001), yet it is currently unclear if any of these transporters are involved in the uptake of mercuric species at the sinusoidal membrane.
The transport of Hg2+ across the canalicular plasma membrane of hepatocytes has been studied extensively. A fraction of the Hg2+ that is transported across the canalicular membrane into bile appears to be dependent upon the concentration of GSH within hepatocytes (Ballatori and Clarkson 1983, 1984, 1985a, b; Dutczak and Ballatori 1992; Zalups and Lash 1997). A reduction in GSH levels in rats exposed intravenously to Hg2+ led to an accumulation of mercuric ions within hepatocytes (Zalups and Lash 1997). These findings suggest that intracellular mercuric ions may bind with intracellular GSH to form G-S-Hg-S-G, which may be transported out of hepatocytes at the canalicular membrane. Possible mechanisms for this transport include MRP2 and BCRP, which are both present in the canalicular membrane of hepatocytes (Buchler et al. 1996; Maliepaard et al. 2001). Since MRP2 appears to utilize GSH as a co-substrate for other compounds (Akerboom et al. 1991; Keppler et al. 1997), it was hypothesized that this carrier may transport G-S-Hg-S-G, and possibly other species of Hg2+. Recent studies using TR− rats, Mrp2- knockout mice, and inside-out membrane vesicles expressing MRP2 indicate that this carrier is capable of transporting Hg2+ as a conjugate of Cys, DMPS, or DMSA (Bridges et al. 2008a, b, 2011, 2013). Similarly, studies using Bcrp-knockout rats and inside-out membrane vesicles expressing BCRP have recently implicated BCRP in the transport of various mercuric species (Bridges et al. 2015).
Additional transport proteins on the canalicular membrane that may be involved in the transmembrane transport of mercuric species into bile include the multidrug resistance protein 1 (MDR1; P-glycoprotein; ABCB1) and MRP3. Both of these carriers are localized in the canalicular membrane of hepatocytes and have been shown to transport a wide variety of substrates (Konig et al. 1999; Thiebaut et al. 1987). Given that the substrate specificity of these carriers is similar to that of MRP2 (Pfeifer et al. 2014), it is logical to postulate that MDR1 and MRP3 may be capable of mediating the transport of mercuric species across the canalicular membrane.
Transport of Hg2+ in the placenta
Despite previous claims that Hg2+ does not cross the blood–placental barrier, several in vivo and in vitro studies have provided evidence for the transport and accumulation of Hg2+ in placentas and fetuses (Ask et al. 2002; Inouye and Kajiwara 1990; Oliveira et al. 2015). A recent study in pregnant Wistar rats exposed intravenously to two different doses of HgCl2 showed that the accumulation of mercuric ions in the placental and fetal organs was dependent on the time of exposure and the dose to which pregnant rats were exposed (Oliveira et al. 2015). Although maternal exposure to mercuric compounds during pregnancy can have significant detrimental effects on fetal development, the specific molecular mechanisms by which Hg2+ is transported across placental syncytiotrophoblasts have not been well defined. Experiments using brush-border membrane vesicles from human placenta have implicated amino acid transporters in the placental uptake of Hg2+ (Iioka et al. 1987). Considering the prevalence of amino acid transporters in the placenta (Jansson 2001; Kudo and Boyd 2002), it is possible that transportable forms of Hg2+ may utilize one or more amino acid transporters to gain entry to the intracellular compartment of placental syncytiotrophoblasts. Candidates for this transport include System b0,+, System L (LAT1; SLC7A5), and System B0,+ (ATB0,+; SLC6A14; Fig. 3). Additional studies are clearly needed to elucidate fully the mechanisms involved in the cellular uptake of mercuric species by placental syncytiotrophoblasts.
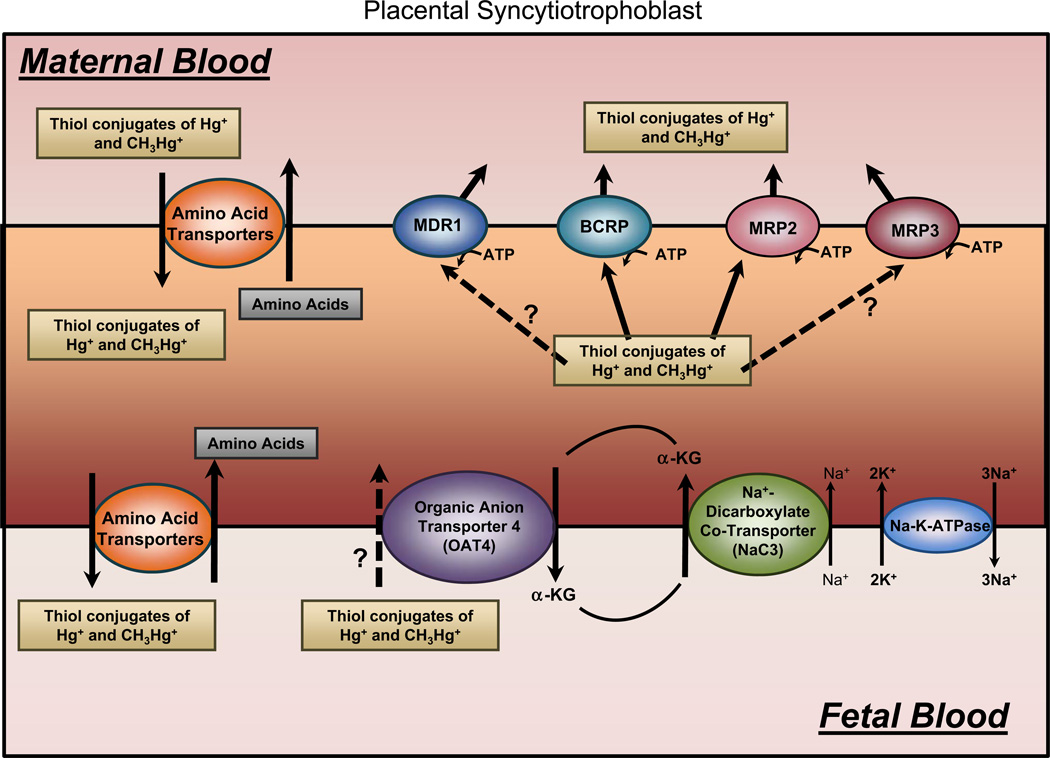
Fig. 3.
Diagram of a placental syncytiotrophoblast outlining the mechanisms potentially involved in the uptake and export of Hg2+ and CH3Hg+. Mercuric species may be taken up by placental syncytiotrophoblasts via amino acid transporters on the apical (maternal) side of the cell. Conjugates of Hg2+ and CH3Hg+ may remain in the intracellular compartment of the cell, or they may be exported into the interstitial space surrounding the fetal circulation via amino acid transporters on the basolateral side. In order to eliminate mercuric species from placental and fetal tissues, mercuric ions may be taken up by the organic anion transporter (OAT) 4 located in the basolateral membrane. Export at the luminal membrane may involve the multidrug resistance protein (MDR1), the breast cancer resistance protein (BCRP), and/or the multidrug resistance-associated protein 2 (MRP2) and MRP3
Currently, there are no data implicating a specific mechanism for the transport of Hg2+ on the basolateral membrane of syncytiotrophoblasts. However, based on data from studies using proximal tubular cells from the kidney, we suggest that carriers such as OAT4 (SLC22A11), System L (LAT2; SLC7A8), and System B0,+ (Fig. 3) may be involved in the transport of mercuric ions across the basolateral membrane of syncytiotrophoblasts into the fetal capillaries.
Transport of methylmercury
Intestinal transport of methylmercury
As mentioned previously, humans are exposed to methylmercury (CH3Hg+) primarily through the ingestion of contaminated food. Therefore, it is critical that the mechanisms involved in the intestinal absorption of CH3Hg+ are understood thoroughly.
X-ray absorption spectroscopy (XAS) showed that the primary species of CH3Hg+ in muscle tissue of fish is a Cys S-conjugate of CH3Hg+ (CH3Hg-S-Cys) (Harris et al. 2003). Therefore, following ingestion of fish, CH3Hg- S-Cys will likely be present in the lumen of the intestine. Moreover, Cys S-CH3Hg also appears to enter the lumen of the intestine via secretion into bile (Norseth and Clarkson 1971). Even so, recent in vivo studies suggest that the components of ingested food have a significant influence on the ability of mercuric ions to be taken up by enterocytes (Vazquez et al. 2014). Ingested food has a high concentration of thiol-containing molecules, which may combine with ingested CH3Hg+ to form various thiol S-conjugates. GSH S-conjugates of CH3Hg (CH3Hg-S-G) may also be present in the intestinal lumen (Urano et al. 1990). Experiments using ligated segments of rat intestine suggest that CH3Hg-S-G in the intestinal lumen is broken down by γ-glutamyltransferase to form cysteinylglycine (CysGly) conjugates of CH3Hg+ (CH3Hg-S-CysGly), which may be degraded further to yield CH3Hg-S-Cys (Urano et al. 1990). The majority of CH3Hg+ taken up by enterocytes appears to be in the form of CH3Hg-S-Cys (Vazquez et al. 2013, 2014). However, any CH3Hg-S-CysGly that escapes degradation may be taken up into cells via one of the peptide transporters such as the oligopeptide transporter, PEPT1 (SLC15A), which is present in the luminal plasma membrane of enterocytes (Estudante et al. 2013). The remaining CH3Hg-S-Cys may be taken up via intestinal amino acid transporters or organic anion transporters (Urano et al. 1990). Possible candidates for this transport include System b0,+ and the organic anion transporting polypeptide (OATP2B1; SLC22O), which have both been localized in the apical membrane of enterocytes (Dave et al. 2004; Estudante et al. 2013; Klaassen and Aleksunes 2010; Tamai and Nakanishi 2013). Currently, there are no published data linking PEPT1, OAT2B1, or System b0,+ to the intestinal uptake of any species of CH3Hg+.
CH3Hg+ gains access to the systemic circulation via carrier proteins in the basolateral membrane of enterocytes (Leaner and Mason 2002). MRP1, MRP3, MRP4, and MRP5 have been identified on the basolateral membrane of enterocytes (Estudante et al. 2013), and since conjugates of CH3Hg+ appear to be substrates of MRP2, it is possible that one or more MRPs are involved in export of mercuric species across the basolateral membrane of enterocytes. It has also been suggested that the efflux of CH3Hg+ at the basolateral membrane is regulated by the intracellular concentration of GSH (Foulkes 1993). Since some members of the MRP family require GSH as a co-substrate, it is feasible to suggest that the export of CH3Hg+ across the basolateral membrane of enterocytes may involve one or more MRPs.
Alternatively, indirect evidence from isolated, perfused catfish intestines suggests that a neutral amino acid transporter is involved in the export of CH3Hg-S-Cys at the basolateral membrane of enterocytes. Given that LAT2 is localized in the basolateral membrane of enterocytes (Dave et al. 2004) and because it appears to be involved in the transport of CH3Hg-S-Cys in other cell types, this carrier may be involved in the export of mercuric species across the basolateral membrane of enterocytes.
Transport of methylmercury in erythrocytes
Studies in Wistar rats exposed intravenously to a nonnephrotoxic dose of CH3Hg+ for 24 h have demonstrated that approximately 30 % of the administered dose of CH3Hg+ is detected in blood. Approximately 99 % of this CH3Hg+ is associated with erythrocytes, while the remaining 1 % is associated with the plasma fraction (Zalups and Bridges 2009). One possible explanation for this distribution could be the binding of CH3Hg+ to some component of the erythrocyte membrane. Indeed, studies in human erythrocyte ghosts demonstrated that the Na+, K+-ATPase is capable of binding seven molecules of CH3Hg+ while the Mg+, Ca+2-ATPase is capable of binding one molecule of CH3Hg+ (Berg and Miles 1979). Interestingly, binding of mercuric ions to the Na+, K+-ATPase has been shown to significantly inhibit the activity of this enzyme (Hahn et al. 1990).
Another possible explanation for this pattern of distribution is that mercuric ions are actively taken up into erythrocytes. Multiple transport systems appear to be involved in the uptake of CH3Hg+. It has been hypothesized that the primary mechanism involved in this transport is an organic anion transporter (Wu 1995, 1996). Additional mechanisms may include a d-glucose diffusive transporter, a cysteine-facilitated transporter, and/or a Cl− transporter (Wu 1995). Other studies showed that when erythrocytes were exposed to CH3Hg-S-G in the presence of probenecid, the uptake of CH3Hg+ was reduced significantly. This finding suggests that one or more OATs may play a role in the uptake of mercuric ions into erythrocytes (Wu 1997). The transport of CH3Hg+ into erythrocytes is an area of study that clearly needs further characterization.
Hepatic transport of methylmercury
Following absorption by the intestine, CH3Hg+ is delivered to the liver via portal blood. Little is known about the mechanisms by which CH3Hg+ is transported into hepatocytes at the sinusoidal membrane. In vivo studies in rats have shown that hepatic uptake and accumulation of CH3Hg+ is enhanced when Cys or GSH is either co-administered with or administered subsequently to CH3Hg+ (Thomas and Smith 1982). More recently, an in vitro study using cultured human hepatocytes (HepG2 cells) demonstrated that cellular uptake of CH3Hg+ occurs more rapidly when cells are exposed to CH3Hg+ as a conjugate of Cys than when exposed to CH3Hg+ alone (Wang et al. 2000). The actual mechanisms involved in this transport remain unknown.
The transport of CH3Hg+ across the canalicular membrane has been studied more extensively and is better defined. Findings from numerous studies indicate that transport of CH3Hg+ from hepatocytes into the biliary canaliculus occurs in association with GSH (Ballatori and Clarkson 1982, 1983, 1985a; Refsvik 1982; Refsvik and Norseth 1975). This is not surprising since the majority of CH3Hg+ within hepatocytes appears to be bound to GSH (Omata et al. 1978). Interestingly, increased hepatocellular levels of GSH correspond to an increase in the excretion of GSH and CH3Hg+ into bile (Magos et al. 1978). In contrast, a reduction in the hepatic and biliary levels of GSH corresponds to a reduced accumulation of CH3Hg+ in liver (Refsvik 1978). Therefore, it seems that the intracellular concentration of GSH significantly impacts the hepatobiliary transport of CH3Hg+. It has been postulated that CH3Hg-S-G, formed within hepatocytes, is transported across the canalicular membrane into bile. Since CH3Hg-S-G is similar in shape to GSH, it is possible that this conjugate may utilize a GSH transporter in the canalicular membrane for export out of hepatocytes. Indeed, it has been suggested that a GSH transport system in the canalicular membrane plays a significant role in the hepatobiliary secretion of CH3Hg-S-G (Dutczak and Ballatori 1994). MRP2, which is capable of transporting GSH, has since been identified in the canalicular membrane of hepatocytes (Ballatori and Dutczak 1994; Ballatori and Truong 1995; Fernandez-Checa et al. 1992, 1993; Garcia-Ruiz et al. 1992), and thus, it probably plays an important role in the export of CH3Hg+. Indeed, recent studies in TR− rats indicate that MRP2 is involved in the hepatobiliary elimination of mercuric ions following exposure to CH3Hg+ (Madejczyk et al. 2007; Zalups and Bridges 2009).
After secretion into bile, CH3Hg-S-G appears to be broken down by γ-glutamyltransferase and cysteinylglycinase to yield CH3Hg-S-Cys, which can be reabsorbed by cells lining the bile ducts as well as by enterocytes (Ballatori 1994; Dutczak and Ballatori 1992; Dutczak et al. 1991). Although the actual mechanisms involved in the uptake of mercuric ions along the biliary tree have not been determined, it is reasonable to hypothesize that CH3Hg-S-Cys utilizes one or more amino acid transporters in order to gain access to cells. A number of various amino acid transporters have been identified in the biliary tree (Bode 2001; Wagner et al. 2001); however, the exact membrane localization of each carrier remains unknown.
Transport of methylmercury in the brain
Similar to Hg2+, methylmercury (CH3Hg+) does not exist as a free, unbound cation in biological systems (Hughes 1957), but rather, it is found conjugated to protein and non-protein thiols (Clarkson 1993; Simpson et al. 1973; Simpson 1961). Exposure to CH3Hg+ can cause significant adverse effects in the brain and central nervous system (CNS) (ATSDR 1999; WHO 2000). Clinical signs and symptoms of CH3Hg+ intoxication are associated with the death of neuronal cells in specific regions of the brain. In adults, the visual cortex and the cerebellum, specifically the cerebellar granule cells, appear to be most sensitive to the effects of CH3Hg+ (Aschner and Syversen 2005). Owing to the severe neurological toxicity associated with exposure to CH3− Hg+, it is important to understand the cellular mechanisms that facilitate the movement of mercuric ions across the blood brain barrier. A number of studies have focused on the transport of CH3Hg+ in astrocytes, which are glial cells that provide support to the endothelial cells of the blood–brain barrier. Early studies utilizing homogenates of rat cerebrum demonstrated that GSH is the primary nonprotein thiol bound to CH3Hg+ (Thomas and Smith 1979). Subsequent studies in rats and primary cultures of bovine brain endothelial cells showed that co-administration of Cys with CH3Hg+ increased the uptake of CH3Hg+ into capillary endothelial cells of the blood–brain barrier (Aschner and Clarkson 1988, 1989; Hirayama 1980; Roos et al. 2010). Interestingly, the uptake of CH3Hg+ into endothelial cells was inhibited significantly by the neutral amino acid, phenylalanine (Hirayama 1975, 1980, 1985; Thomas and Smith 1982). In vivo studies in rat brain (Aschner and Clarkson 1988) and in vitro studies in bovine cerebral capillary endothelial cells (Aschner and Clarkson 1989; Heggland et al. 2009) demonstrated that neutral amino acids are capable of inhibiting the uptake of CH3Hg-S-Cys. These data collectively led to the hypothesis that CH3Hg-S-Cys is a transportable substrate of one or more neutral amino acid transporters in the luminal membrane of capillary endothelial cells. It was suggested that the structural similarities between CH3Hg-S-Cys and methionine (Jernelov 1973; Landner 1971) serve as an important factor in the transport of CH3Hg+ across the blood–brain barrier. One possible mechanism for this transport is the amino acid carrier, System L (Wagner et al. 2001).
System L is a heterodimeric protein, comprised of a light chain (either LAT1, SLC7A5, or LAT2, SLC7A8) and a heavy chain (4F2hc, SLC3A2) bound together with a disulfide bond (Chillaron et al. 2001; Wagner et al. 2001). LAT1 and LAT2 have been localized in the apical and basolateral plasma membranes, respectively, of endothelial cells lining the blood–brain barrier (Betz and Goldstein 1978). In addition, LAT1 and LAT2 have also been identified in cultured glial cells (Kim et al. 2004). System L is capable of transporting a broad range of neutral amino acids such as methionine (Met) and leucine (Leu) (Oldendorf 1973). Since CH3Hg-S-Cys and Met are similar in shape and size (Bridges and Zalups 2010) and because Met is a substrate of System L, it was hypothesized that CH3Hg-S-Cys may also be a substrate for System L. Indeed, in vivo and in vitro studies have provided strong evidence supporting the idea that CH3Hg-S-Cys is a transportable substrate of System L (Aschner et al. 1990, 1991; Kerper et al. 1992; Simmons-Willis et al. 2002; Zimmermann et al. 2013). Studies using epithelial cells from hamster ovary (CHOk1) demonstrated that overexpression of LAT1 enhances uptake of CH3Hg-S-Cys into cells. When the expression of LAT1 was reduced with small interference RNA (siRNA), a corresponding reduction in the intracellular burden of CH3Hg+ was observed, suggesting that CH3Hg-S-Cys is a transportable substrate of LAT1 (Yin et al. 2008). Site-directed mutagenesis of LAT1 has been shown to cause a reduction in the cellular uptake of CH3Hg+, providing additional evidence for the hypothesis that CH3Hg-S-Cys is a substrate of LAT1 (Boado et al. 2005). Taken together, these data provide strong support for the hypothesis that CH3Hg-S-Cys is taken into cells via one or both isoforms of System L. Interestingly, a recent study indicated that co-administration of methionine and CH3Hg+ enhanced motor impairments in mice compared with administration of CH3Hg+ alone (Zimmermann et al. 2014). This finding suggests that a trans-stimulatory phenomenon may occur whereby the presence of additional substrate enhances the activity of the transporter so that more substrate, including CH3Hg+, is taken up by the carrier. Indeed, the activities of System L and System b0,+ have been shown previously to be enhanced by trans-stimulation with amino acids (Bridges et al. 2004; Fraga et al. 2002; Segel et al. 1988). It is also important to note that Hcy S-conjugates of CH3Hg+ (CH3Hg-S-Hcy) may also be substrates of System L (Mokrzan et al. 1995).
Renal transport of methylmercury
Although CH3Hg+ does not accumulate in the kidney to the same degree as Hg2+, this form of Hg still accumulates in proximal tubular cells at significant levels and is capable of inducing significant detrimental effects (Friberg 1959; Magos et al. 1985; Magos and Butler 1976; Magos et al. 1981; McNeil et al. 1988; Norseth and Clarkson 1970a, b; Prickett et al. 1950). The majority of published evidence indicates that CH3Hg-S-Cys is the primary species of CH3Hg+ taken up by renal epithelial cells. It has been proposed that CH3Hg+, as a conjugate of GSH, enters the proximal tubular lumen where γ-glutamyltransferase and cysteinylglycinase act on the GSH molecule to yield CH3Hg-S-Cys (Berndt et al. 1985; de Ceaurriz and Ban 1990; Di Simplicio et al. 1990; Gregus et al. 1987; Mulder and Kostyniak 1985; Naganuma et al. 1988; Tanaka et al. 1990, 1991, 1992; Yasutake et al. 1989).
Given that CH3Hg-S-Cys is similar in shape and size to methionine, it is reasonable to suggest that CH3Hg-S-Cys can be taken up into proximal tubular cells via transport mechanisms that mediate the uptake of methionine. Indeed, data from an in vivo study utilizing oocytes from Xenopus laevis indicate that the amino acid transporter, System B0,+, is capable of mediating the transport of CH3Hg-S-Cys or a CH3Hg+ S-conjugate of Hcy (CH3Hg-S-Hcy) (Bridges and Zalups 2006). System B0,+ is localized in the luminal plasma membrane of proximal tubular cells (Gonska et al. 2000) and mediates the Na+-dependent transport of many neutral and cationic amino acids, including methionine (Nakanishi et al. 2001; Sloan and Mager 1999). It is interesting to note that the substrate specificity of System B0,+ is similar to that of System b0,+, which has been shown to mediate the Na+-independent transport of Cys-S-Hg-S-Cys and Hcy-S-Hg-S-Hcy (Bridges et al. 2004; Bridges and Zalups 2004).
CH3Hg+ present in peritubular blood may be taken up into renal tubular cells via mechanisms on the basolateral membrane (Tanaka et al. 1992). Studies utilizing cultured renal epithelial cells indicate that OAT1, which is localized in the basolateral membrane of proximal tubular epithelial cells (Kojima et al. 2002; Motohashi et al. 2002), mediates the uptake of Cys-, Hcy-, and NAC-S-conjugates of CH3Hg+ (Koh et al. 2002; Zalups and Ahmad 2005a, b, c).
Once mercuric ions gain access to the intracellular compartments of cells, they tend to form strong bonds with intracellular thiols. Therefore, in order to facilitate the export of mercuric ions from within cells, metal chelators/ complexing agents must be utilized. The ability of the metal chelators, DMPS and DMSA, to extract mercuric ions from cells following exposure to CH3Hg+ has been well documented (Aposhian 1983; Aposhian et al. 1992). However, the mechanisms by which this extraction occurs have been identified only recently. Data from in vivo studies using TR− rats exposed to CH3Hg+ and treated subsequently with NAC, DMPS, or DMSA indicate that MRP2 plays an important role in the transport of CH3Hg+ from within proximal tubular cells into the tubular lumen (Madejczyk et al. 2007; Zalups and Bridges 2009). Even in the absence of a metal chelator, MRP2 has been shown to play a role in the renal handling of Hg2+ (Bridges et al. 2011). More direct evidence was obtained from studies using membrane vesicles isolated from kidneys of TR− rats. Data from this study suggest that CH3Hg-S-NAC is a transportable substrate of MRP2 (Madejczyk et al. 2007). Additional experiments using inside-out membrane vesicles from Sf9 cells transfected with human MRP2 provide evidence indicating that MRP2 is capable of mediating the transport of DMPS- and DMSA-S-conjugates of CH3Hg+ (Zalups and Bridges 2009). Taken together, the data from these studies provide strong support for the hypothesis that MRP2 mediates the cellular export of mercuric ions following exposure to CH3Hg+ with subsequent chelation therapy.
Transport of methylmercury in the placenta
The deleterious effects of CH3Hg+ on fetal development have been recognized widely as one of the most serious toxicological consequences of CH3Hg+ exposure (Amin-Zaki et al. 1974; Davidson et al. 2008; Harada 1978, 1995; Inouye and Kajiwara 1988; Kajiwara and Inouye 1986, 1992; Matsumoto et al. 1965). Following maternal exposure to CH3Hg+, mercuric ions are taken up readily by the placenta and accumulate subsequently in both placental and fetal tissues (Ask et al. 2002; Inouye and Kajiwara 1988; Inouye et al. 1985). Despite the fact that fetal exposure to CH3Hg+ is highly significant clinically, little is known about the mechanism(s) by which mercuric ions are taken up and transported across the placenta. In vivo studies (Kajiwara et al. 1996) have shown that CH3Hg+ is transported across the rat placenta by a neutral amino acid carrier in a time- and dose-dependent manner. Interestingly, co-administration of CH3Hg+ with methionine increased the placental burden of CH3Hg+. It was proposed that this increase in uptake may be the result of the intracellular conversion of methionine to Cys, which may subsequently combine with CH3Hg+ to form a transportable species of CH3Hg+, i.e., CH3Hg-S-Cys. This conjugate may utilize a neutral amino acid carrier such as System L in order to gain access to placenta trophoblasts. Since the two isoforms of System L, LAT1 and LAT2, have been shown to mediate the transport of CH3Hg-S-Cys in astrocytes and endothelial cells of the blood–brain barrier (Aschner et al. 1990; Kerper et al. 1992; Mokrzan et al. 1995; Simmons-Willis et al. 2002), it is logical to hypothesize that this same carrier may also be involved in the transport of CH3Hg-S-Cys across the placenta. In the placenta, LAT1 is localized in the apical (maternal) plasma membrane of trophoblasts, while LAT2 is found in the basolateral (fetal) membrane (Kudo and Boyd 2002). A number of other carrier systems (e.g., amino acid, organic anion) have been identified in the placenta (Leazer and Klaassen 2003), and although the roles of these other transporters in the transport of CH3Hg+ have not been examined, they should be considered as possible mechanisms for this transport.
The mechanisms on the basolateral membrane of trophoblasts that mediate the uptake of mercuric ions from fetal circulation into the placenta have not yet been identified with certainty. One possible mechanism is OAT4, which has been localized in the basolateral membrane of placental trophoblasts (Cha et al. 2000; St-Pierre et al. 2000). Since other members of the OAT family have been shown to transport mercuric ions, it is possible that OAT4 may also be capable of mediating the transport of mercuric species. To date, the ability of OAT4 to transport mercuric ions has not been reported.
Recently, the ability of different chelators to extract mercuric ions from placental and fetal tissues was examined. In pregnant rats exposed to CH3Hg+, treatment with NAC, DMPS, or DMSA has been shown to facilitate the extraction of mercuric ions from fetal and placental tissues (Aremu et al. 2008; Bridges et al. 2009). As mentioned above, OAT4 may mediate the basolateral uptake of mercuric species into trophoblasts. MRP2, which is localized in the apical membrane of placental trophoblasts (St-Pierre et al. 2000), may mediate the export of mercuric ions from within trophoblasts into the maternal circulation. Currently, there are no direct data to support these theories.
Conclusions
Every effort has been made to summarize the current body of literature related to the transport of mercuric ions in key target organ systems and tissues. Despite the growing body of literature pertaining to the handling of various species of mercury within the body, there are numerous gaps in our knowledge. A great deal of research is still required in order to understand more completely the mechanisms involved in the movement of mercuric ions across cellular plasma membranes. A thorough understanding of the mechanisms participating in the transport of mercuric ions at the cellular and molecular levels may lead to advances in treatment regimens for patients exposed to or intoxicated by mercury.
Acknowledgments
This work was supported by Grants from the National Institutes of Health (National Institute of Environmental Health Sciences) awarded to C.C. Bridges (ES015511) and R.K. Zalups (ES05980 and ES11288).
References
- Afonso JF, De Alvarez RR. Effects of mercury on human gestation. Am J Obstet Gynecol. 1960;80:145–154. doi: 10.1016/s0002-9378(16)36432-8. [DOI] [PubMed] [Google Scholar]
- Akerboom TP, Narayanaswami V, Kunst M, Sies H. ATP-dependent S-(2,4-dinitrophenyl)glutathione transport in canalicular plasma membrane vesicles from rat liver. J Biol Chem. 1991;266(20):13147–13152. [PubMed] [Google Scholar]
- Ambrose JL, Gratz LE, Jaffe DA, et al. Mercury emission ratios from coal-fired power plants in the Southeastern United States during NOMADSS. Environ Sci Technol. 2015;49(17):10389–10397. doi: 10.1021/acs.est.5b01755. [DOI] [PubMed] [Google Scholar]
- Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Intra-uterine methylmercury poisoning in Iraq. Pediatrics. 1974;54(5):587–595. [PubMed] [Google Scholar]
- Andres S, Laporte JM, Mason RP. Mercury accumulation and flux across the gills and the intestine of the blue crab (Callinectes sapidus) Aquat Toxicol. 2002;56(4):303–320. doi: 10.1016/s0166-445x(01)00228-4. [DOI] [PubMed] [Google Scholar]
- Anzai N, Jutabha P, Enomoto A, et al. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther. 2005;315(2):534–544. doi: 10.1124/jpet.105.088583. [DOI] [PubMed] [Google Scholar]
- Aposhian HV. DMSA and DMPS—water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983;23:193–215. doi: 10.1146/annurev.pa.23.040183.001205. [DOI] [PubMed] [Google Scholar]
- Aposhian HV, Maiorino RM, Rivera M, et al. Human studies with the chelating agents, DMPS and DMSA. J Toxicol Clin Toxicol. 1992;30(4):505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- Aremu DA, Madejczyk MS, Ballatori N. N-acetylcysteine as a potential antidote and biomonitoring agent of methylmercury exposure. Environ Health Perspect. 2008;116(1):26–31. doi: 10.1289/ehp.10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462(1):31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol. 1989;64(3):293–297. doi: 10.1111/j.1600-0773.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T. Methylmercury: recent advances in the understanding of its neurotoxicity. Ther Drug Monit. 2005;27(3):278–283. doi: 10.1097/01.ftd.0000160275.85450.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Goderie S, Kimelberg HK. Methyl-mercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res. 1990;521(1–2):221–228. doi: 10.1016/0006-8993(90)91546-s. [DOI] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Kimelberg HK. Interactions of methylmercury with rat primary astrocyte cultures: methylmercury efflux. Brain Res. 1991;554(1–2):10–14. doi: 10.1016/0006-8993(91)90165-r. [DOI] [PubMed] [Google Scholar]
- Ashe WF, Largent EJ, Dutra FR, Hubbard DM, Blackstone M. Behavior of mercury in the animal organism following inhalation. AMA Arch Ind Hyg Occup Med. 1953;7(1):19–43. [PubMed] [Google Scholar]
- Ask K, Akesson A, Berglund M, Vahter M. Inorganic mercury and methylmercury in placentas of Swedish women. Environ Health Perspect. 2002;110(5):523–526. doi: 10.1289/ehp.02110523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslamkhan AG, Han YH, Yang XP, Zalups RK, Pritchard JB. Human renal organic anion transporter 1-dependent uptake and toxicity of mercuric-thiol conjugates in Madin-Darby canine kidney cells. Mol Pharmacol. 2003;63(3):590–596. doi: 10.1124/mol.63.3.590. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) U.S. Department of Health and Humans Services. Atlanta, GA: Public Health Service; 1999. Toxicological profile for Mercury. [Google Scholar]
- Bahn A, Knabe M, Hagos Y, et al. Interaction of the metal chelator 2,3-dimercapto-1-propanesulfonate with the rabbit multi-specific organic anion transporter 1 (rbOAT1) Mol Pharmacol. 2002;62(5):1128–1136. doi: 10.1124/mol.62.5.1128. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Glutathione mercaptides as transport forms of metals. Adv Pharmacol. 1994;27:271–298. doi: 10.1016/s1054-3589(08)61036-4. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Developmental changes in the biliary excretion of methylmercury and glutathione. Science. 1982;216(4541):61–63. doi: 10.1126/science.7063871. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Biliary transport of glutathione and methylmercury. Am J Physiol. 1983;244(4):G435–G441. doi: 10.1152/ajpgi.1983.244.4.G435. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Dependence of biliary secretion of inorganic mercury on the biliary transport of glutathione. Biochem Pharmacol. 1984;33(7):1093–1098. doi: 10.1016/0006-2952(84)90519-7. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Biliary secretion of glutathione and of glutathione-metal complexes. Fundam Appl Toxicol. 1985a;5(5):816–831. doi: 10.1016/0272-0590(85)90165-4. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Clarkson TW. Sulfobromophthalein inhibition of glutathione and methylmercury secretion into bile. Am J Physiol. 1985b;248(2 Pt 1):G238–G245. doi: 10.1152/ajpgi.1985.248.2.G238. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Dutczak WJ. Identification and characterization of high and low affinity transport systems for reduced glutathione in liver cell canalicular membranes. J Biol Chem. 1994;269(31):19731–19737. [PubMed] [Google Scholar]
- Ballatori N, Truong AT. Multiple canalicular transport mechanisms for glutathione S-conjugates. Transport on both ATP- and voltage-dependent carriers. J Biol Chem. 1995;270(8):3594–3601. doi: 10.1074/jbc.270.8.3594. [DOI] [PubMed] [Google Scholar]
- Berg GG, Miles EF. Mechanisms of inhibition of active transport ATPases by mercurials. Chem Biol Interact. 1979;27(2–3):199–219. doi: 10.1016/0009-2797(79)90126-1. [DOI] [PubMed] [Google Scholar]
- Berlin M, Gibson S. Renal uptake, excretion, and retention of mercury. I. A study in the rabbit during infusion of mercuric chloride. Arch Environ Health. 1963;6:617–625. doi: 10.1080/00039896.1963.10663450. [DOI] [PubMed] [Google Scholar]
- Berlin M, Ullberg S. Accumulation and retention of mercury in the mouse. I. An autoradiographic study after a single intravenous injection of mercuric chloride. Arch Environ Health. 1963;6:589–601. doi: 10.1080/00039896.1963.10663447. [DOI] [PubMed] [Google Scholar]
- Berndt WO, Baggett JM, Blacker A, Houser M. Renal glutathione and mercury uptake by kidney. Fundam Appl Toxicol. 1985;5(5):832–839. doi: 10.1016/0272-0590(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Betz AL, Goldstein GW. Polarity of the blood–brain barrier: neutral amino acid transport into isolated brain capillaries. Science. 1978;202(4364):225–227. doi: 10.1126/science.211586. [DOI] [PubMed] [Google Scholar]
- Bluhm RE, Breyer JA, Bobbitt RG, Welch LW, Wood AJ, Branch RA. Elemental mercury vapour toxicity, treatment, and prognosis after acute, intensive exposure in chloralkali plant workers. Part II: hyperchloraemia and genitourinary symptoms. Hum Exp Toxicol. 1992;11(3):211–215. doi: 10.1177/096032719201100309. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Chu C, Ogoshi F, Wise P, Pardridge WM. Site-directed mutagenesis of cysteine residues of large neutral amino acid transporter LAT1. Biochim Biophys Acta. 2005;1715(2):104–110. doi: 10.1016/j.bbamem.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Bode BP. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131(9 Suppl):2475S–2485S. doi: 10.1093/jn/131.9.2475S. discussion 2486S-7S. [DOI] [PubMed] [Google Scholar]
- Breljak D, Ljubojevic M, Hagos Y, et al. Distribution of the organic anion transporters NaDC3 and OAT1-3 along the human nephron. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00113.2016. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Homocysteine, system b0,+ and the renal epithelial transport and toxicity of inorganic mercury. Am J Pathol. 2004;165(4):1385–1394. doi: 10.1016/S0002-9440(10)63396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. System B0,+ and the transport of thiol-s-conjugates of methylmercury. J Pharmacol Exp Ther. 2006;319(2):948–956. doi: 10.1124/jpet.106.109371. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Transport of inorganic mercury and methylmercury in target tissues and organs. J Toxicol Environ Health Part B. 2010;13(5):385–410. doi: 10.1080/10937401003673750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Bauch C, Verrey F, Zalups RK. Mercuric conjugates of cysteine are transported by the amino acid transporter system b(0, +): implications of molecular mimicry. J Am Soc Nephrol. 2004;15(3):663–673. doi: 10.1097/01.ASN.0000113553.62380.F5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the DMPS− and DMSA-mediated elimination of mercury in TR(−) and control rats exposed to thiol S-conjugates of inorganic mercury. Toxicol Sci. 2008a;105(1):211–220. doi: 10.1093/toxsci/kfn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Multidrug resistance proteins and the renal elimination of inorganic mercury mediated by 2,3-dimercaptopropane-1-sulfonic acid and meso-2,3-dimercaptosuccinic acid. J Pharmacol Exp Ther. 2008b;324(1):383–390. doi: 10.1124/jpet.107.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Effect of DMPS and DMSA on the placental and fetal disposition of methylmercury. Placenta. 2009;30(9):800–805. doi: 10.1016/j.placenta.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. MRP2 and the handling of mercuric ions in rats exposed acutely to inorganic and organic species of mercury. Toxicol Appl Pharmacol. 2011;251(1):50–58. doi: 10.1016/j.taap.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, van den Heuvel JJ, Russel FG, Zalups RK. Glutathione status and the renal elimination of inorganic mercury in the Mrp2(−−) mouse. PLoS One. 2013;8(9):e73559. doi: 10.1371/journal.pone.0073559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Joshee L, Zalups RK. Aging and the disposition and toxicity of mercury in rats. Exp Gerontol. 2014;53:31–39. doi: 10.1016/j.exger.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK, Joshee L. Toxicological significance of renal Bcrp: another potential transporter in the elimination of mercuric ions from proximal tubular cells. Toxicol Appl Pharmacol. 2015;285(2):110–117. doi: 10.1016/j.taap.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchler M, Konig J, Brom M, et al. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271(25):15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- Burckhardt BC, Drinkuth B, Menzel C, et al. The renal Na(+)-dependent dicarboxylate transporter, NaDC-3, translocates dimethyl- and disulfhydryl-compounds and contributes to renal heavy metal detoxification. J Am Soc Nephrol. 2002;13(11):2628–2638. doi: 10.1097/01.asn.0000033463.58641.f9. [DOI] [PubMed] [Google Scholar]
- Cannon VT, Barfuss DW, Zalups RK. Molecular homology and the luminal transport of Hg2+ in the renal proximal tubule. J Am Soc Nephrol. 2000;11(3):394–402. doi: 10.1681/ASN.V113394. [DOI] [PubMed] [Google Scholar]
- Cannon VT, Zalups RK, Barfuss DW. Amino acid transporters involved in luminal transport of mercuric conjugates of cysteine in rabbit proximal tubule. J Pharmacol Exp Ther. 2001;298(2):780–789. [PubMed] [Google Scholar]
- Canonne-Hergaux F, Gruenheid S, Ponka P, Gros P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood. 1999;93(12):4406–4417. [PubMed] [Google Scholar]
- Carmignani M, Boscolo P, Artese L, et al. Renal mechanisms in the cardiovascular effects of chronic exposure to inorganic mercury in rats. Br J Ind Med. 1992;49(4):226–232. doi: 10.1136/oem.49.4.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cember H, Gallagher P, Faulkner A. Distribution of mercury among blood fractions and serum proteins. Am Ind Hyg Assoc J. 1968;29(3):233–237. doi: 10.1080/00028896809342994. [DOI] [PubMed] [Google Scholar]
- Cha SH, Sekine T, Kusuhara H, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275(6):4507–4512. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- Chillaron J, Roca R, Valencia A, Zorzano A, Palacin M. Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am J Physiol Renal Physiol. 2001;281(6):F995–F1018. doi: 10.1152/ajprenal.2001.281.6.F995. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol. 1993;33:545–571. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. Studies on the binding of mercury in tissue homogenates. Biochem J. 1966;99(1):62–70. doi: 10.1042/bj0990062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36(8):609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F. Expression of heteromeric amino acid transporters along the murine intestine. J Physiol. 2004;558(Pt 2):597–610. doi: 10.1113/jphysiol.2004.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Strain JJ, Myers GJ, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology. 2008;29(5):767–775. doi: 10.1016/j.neuro.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ceaurriz J, Ban M. Role of gamma-glutamyltranspeptidase and beta-lyase in the nephrotoxicity of hexachloro-1,3-butadiene and methyl mercury in mice. Toxicol Lett. 1990;50(2–3):249–256. doi: 10.1016/0378-4274(90)90017-g. [DOI] [PubMed] [Google Scholar]
- de Ceaurriz J, Payan JP, Morel G, Brondeau MT. Role of extracellular glutathione and gamma-glutamyltranspeptidase in the disposition and kidney toxicity of inorganic mercury in rats. J Appl Toxicol. 1994;14(3):201–206. doi: 10.1002/jat.2550140310. [DOI] [PubMed] [Google Scholar]
- Di Simplicio P, Gorelli M, Ciuffreda P, Leonzio C. The relationship between gamma-glutamyl transpeptidase and Hg levels in Se/Hg antagonism in mouse liver and kidney. Pharmacol Res. 1990;22(4):515–526. doi: 10.1016/1043-6618(90)90757-5. [DOI] [PubMed] [Google Scholar]
- Diamond GL, Klotzbach JM, Stewart JR. Complexing activity of 2,3-dimercapto-1-propanesulfonate and its disulfide auto-oxidation product in rat kidney. J Pharmacol Exp Ther. 1988;246(1):270–274. [PubMed] [Google Scholar]
- Dutczak WJ, Ballatori N. gamma-glutamyltransferase-dependent biliary-hepatic recycling of methyl mercury in the guinea pig. J Pharmacol Exp Ther. 1992;262(2):619–623. [PubMed] [Google Scholar]
- Dutczak WJ, Ballatori N. Transport of the glutathione-methylmercury complex across liver canalicular membranes on reduced glutathione carriers. J Biol Chem. 1994;269(13):9746–9751. [PubMed] [Google Scholar]
- Dutczak WJ, Clarkson TW, Ballatori N. Biliary-hepatic recycling of a xenobiotic: gallbladder absorption of methyl mercury. Am J Physiol. 1991;260(6 Pt 1):G873–G880. doi: 10.1152/ajpgi.1991.260.6.G873. [DOI] [PubMed] [Google Scholar]
- Endo T, Nakaya S, Kimura R, Murata T. Gastrointestinal absorption of inorganic mercuric compounds in vivo and in situ. Toxicol Appl Pharmacol. 1984;74(2):223–229. doi: 10.1016/0041-008x(84)90146-7. [DOI] [PubMed] [Google Scholar]
- Estudante M, Morais JG, Soveral G, Benet LZ. Intestinal drug transporters: an overview. Adv Drug Deliv Rev. 2013;65(10):1340–1356. doi: 10.1016/j.addr.2012.09.042. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Takikawa H, Horie T, Ookhtens M, Kaplowitz N. Canalicular transport of reduced glutathione in normal and mutant Eisai hyperbilirubinemic rats. J Biol Chem. 1992;267(3):1667–1673. [PubMed] [Google Scholar]
- Fernandez-Checa JC, Ookhtens M, Kaplowitz N. Selective induction by phenobarbital of the electrogenic transport of glutathione and organic anions in rat liver canalicular membrane vesicles. J Biol Chem. 1993;268(15):10836–10841. [PubMed] [Google Scholar]
- Foulkes EC. Metallothionein and glutathione as determinants of cellular retention and extrusion of cadmium and mercury. Life Sci. 1993;52(20):1617–1620. doi: 10.1016/0024-3205(93)90042-2. [DOI] [PubMed] [Google Scholar]
- Foulkes EC. Transport of toxic heavy metals across cell membranes. Proc Soc Exp Biol Med. 2000;223(3):234–240. doi: 10.1046/j.1525-1373.2000.22334.x. [DOI] [PubMed] [Google Scholar]
- Fraga S, Serrao MP, Soares-da-Silva P. L-type amino acid transporters in two intestinal epithelial cell lines function as exchangers with neutral amino acids. J Nutr. 2002;132(4):733–738. doi: 10.1093/jn/132.4.733. [DOI] [PubMed] [Google Scholar]
- Friberg L. Studies on the metabolism of mercuric chloride and methyl mercury dicyandiamide; experiments on rats given subcutaneous injections with radioactive mercury (Hg203) AMA Arch Ind Health. 1959;20(1):42–49. [PubMed] [Google Scholar]
- Friberg L, Odeblad E, Forssman S. Distribution of two mercury compounds in rabbits after a single subcutaneous injection; a radiometric and autoradiographic study of the distribution of mercuric chloride and phenylmercuric acetate. AMA Arch Ind Health. 1957;16(2):163–168. [PubMed] [Google Scholar]
- Friedman HL. Relationship between chemical structure and biological activity in mercurial compounds. Ann N Y Acad Sci. 1957;65(5):461–470. doi: 10.1111/j.1749-6632.1956.tb36651.x. [DOI] [PubMed] [Google Scholar]
- Fuhr BJ, Rabenstein DL. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. The binding of cadmium, zinc, lead, and mercury by glutathione. J Am Chem Soc. 1973;95(21):6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Ganapathy ME, Leibach FH. Intestinal transport of peptides and amino acids. New York: Academic Press; 2001. [Google Scholar]
- Garcia-Ruiz C, Fernandez-Checa JC, Kaplowitz N. Bidirectional mechanism of plasma membrane transport of reduced glutathione in intact rat hepatocytes and membrane vesicles. J Biol Chem. 1992;267(31):22256–22264. [PubMed] [Google Scholar]
- Gibb H, O’Leary KG. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ Health Perspect. 2014;122(7):667–672. doi: 10.1289/ehp.1307864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonska T, Hirsch JR, Schlatter E. Amino acid transport in the renal proximal tubule. Amino Acids. 2000;19(2):395–407. doi: 10.1007/s007260070019. [DOI] [PubMed] [Google Scholar]
- Gregus Z, Stein AF, Klaassen CD. Effect of inhibition of gamma-glutamyltranspeptidase on biliary and urinary excretion of glutathione-derived thiols and methylmercury. J Pharmacol Exp Ther. 1987;242(1):27–32. [PubMed] [Google Scholar]
- Hahn LJ, Kloiber R, Leininger RW, Vimy MJ, Lorscheider FL. Whole-body imaging of the distribution of mercury released from dental fillings into monkey tissues. FASEB J. 1990;4(14):3256–3260. doi: 10.1096/fasebj.4.14.2227216. [DOI] [PubMed] [Google Scholar]
- Harada M. Congenital minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18(2):285–288. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25(1):1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Harris HH, Pickering IJ, George GN. The chemical form of mercury in fish. Science. 2003;301(5637):1203. doi: 10.1126/science.1085941. [DOI] [PubMed] [Google Scholar]
- Heggland I, Kaur P, Syversen T. Uptake and efflux of methylmercury in vitro: comparison of transport mechanisms in C6, B35 and RBE4 cells. Toxicology In Vitro. 2009;23(6):1020–1027. doi: 10.1016/j.tiv.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Henjakovic M, Hagos Y, Krick W, Burckhardt G, Burckhardt BC. Human organic anion transporter 2 is distinct from organic anion transporters 1 and 3 with respect to transport function. Am J Physiol Renal Physiol. 2015;309(10):F843–F851. doi: 10.1152/ajprenal.00140.2015. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Transport mechanism of methyl mercury. Intestinal absorption, biliary excretion and distribution of methyl mercury. Kumamoto Med J. 1975;28(4):151–163. [PubMed] [Google Scholar]
- Hirayama K. Effect of amino acids on brain uptake of methyl mercury. Toxicol Appl Pharmacol. 1980;55(2):318–323. doi: 10.1016/0041-008x(80)90093-9. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Effects of combined administration of thiol compounds and methylmercury chloride on mercury distribution in rats. Biochem Pharmacol. 1985;34(11):2030–2032. doi: 10.1016/0006-2952(85)90328-4. [DOI] [PubMed] [Google Scholar]
- Holzman DC. Mercury emissions not shrinking as forecast. Environ Health Perspect. 2010;118(5):A198. doi: 10.1289/ehp.118-a198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999;276(1 Pt 2):F122–F128. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- Hoyle I, Handy RD. Dose-dependent inorganic mercury absorption by isolated perfused intestine of rainbow trout, Oncorhynchus mykiss, involves both amiloride-sensitive and energy-dependent pathways. Aquat Toxicol. 2005;72(1–2):147–159. doi: 10.1016/j.aquatox.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Hughes WL. A physicochemical rationale for the biological activity of mercury and its compounds. Ann N Y Acad Sci. 1957;65(5):454–460. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- Hultman P, Enestrom S. Localization of mercury in the kidney during experimental acute tubular necrosis studied by the cytochemical Silver Amplification method. Br J Exp Pathol. 1986;67(4):493–503. [PMC free article] [PubMed] [Google Scholar]
- Hultman P, Enestrom S. Dose–response studies in murine mercury-induced autoimmunity and immune-complex disease. Toxicol Appl Pharmacol. 1992;113(2):199–208. doi: 10.1016/0041-008x(92)90115-9. [DOI] [PubMed] [Google Scholar]
- Hultman P, Enestrom S, von Schenck H. Renal handling of inorganic mercury in mice. The early excretion phase following a single intravenous injection of mercuric chloride studied by the silver amplification method. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(3):209–224. [PubMed] [Google Scholar]
- Hultman P, Bell LJ, Enestrom S, Pollard KM. Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol. 1992;65(2):98–109. doi: 10.1016/0090-1229(92)90212-7. [DOI] [PubMed] [Google Scholar]
- Iioka H, Moriyama I, Oku M, et al. The effect of inorganic mercury on placental amino acid transport using microvillous membrane vesicles. Nippon Sanka Fujinka Gakkai Zasshi. 1987;39(2):202–206. [PubMed] [Google Scholar]
- Ilback NG, Frisk P, Tallkvist J, Gadhasson IL, Blomberg J, Friman G. Gastrointestinal uptake of trace elements are changed during the course of a common human viral (Coxsackievirus B3) infection in mice. J Trace Elem Med Biol. 2008;22(2):120–130. doi: 10.1016/j.jtemb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Inouye M, Kajiwara Y. Developmental disturbances of the fetal brain in guinea-pigs caused by methylmercury. Arch Toxicol. 1988;62(1):15–21. doi: 10.1007/BF00316251. [DOI] [PubMed] [Google Scholar]
- Inouye M, Kajiwara Y. Strain difference of the mouse in manifestation of hydrocephalus following prenatal methylmercury exposure. Teratology. 1990;41(2):205–210. doi: 10.1002/tera.1420410212. [DOI] [PubMed] [Google Scholar]
- Inouye M, Murao K, Kajiwara Y. Behavioral and neuropathological effects of prenatal methylmercury exposure in mice. Neurobehav Toxicol Teratol. 1985;7(3):227–232. [PubMed] [Google Scholar]
- Islinger F, Gekle M, Wright SH. Interaction of 2,3-dimercapto1-propane sulfonate with the human organic anion transporter hOAT1. J Pharmacol Exp Ther. 2001;299(2):741–747. [PubMed] [Google Scholar]
- Itagaki S, Chiba M, Kobayashi M, Hirano T, Iseki K. Contribution of multidrug resistance-associated protein 2 to secretory intestinal transport of organic anions. Biol Pharm Bull. 2008;31(1):146–148. doi: 10.1248/bpb.31.146. [DOI] [PubMed] [Google Scholar]
- Jaffe KM, Shurtleff DB, Robertson WO. Survival after acute mercury vapor poisoning. Am J Dis Child. 1983;137(8):749–751. doi: 10.1001/archpedi.1983.02140340033008. [DOI] [PubMed] [Google Scholar]
- Jansson T. Amino acid transporters in the human placenta. Pediatr Res. 2001;49(2):141–147. doi: 10.1203/00006450-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Jernelov AA. A new biochemical pathway for the methylation of mercury and some ecolgical implications. Springfield: Thomas; 1973. [Google Scholar]
- Joshi JG, Sczekan SR, Fleming JT. Ferritin—a general metal detoxicant. Biol Trace Elem Res. 1989;21:105–110. doi: 10.1007/BF02917242. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Inouye M. Effects of methylmercury and mercuric chloride on preimplantation mouse embryos in vivo. Teratology. 1986;33(2):231–237. doi: 10.1002/tera.1420330210. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Inouye M. Inhibition of implantation caused by methylmercury and mercuric chloride in mouse embryos in vivo. Bull Environ Contam Toxicol. 1992;49(4):541–546. doi: 10.1007/BF00196296. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70(5):310–314. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- Keppler D, Leier I, Jedlitschky G. Transport of glutathione conjugates and glucuronides by the multidrug resistance proteins MRP1 and MRP2. Biol Chem. 1997;378(8):787–791. [PubMed] [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood–brain barrier by an amino acid carrier. Am J Physiol. 1992;262(5 Pt 2):R761–R765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Kilberg MS. Amino acid transport in isolated rat hepatocytes. J Membr Biol. 1982;69(1):1–12. doi: 10.1007/BF01871236. [DOI] [PubMed] [Google Scholar]
- Kim DK, Kim IJ, Hwang S, et al. System L-amino acid transporters are differently expressed in rat astrocyte and C6 glioma cells. Neurosci Res. 2004;50(4):437–446. doi: 10.1016/j.neures.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh AS, Simmons-Willis TA, Pritchard JB, Grassl SM, Ballatori N. Identification of a mechanism by which the methylmercury antidotes N-acetylcysteine and dimercaptopropanesulfonate enhance urinary metal excretion: transport by the renal organic anion transporter-1. Mol Pharmacol. 2002;62(4):921–926. doi: 10.1124/mol.62.4.921. [DOI] [PubMed] [Google Scholar]
- Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol. 2002;13(4):848–857. doi: 10.1681/ASN.V134848. [DOI] [PubMed] [Google Scholar]
- Konig J, Rost D, Cui Y, Keppler D. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29(4):1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- Kristensen AK, Thomsen JF, Mikkelsen S. A review of mercury exposure among artisanal small-scale gold miners in developing countries. Int Arch Occup Environ Health. 2014;87(6):579–590. doi: 10.1007/s00420-013-0902-9. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CA. Human placental amino acid transporter genes: expression and function. Reproduction. 2002;124(5):593–600. doi: 10.1530/rep.0.1240593. [DOI] [PubMed] [Google Scholar]
- Landner L. Biochemical model for the biological methylation of mercury suggested from methylation studies in vivo with Neurospora crassa. Nature. 1971;230(5294):452–454. doi: 10.1038/230452a0. [DOI] [PubMed] [Google Scholar]
- Laporte JM, Andres S, Mason RP. Effect of ligands and other metals on the uptake of mercury and methylmercury across the gills and the intestine of the blue crab (Callinectes sapidus) Comp Biochem Physiol C Toxicol Pharmacol. 2002;131(2):185–196. doi: 10.1016/s1532-0456(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Lau S, Sarkar B. Inorganic mercury(II)-binding components in normal human blood serum. J Toxicol Environ Health. 1979;5(5):907–916. doi: 10.1080/15287397909529800. [DOI] [PubMed] [Google Scholar]
- Leaner JJ, Mason RP. Methylmercury accumulation and fluxes across the intestine of channel catfish, Ictalurus punctatus. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132(2):247–259. doi: 10.1016/s1532-0456(02)00072-8. [DOI] [PubMed] [Google Scholar]
- Leazer TM, Klaassen CD. The presence of xenobiotic transporters in rat placenta. Drug Metab Dispos. 2003;31(2):153–167. doi: 10.1124/dmd.31.2.153. [DOI] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Liang S, Wang Y, Cinnirella S, Pirrone N. Atmospheric mercury footprints of nations. Environ Sci Technol. 2015;49(6):3566–3574. doi: 10.1021/es503977y. [DOI] [PubMed] [Google Scholar]
- Lin JL, Lim PS. Massive oral ingestion of elemental mercury. J Toxicol Clin Toxicol. 1993;31(3):487–492. doi: 10.3109/15563659309000417. [DOI] [PubMed] [Google Scholar]
- Lundgren KD, Swensson A. Occupational poisoning by alkyl mercury compounds. J Ind Hyg Toxicol. 1949;31(4):190–200. [PubMed] [Google Scholar]
- Madejczyk MS, Aremu DA, Simmons-Willis TA, Clarkson TW, Ballatori N. Accelerated urinary excretion of methylmercury following administration of its antidote N-acetylcysteine requires Mrp2/Abcc2, the apical multidrug resistance-associated protein. J Pharmacol Exp Ther. 2007;322(1):378–384. doi: 10.1124/jpet.107.122812. [DOI] [PubMed] [Google Scholar]
- Magos L, Butler WH. The kinetics of methylmercury administered repeatedly to rats. Arch Toxicol. 1976;35(1):25–39. doi: 10.1007/BF00333983. [DOI] [PubMed] [Google Scholar]
- Magos L, Clarkson TW, Allen J. The interrelationship between non-protein bound thiols and the biliary excretion of methylmercury. Biochem Pharmacol. 1978;27(18):2203–2208. doi: 10.1016/0006-2952(78)90078-3. [DOI] [PubMed] [Google Scholar]
- Magos L, Peristianis GC, Clarkson TW, Brown A, Preston S, Snowden RT. Comparative study of the sensitivity of male and female rats to methylmercury. Arch Toxicol. 1981;48(1):11–20. doi: 10.1007/BF00297071. [DOI] [PubMed] [Google Scholar]
- Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR. The comparative toxicology of ethyl- and methylmercury. Arch Toxicol. 1985;57(4):260–267. doi: 10.1007/BF00324789. [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33(7):947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- Maliepaard M, Scheffer GL, Faneyte IF, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–3464. [PubMed] [Google Scholar]
- Marbet P, Rahner C, Stieger B, Landmann L. Quantitative microscopy reveals 3D organization and kinetics of endocytosis in rat hepatocytes. Microsc Res Tech. 2006;69(9):693–707. doi: 10.1002/jemt.20337. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Koya G, Takeuchi T. Fetal minamata disease. A neuropathological study of two cases of intrauterine intoxication by a methyl mercury compound. J Neuropathol Exp Neurol. 1965;24(4):563–574. [PubMed] [Google Scholar]
- McNeil SI, Bhatnagar MK, Turner CJ. Combined toxicity of ethanol and methylmercury in rat. Toxicology. 1988;53(2–3):345–363. doi: 10.1016/0300-483x(88)90226-0. [DOI] [PubMed] [Google Scholar]
- Ming X, Thakker DR. Role of basolateral efflux transporter MRP4 in the intestinal absorption of the antiviral drug adefovir dipivoxil. Biochem Pharmacol. 2010;79(3):455–462. doi: 10.1016/j.bcp.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Mokrzan EM, Kerper LE, Ballatori N, Clarkson TW. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther. 1995;272(3):1277–1284. [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13(4):866–874. doi: 10.1681/ASN.V134866. [DOI] [PubMed] [Google Scholar]
- Mulder KM, Kostyniak PJ. Effect of l-(alpha S,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid on urinary excretion of methylmercury in the mouse. J Pharmacol Exp Ther. 1985;234(1):156–160. [PubMed] [Google Scholar]
- Murphy MJ, Culliford EJ, Parsons V. A case of poisoning with mercuric chloride. Resuscitation. 1979;7(1):35–44. doi: 10.1016/0300-9572(79)90013-3. [DOI] [PubMed] [Google Scholar]
- Mussini E. Distribution in the organism and diuretic activity of p-chlorobenzoic acid mercury salt. Boll Soc Ital Biol Sper. 1958;34(22):1586–1588. [PubMed] [Google Scholar]
- Nagamori S, Wiriyasermkul P, Guarch ME, et al. Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria-related plasma membrane protein rBAT/SLC3A1. Proc Natl Acad Sci USA. 2016;113(3):775–780. doi: 10.1073/pnas.1519959113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma A, Oda-Urano N, Tanaka T, Imura N. Possible role of hepatic glutathione in transport of methylmercury into mouse kidney. Biochem Pharmacol. 1988;37(2):291–296. doi: 10.1016/0006-2952(88)90731-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Hatanaka T, Huang W, et al. Na+ - and Cl-coupled active transport of carnitine by the amino acid transporter ATB(0, +) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. 2001;532(Pt 2):297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Nagafuchi O, Kawakami T, et al. Human health risk assessment of mercury vapor around artisanal small-scale gold mining area, Palu city, Central Sulawesi, Indonesia. Ecotoxicol Environ Saf. 2016;124:155–162. doi: 10.1016/j.ecoenv.2015.09.042. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Biotransformation of methylmercury salts in the rat studied by specific determination of inorganic mercury. Biochem Pharmacol. 1970a;19(10):2775–2783. doi: 10.1016/0006-2952(70)90104-8. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Studies on the biotransformation of 203Hg-labeled methyl mercury chloride in rats. Arch Environ Health. 1970b;21(6):717–727. doi: 10.1080/00039896.1970.10667325. [DOI] [PubMed] [Google Scholar]
- Norseth T, Clarkson TW. Intestinal transport of 203Hg-labeled methyl mercury chloride. Role of biotransformation in rats. Arch Environ Health. 1971;22(5):568–577. doi: 10.1080/00039896.1971.10665903. [DOI] [PubMed] [Google Scholar]
- Ohlander J, Huber SM, Schomaker M, et al. Mercury and neuromotor function among children in a rural town in Chile. Int J Occup Environ Health. 2016 doi: 10.1080/10773525.2015.1125585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldendorf WH. Stereospecificity of blood–brain barrier permeability to amino acids. Am J Physiol. 1973;224(4):967–969. doi: 10.1152/ajplegacy.1973.224.4.967. [DOI] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Zalups RK, Pereira ME, Bridges CC. Disposition of inorganic mercury in pregnant rats and their offspring. Toxicology. 2015;335:62–71. doi: 10.1016/j.tox.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira CS, Joshee L, Zalups RK, Bridges CC. Compensatory renal hypertrophy and the handling of an acute nephrotoxicant in a model of aging. Exp Gerontol. 2016;75:16–23. doi: 10.1016/j.exger.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata S, Sakimura K, Ishii T, Sugano H. Chemical nature of a methylmercury complex with a low molecular weight in the liver cytosol of rats exposed to methylmercury chloride. Biochem Pharmacol. 1978;27(12):1700–1702. doi: 10.1016/0006-2952(78)90184-3. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78(4):969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Palacin M, Fernandez E, Chillaron J, Zorzano A. The amino acid transport system b(o, +) and cystinuria. Mol Membr Biol. 2001;18(1):21–26. [PubMed] [Google Scholar]
- Pfeifer ND, Hardwick RN, Brouwer KL. Role of hepatic efflux transporters in regulating systemic and hepatocyte exposure to xenobiotics. Annu Rev Pharmacol Toxicol. 2014;54:509–535. doi: 10.1146/annurev-pharmtox-011613-140021. [DOI] [PubMed] [Google Scholar]
- Planas-Bohne F. The effect of 2,3-dimercaptorpropane-1-sulfonate and dimercaptosuccinic acid on the distribution and excretion of mercuric chloride in rats. Toxicology. 1981;19(3):275–278. doi: 10.1016/0300-483x(81)90138-4. [DOI] [PubMed] [Google Scholar]
- Prickett CS, Laug EP, Kunze FM. Distribution of mercury in rats following oral and intravenous administration of mercuric acetate and phenylmercuric acetate. Proc Soc Exp Biol Med. 1950;73(4):585–588. doi: 10.3181/00379727-73-17752. [DOI] [PubMed] [Google Scholar]
- Prime-Chapman HM, Fearn RA, Cooper AE, Moore V, Hirst BH. Differential multidrug resistance-associated protein 1 through 6 isoform expression and function in human intestinal epithelial Caco-2 cells. J Pharmacol Exp Ther. 2004;311(2):476–484. doi: 10.1124/jpet.104.068775. [DOI] [PubMed] [Google Scholar]
- Rahner C, Stieger B, Landmann L. Apical endocytosis in rat hepatocytes in situ involves clathrin, traverses a subapical compartment, and leads to lysosomes. Gastroenterology. 2000;119(6):1692–1707. doi: 10.1053/gast.2000.20233. [DOI] [PubMed] [Google Scholar]
- Rallo M, Lopez-Anton MA, Contreras ML, Maroto-Valer MM. Mercury policy and regulations for coal-fired power plants. Environ Sci Pollut Res Int. 2012;19(4):1084–1096. doi: 10.1007/s11356-011-0658-2. [DOI] [PubMed] [Google Scholar]
- Refsvik T. Excretion of methyl mercury in rat bile: the effect of diethylmaleate, cyclohexene oxide and acrylamide. Acta Pharmacol Toxicol (Copenh) 1978;42(2):135–141. doi: 10.1111/j.1600-0773.1978.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Refsvik T. Excretion of methyl mercury in rat bile: the effect of thioctic acid, thionalide, hexadecyl- and octadecylmercaptoacetate. Acta Pharmacol Toxicol (Copenh) 1982;50(3):196–205. doi: 10.1111/j.1600-0773.1982.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Refsvik T, Norseth T. Methyl mercuric compounds in rat bile. Acta Pharmacol Toxicol (Copenh) 1975;36(1):67–78. doi: 10.1111/j.1600-0773.1975.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Risher JF, De Rosa CT. Inorganic: the other mercury. J Environ Health. 2007;70(4):9–16. discussion 40. [PubMed] [Google Scholar]
- Rodier PM, Kates B, Simons R. Mercury localization in mouse kidney over time: autoradiography versus silver staining. Toxicol Appl Pharmacol. 1988;92(2):235–245. doi: 10.1016/0041-008x(88)90383-3. [DOI] [PubMed] [Google Scholar]
- Rodiger M, Zhang X, Ugele B, et al. Organic anion transporter 3 (OAT3) and renal transport of the metal chelator 2,3-dimercapto-1-propanesulfonic acid (DMPS) Can J Physiol Pharmacol. 2010;88(2):141–146. doi: 10.1139/Y09-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Lugokenski TH, et al. Complex methylmercury-cysteine alters mercury accumulation in different tissues of mice. Basic Clin Pharmacol Toxicol. 2010;107(4):789–792. doi: 10.1111/j.1742-7843.2010.00577.x. [DOI] [PubMed] [Google Scholar]
- Rost D, Mahner S, Sugiyama Y, Stremmel W. Expression and localization of the multidrug resistance-associated protein 3 in rat small and large intestine. Am J Physiol Gastrointest Liver Physiol. 2002;282(4):G720–G726. doi: 10.1152/ajpgi.00318.2001. [DOI] [PubMed] [Google Scholar]
- Rowens B, Guerrero-Betancourt D, Gottlieb CA, Boyes RJ, Eichenhorn MS. Respiratory failure and death following acute inhalation of mercury vapor. A clinical and histologic perspective. Chest. 1991;99(1):185–190. doi: 10.1378/chest.99.1.185. [DOI] [PubMed] [Google Scholar]
- Rubino FM, Verduci C, Giampiccolo R, Pulvirenti S, Brambilla G, Colombi A. Molecular characterization of homo- and heterodimeric mercury(II)-bis-thiolates of some biologically relevant thiols by electrospray ionization and triple quadrupole tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15(3):288–300. doi: 10.1016/j.jasms.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Samuels ER, Heick HM, McLaine PN, Farant JP. A case of accidental inorganic mercury poisoning. J Anal Toxicol. 1982;6(3):120–122. doi: 10.1093/jat/6.3.120. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, Konig J, et al. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8(8):1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, Konig J, et al. Expression of the MRP2 gene-encoded conjugate export pump in human kidney proximal tubules and in renal cell carcinoma. J Am Soc Nephrol. 1999;10(6):1159–1169. doi: 10.1681/ASN.V1061159. [DOI] [PubMed] [Google Scholar]
- Seccatore J, Veiga M, Origliasso C, Marin T, De Tomi G. An estimation of the artisanal small-scale production of gold in the world. Sci Total Environ. 2014;496:662–667. doi: 10.1016/j.scitotenv.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Segel GB, Woodlock TJ, Lichtman MA. Trans-stimulation of l-system amino acid transport in normal and chronic leukemic human lymphocytes: phorbol ester restores function in CLL. J Cell Physiol. 1988;134(3):503–508. doi: 10.1002/jcp.1041340325. [DOI] [PubMed] [Google Scholar]
- Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272(30):18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- Sekine T, Miyazaki H, Endou H. Molecular physiology of renal organic anion transporters. Am J Physiol Renal Physiol. 2006;290(2):F251–F261. doi: 10.1152/ajprenal.00439.2004. [DOI] [PubMed] [Google Scholar]
- Shoji T, Suzuki H, Kusuhara H, Watanabe Y, Sakamoto S, Sugiyama Y. ATP-dependent transport of organic anions into isolated basolateral membrane vesicles from rat intestine. Am J Physiol Gastrointest Liver Physiol. 2004;287(4):G749–G756. doi: 10.1152/ajpgi.00065.2003. [DOI] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury-l-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Biochem J. 2002;367(Pt 1):239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci. 1994;107(Pt 4):1065–1072. doi: 10.1242/jcs.107.4.1065. [DOI] [PubMed] [Google Scholar]
- Simpson RB. Association constants of methylmercury with sulfhydryl and other bases. J Am Soc Chem. 1961;83(23):4711–4717. [Google Scholar]
- Simpson PG, Hopkins TE, Haque R. Binding of methylmercury chloride to the model peptide, N-acetyl-l-cysteine. A proton magnetic resonance study. J Phys Chem. 1973;77(19):2282–2285. doi: 10.1021/j100638a005. [DOI] [PubMed] [Google Scholar]
- Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl(−)-dependent neutral and cationic amino B(0+) J Biol Chem. 1999;274(34):23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- SOER. The European environment—state and outlook 2015. In: Agency EE, editor. Global megatrends: increasing environmental pollution (GMT 10) Copenhagen, Denmark: European Environment Agency (EEA); 2015. [Google Scholar]
- Soni JP, Singhania RU, Bansal A, Rathi G. Acute mercury vapor poisoning. Indian Pediatr. 1992;29(3):365–368. [PubMed] [Google Scholar]
- St-Pierre MV, Serrano MA, Macias RI, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- Sugawara N, Lai YR, Sugaware C, Arizono K. Decreased hepatobiliary secretion of inorganic mercury, its deposition and toxicity in the Eisai hyperbilirubinemic rat with no hepatic canalicular organic anion transporter. Toxicology. 1998;126(1):23–31. doi: 10.1016/s0300-483x(97)00170-4. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nakanishi T. OATP transporter-mediated drug absorption and interaction. Curr Opin Pharmacol. 2013;13(6):859–863. doi: 10.1016/j.coph.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Naganuma A, Imura N. Role of gamma-glutamyltranspeptidase in renal uptake and toxicity of inorganic mercury in mice. Toxicology. 1990;60(3):187–198. doi: 10.1016/0300-483x(90)90142-4. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Naganuma A, Kobayashi K, Imura N. An explanation for strain and sex differences in renal uptake of methylmercury in mice. Toxicology. 1991;69(3):317–329. doi: 10.1016/0300-483x(91)90190-c. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Naganuma A, Miura N, Imura N. Role of testosterone in gamma-glutamyltranspeptidase-dependent renal methylmercury uptake in mice. Toxicol Appl Pharmacol. 1992;112(1):58–63. doi: 10.1016/0041-008x(92)90279-2. [DOI] [PubMed] [Google Scholar]
- Tanaka-Kagawa T, Naganuma A, Imura N. Tubular secretion and reabsorption of mercury compounds in mouse kidney. J Pharmacol Exp Ther. 1993;264(2):776–782. [PubMed] [Google Scholar]
- Taugner R. On the renal uptake and intrarenal distribution of sublimate and Hg-cysteine. Arzneimittelforschung. 1966;16(8):1120–1121. [PubMed] [Google Scholar]
- Taugner R, Kz W, Iravani J. On the localization of mercuric chloride concentration in the rat kidney. Virchows Arch Pathol Anat Physiol Klin Med. 1966;340(4):369–383. [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Smith JC. Partial characterization of a low-molecular weight methylmercury complex in rat cerebrum. Toxicol Appl Pharmacol. 1979;47(3):547–556. doi: 10.1016/0041-008x(79)90525-8. [DOI] [PubMed] [Google Scholar]
- Thomas DJ, Smith JC. Effects of coadministered low-molecular-weight thiol compounds on short-term distribution of methyl mercury in the rat. Toxicol Appl Pharmacol. 1982;62(1):104–110. doi: 10.1016/0041-008x(82)90106-5. [DOI] [PubMed] [Google Scholar]
- Urano T, Iwasaki A, Himeno S, Naganuma A, Imura N. Absorption of methylmercury compounds from rat intestine. Toxicol Lett. 1990;50(2–3):159–164. doi: 10.1016/0378-4274(90)90006-8. [DOI] [PubMed] [Google Scholar]
- USEPA. Mercury study report to congress. Volume II: an inventory of anthropognenic mercury emissions in the United States. Environmental Protection Agency, Emissions standards division/air quality strategies and standards division. 1997 [Google Scholar]
- USEPA. A study of hazardous air pollutant emissions from electric utility steam generating units. Study of hazardous air pollutant emissions from Electric Utility Steam Generating Units— final report to congress. Environmental Protection Agency, Emissions standards division/air quality strategies and standards division. 1998 [Google Scholar]
- Vazquez M, Calatayud M, Velez D, Devesa V. Intestinal transport of methylmercury and inorganic mercury in various models of Caco-2 and HT29-MTX cells. Toxicology. 2013;311(3):147–153. doi: 10.1016/j.tox.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Velez D, Devesa V. In vitro characterization of the intestinal absorption of methylmercury using a Caco-2 cell model. Chem Res Toxicol. 2014;27(2):254–264. doi: 10.1021/tx4003758. [DOI] [PubMed] [Google Scholar]
- Vazquez M, Velez D, Devesa V, Puig S. Participation of divalent cation transporter DMT1 in the uptake of inorganic mercury. Toxicology. 2015;331:119–124. doi: 10.1016/j.tox.2015.03.005. [DOI] [PubMed] [Google Scholar]
- Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61(1):14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol. 2001;281(4):C1077–C1093. doi: 10.1152/ajpcell.2001.281.4.C1077. [DOI] [PubMed] [Google Scholar]
- Wakita Y. Hypertension induced by methyl mercury in rats. Toxicol Appl Pharmacol. 1987;89(1):144–147. doi: 10.1016/0041-008x(87)90185-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Clarkson TW, Ballatori N. gamma-glutamyl transpeptidase and l-cysteine regulate methylmercury uptake by HepG2 cells, a human hepatoma cell line. Toxicol Appl Pharmacol. 2000;168(1):72–78. doi: 10.1006/taap.2000.9018. [DOI] [PubMed] [Google Scholar]
- Ward DM, Nislow KH, Folt CL. Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann N Y Acad Sci. 2010;1195:62–83. doi: 10.1111/j.1749-6632.2010.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkany J, Hubbard DM. Acrodynia and mercury. J Pediatr. 1953;42(3):365–386. doi: 10.1016/s0022-3476(53)80195-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Mercury and Health Fact Sheet. Switzerland: Geneva; 2000. [Google Scholar]
- WHO. WHO European Centre for Environment and Health. Bonn: WHO Regional Office for Europe; 2016. Review of evidence on health aspects of air pollution— REVIHAAP Project. [PubMed] [Google Scholar]
- Wu G. Screening of potential transport systems for methyl mercury uptake in rat erythrocytes at 5 degrees by use of inhibitors and substrates. Pharmacol Toxicol. 1995;77(3):169–176. doi: 10.1111/j.1600-0773.1995.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Wu G. Methylmercury-cysteine uptake by rat erythrocytes: evidence for several transport systems. J Appl Toxicol. 1996;16(1):77–83. doi: 10.1002/(SICI)1099-1263(199601)16:1<77::AID-JAT319>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wu G. Effect of probenecid on the transport of methyl mercury in erythrocytes by the organic anion transport system. Arch Toxicol. 1997;71(4):218–222. doi: 10.1007/s002040050379. [DOI] [PubMed] [Google Scholar]
- Yasutake A, Hirayama K, Inoue M. Mechanism of urinary excretion of methylmercury in mice. Arch Toxicol. 1989;63(6):479–483. doi: 10.1007/BF00316452. [DOI] [PubMed] [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. The methylmercury-l-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem. 2008;107(4):1083–1090. doi: 10.1111/j.1471-4159.2008.05683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokooji T, Murakami T, Yumoto R, Nagai J, Takano M. Site-specific bidirectional efflux of 2,4-dinitrophenyl-S-glutathione, a substrate of multidrug resistance-associated proteins, in rat intestine and Caco-2 cells. J Pharm Pharmacol. 2007;59(4):513–520. doi: 10.1211/jpp.59.4.0005. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Autometallographic localization of inorganic mercury in the kidneys of rats: effect of unilateral nephrectomy and compensatory renal growth. Exp Mol Pathol. 1991;54(1):10–21. doi: 10.1016/0014-4800(91)90039-z. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Early aspects of the intrarenal distribution of mercury after the intravenous administration of mercuric chloride. Toxicology. 1993a;79(3):215–228. doi: 10.1016/0300-483x(93)90213-c. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Influence of 2,3-dimercaptopropane-1-sulfonate (DMPS) and meso-2,3-dimercaptosuccinic acid (DMSA) on the renal disposition of mercury in normal and uninephrectomized rats exposed to inorganic mercury. J Pharmacol Exp Ther. 1993b;267(2):791–800. [PubMed] [Google Scholar]
- Zalups RK. Organic anion transport and action of gamma-glutamyl transpeptidase in kidney linked mechanistically to renal tubular uptake of inorganic mercury. Toxicol Appl Pharmacol. 1995;132(2):289–298. doi: 10.1006/taap.1995.1110. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Intestinal handling of mercury in the rat: implications of intestinal secretion of inorganic mercury following biliary ligation or cannulation. J Toxicol Environ Health A. 1998;53(8):615–636. doi: 10.1080/009841098159079. [DOI] [PubMed] [Google Scholar]
- Zalups RK. Molecular interactions with mercury in the kidney. Pharmacol Rev. 2000;52(1):113–143. [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Homocysteine and the renal epithelial transport and toxicity of inorganic mercury: role of basolateral transporter organic anion transporter 1. J Am Soc Nephrol. 2004;15(8):2023–2031. doi: 10.1097/01.ASN.0000135115.63412.A9. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of cysteine S-conjugates of methylmercury in MDCK cells expressing human OAT1. Kidney Int. 2005a;68(4):1684–1699. doi: 10.1111/j.1523-1755.2005.00585.x. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Handling of the homocysteine S-conjugate of methylmercury by renal epithelial cells: role of organic anion transporter 1 and amino acid transporters. J Pharmacol Exp Ther. 2005b;315(2):896–904. doi: 10.1124/jpet.105.090530. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Transport of N-acetylcysteine s-conjugates of methylmercury in Madin-Darby canine kidney cells stably transfected with human isoform of organic anion transporter 1. J Pharmacol Exp Ther. 2005c;314(3):1158–1168. doi: 10.1124/jpet.105.086645. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW. Intrarenal distribution of inorganic mercury and albumin after coadministration. J Toxicol Environ Health. 1993;40(1):77–103. doi: 10.1080/15287399309531777. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. MRP2 involvement in renal proximal tubular elimination of methylmercury mediated by DMPS or DMSA. Toxicol Appl Pharmacol. 2009;235(1):10–17. doi: 10.1016/j.taap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Bridges CC. Relationships between the renal handling of DMPS and DMSA and the renal handling of mercury. Chem Res Toxicol. 2012;25(9):1825–1838. doi: 10.1021/tx3001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG. Renal metallothionein metabolism after a reduction of renal mass. II. Effect of zinc pretreatment on the renal toxicity and intrarenal accumulation of inorganic mercury. Toxicology. 1992;71(1–2):103–117. doi: 10.1016/0300-483x(92)90057-l. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Koropatnick J. Temporal changes in metallothionein gene transcription in rat kidney and liver: relationship to content of mercury and metallothionein protein. J Pharmacol Exp Ther. 2000;295(1):74–82. [PubMed] [Google Scholar]
- Zalups RK, Lash LH. Depletion of glutathione in the kidney and the renal disposition of administered inorganic mercury. Drug Metab Dispos. 1997;25(4):516–523. [PubMed] [Google Scholar]
- Zalups RK, Minor KH. Luminal and basolateral mechanisms involved in the renal tubular uptake of inorganic mercury. J Toxicol Environ Health. 1995;46(1):73–100. doi: 10.1080/15287399509532019. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Cherian MG, Barfuss DW. Mercury-metallothionein and the renal accumulation and handling of mercury. Toxicology. 1993;83(1–3):61–78. doi: 10.1016/0300-483x(93)90092-7. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Barfuss DW, Lash LH. Disposition of inorganic mercury following biliary obstruction and chemically induced glutathione depletion: dispositional changes one hour after the intravenous administration of mercuric chloride. Toxicol Appl Pharmacol. 1999;154(2):135–144. doi: 10.1006/taap.1998.8562. [DOI] [PubMed] [Google Scholar]
- Zalups RK, Aslamkhan AG, Ahmad S. Human organic anion transporter 1 mediates cellular uptake of cysteine-S conjugates of inorganic mercury. Kidney Int. 2004;66(1):251–261. doi: 10.1111/j.1523-1755.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann LT, Santos DB, Naime AA, et al. Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake. Neurotoxicology. 2013;38:1–8. doi: 10.1016/j.neuro.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann LT, dos Santos DB, Colle D, et al. Methionine stimulates motor impairment and cerebellar mercury deposition in methylmercury-exposed mice. J Toxicol Environ Health A. 2014;77(1–3):46–56. doi: 10.1080/15287394.2014.865582. [DOI] [PubMed] [Google Scholar]