Abstract
In the mammalian central nervous system a remarkably small number of connexins is used in electrical synapses, with the majority formed from Cx36. A larger number has been detected in teleosts, with some seeming to serve restricted roles. Here we report the discovery of a new connexin expressed in the zebrafish lens and a limited set of neurons. Zebrafish cx79.8 (gja8a), previously annotated incorrectly as cx50.5 based on a partial cDNA sequence, is a homologue of mammalian Cx50 (Gja8). We examined its expression through transgenic promoter-reporter constructs, in situ hybridization, and immunolabeling, and examined regulation of coupling in transfected HeLa cells. cx79.8 was expressed most strongly in the lens, but expression was also found in several groups of neurons in the cerebellum and related areas at the midbrain-hindbrain boundary, in cone photoreceptors, and in neurons in the retinal inner nuclear and ganglion cell layers. Labeling in the retina with antibodies against two C-terminal epitopes revealed numerous small punctate spots in the inner plexiform layer and along the somata of cones. Abundant gap junctions were labeled in the outer 1/3 of the lens, but were absent from the center, suggesting that the epitopes or the entire protein was absent from the center. Cx79.8 tracer coupling was strongly regulated by phosphorylation, and was extremely low in control conditions in HeLa cells due to protein phosphatase 2A activity. These properties allow coupling to be strongly restricted in situ, a frequently observed property for electrical synapses.
Keywords: Gja8, cx50.5, photoreceptor, retina, cerebellum, transgenic
INTRODUCTION
Electrical synapses are pervasive elements of neural circuits throughout the vertebrate central nervous system (CNS). Formed by gap junctions made of connexin proteins, electrical synapses support direct electrical communication between neighboring cells. Electrical synapses support rapid communication that can expand receptive field size, propagate oscillations, synchronize behaviors within a network, or even propagate electrical signals forward through circuits (O'Brien, 2014; Curti and O'Brien, 2016).
The majority of electrical synapses in the vertebrate CNS appear to be formed from Connexin 35 (Cx35) (O'Brien et al., 1996), the mammalian homologue of which is Cx36 (O'Brien et al., 1998; Sohl et al., 1998). Cx35 and Cx36 are characterized by very small single channel conductance around 15 pS and very low voltage sensitivity (Srinivas et al., 1999; Teubner et al., 2000). Cx34.7, a connexin very closely related to Cx35, is also present in teleost fish (O'Brien et al., 1998). Although far less studied than Cx35/Cx36, its properties appear to be similar (O'Brien et al., 1998). Cx34.7 has been found in isolated electrical synapses in cone photoreceptors (O'Brien et al., 2004) and as the post-synaptic partner to Cx35 in heterotypic mixed synapses on Mauthner cells (Rash et al., 2013). This configuration has recently been found to be widespread in the teleost brain (Rash et al., 2015).
Gap junctions utilizing Cx35/Cx36 are regulated by phosphorylation by various protein kinases (Ouyang et al., 2005; Patel et al., 2006; Del Corsso et al., 2012), showing at least an order of magnitude dynamic range of coupling directly dependent on phosphorylation state (Kothmann et al., 2009; Li et al., 2013). Gap junctions utilizing Cx34.7 also show similar plasticity (Pereda et al., 1998; Li et al., 2009), suggesting that this connexin is regulated in a similar fashion. The combination of small conductance and extensive plasticity endow electrical synapses utilizing these connexins with the ability to vary coupling from vanishingly small to moderate.
A few neural networks are characterized by very strong electrical coupling; retinal horizontal cells are a noteworthy example of such a network. These cells do not use Cx35/Cx36 (Deans and Paul, 2001), but rather several other connexins. Zebrafish horizontal cells have been found to express Cx55.5, Cx52.6 and Cx52.9 (Dermietzel et al., 2000; Zoidl et al., 2004; Klaassen et al., 2011), while mammalian horizontal cells use Cx57 (Hombach et al., 2004; Pan et al., 2012), a homologue of Cx52.6, and Cx50 (O'Brien et al., 2006; Dorgau et al., 2015). These connexins show much larger single channel conductances of 39-57 pS for Cx52.6, Cx55.5, and Cx57 (Dermietzel et al., 2000; Zoidl et al., 2004; Palacios-Prado et al., 2009), and over 200 pS for Cx50 (Srinivas et al., 1999).
In zebrafish retina, some amacrine cells display unusually extensive tracer coupled fields and the ability to pass anionic fluorescent dyes freely through gap junctions (Miller et al., 2008). It is unlikely that these properties are supported by Cx35, in particular because Cx35 gap junctions have been found not to pass anionic dyes in vitro (O'Brien et al., 2004; Ouyang et al., 2005), although its mammalian counterpart has been reported to do so (Srinivas et al., 1999). This suggests that another type of connexin is used in gap junctions in some retinal amacrine cells. To identify candidate connexins, we searched for connexins expressed in the zebrafish retina by rtPCR. Here we report that Cx79.8 (Gja8a), previously annotated erroneously as Cx50.5 based on partial sequence, is expressed in some neurons and we describe some of its functional characteristics.
METHODS
Experimental animals
Wild-type, AB-strain zebrafish of both sexes were used in this study. The AB strain was originally obtained from the Zebrafish International Resource Center at the University of Oregon. Dr. Xinping C. Zhou (University of Texas Health Science Center at Houston) generously provided a Zebrafish opsin-GFP transgenic line used in some experiments. Animals were maintained in a 14 hr light/10 hr dark cycle at 28°C. All procedures performed on animals, including breeding, transgenesis, experimental manipulations and euthanasia, were approved by the institutional animal care and use committee.
rtPCR
Expression of connexin genes in adult zebrafish tissues or whole 1-5 days post-fertilization (dpf) fry was assessed by rtPCR. Animals were sacrificed by chilling in tank water on ice. Total RNA was purified from dissected tissues or whole fry using Tri-reagent (Sigma, St. Louis, MO) using the manufacturer's protocol. Reverse transcription and PCR were performed in a single tube using SuperScript One-step RT-PCR kit (ThermoFisher, Waltham, MA) and 0.5 μg of total RNA template. Primers used include for cx34.1: cx34.1RT-F1 5’-TTGCTGGAGGCTGCGGTACAGCAGC-3’, cx34.1RT-R1 5’-CAACCTCCTTCACGCAAGGGTAGCG-3’; for cx44.1: cx44.1RT-F1 5’-CATTCGGGCGACAAGGATTGGAGGC-3’, cx44.1RT-R1 5’-CTGCCCTTTGTGCTCGTCTCCTTGG-3’; for cx48.5: JS7-Cx48.5CT-F 5’-GCGCCTCCAACCCTCAGCTA-3’, JS8-Cx48.5CT-R 5’-GGGGGAGGTGGCGTTCATCT-3’; for cx79.8: cx50.5RT-F1 5’-CAGGAGTTCAATTCGCCCCACAGGG-3’, cx50.5-ex2-R1 5’-CCTCCATGTGGATGTAATGGACAGC-3’, cx50.5-ex2-R2 5’-GCTTTGTGTTCCACCGACCAGCTCC-3’; for actb1: Zbactin1-ex4F 5’-GTCCGTGACATCAAGGAGAAGC-3’, Zbactin1-ex6R 5’-GGATGGGCCAGACTCATCGTAC-3’.
Cx79.8 cloning and development of transgenic fish
The zebrafish genomic bac DKEY-18P12 (Accession number BX571845) was obtained from Source Bioscience (Nottingham, UK). To make an expression clone, the cx79.8 open reading frame was PCR amplified using Phusion polymerase (New England Biolabs, Ipswich, MA) with primers JOB407 Z79.8-CF-F 5’-CTAGCGTTTAAACTTAAGCTTGTACACAGCTAATACACAACCATG-3’ and primers JOB408 Z79.8-CF-R 5’-GGCCCTCTAGACTCGAGTTGCTGAAATTTCTGTTTCTTGG-3’. The PCR product was cloned into pcDNA 3.1zeo (ThermoFisher) at the HindIII and XhoI sites using Gibson Assembly Master Mix (New England Biolabs). A GFP-tagged expression clone was made in a similar fashion using the pEGFP-N1 vector (Clontech, Mountain View, CA) cut at the XhoI and BamHI sites and DNA amplified using primers JOB346 Z80-EGFP-CF-F 5’-CCGGACTCAGATCTCGAGTACACAGCTAATACACAACCATG-3’ and JOB347 Z80-EGFP-CF-R 5’-GCGACCGGTGGATCCTTGGTCAGTCcTATAGTTAGATCA-3’. Clones were sequenced on both strands.
To develop transgenic zebrafish, we used the Tol2 transposon system (Urasaki et al., 2006). A 5.4 kb genomic fragment upstream of the cx79.8 open reading frame was amplified by PCR using Phusion polymerase (New England Biolabs) with primers JOB356 Z79.8-prom-CF-F 5’-TCACTTGGGCCCGGCTCGAGCACACAAATATGACTCCTTGACTGTTG-3’ and JOB357 Z79.8-prom-CF-R 5’-CTCAGGATCGGTCGACCTGCAGTTGCACAATGTCGGGTCCAC-3’. The amplified fragment was cloned by Gibson Assembly into the Tol2-EGFP reporter plasmid pT2AL200R150G (generously provided by Dr. Koichi Kawakami, National Institute of Genetics, Mishima, Japan) cut at XhoI and PstI sites. The ends of the cloned promoter fragment were confirmed by Sanger sequencing. Tol2 transposase mRNA was made by in vitro transcription from the pCS-TP plasmid (generously provided by Dr. Koichi Kawakami) using the mMessage mMachine kit (ThermoFisher). The transgene construct and Tol2 mRNA were co-injected into 1 cell stage AB strain zebrafish embryos, and the resulting fish were either used at 4 to 7 dpf for imaging studies or were raised to adulthood and bred to establish stable transgenic lines. EGFP expression in the lens was used to screen offspring for expression of the transgene.
EGFP expression in tissue sections was visualized by confocal microscopy as described in the section below for immunofluorescence. Some stable transgenic fry were imaged in a Zeiss (Thornwood, NY) Z.1 lightsheet microscope. Fry were deeply anesthetized with 0.15% Tricaine, immobilized in 0.5% low melt agarose (Sigma) in tank water, and drawn head-down into the imaging capillary. Imaging was performed at room temperature.
In situ hybridization
We performed in situ hybridization to localize cx79.8 mRNA in zebrafish retina. To make cx79.8 RNA probes, a 261 bp DNA fragment from the C-terminal coding sequence was amplified by PCR using the genomic Bac clone as a template and the following primer set: forward 5′taatacgactcactatagggTGGGGAAACAGAGGATAGCAACT 3′, reverse 5′cgatttaggtgacactatagaAGGCGCTATGTCAGGAAGATTTG 3′. T7 or SP6 RNA polymerase promoters (lower case text) were incorporated into the primers, and digoxigenin-labeled antisense and sense strand RNA probes were synthesized by in vitro transcription using a MAXIscript kit (ThermoFisher) in the presence of digoxigenin-dUTP (Roche, Basel, Switzerland) following the manufacturer's instructions.
Adult AB strain zebrafish were sacrificed by immersion in 0.3% Tricaine (Sigma, St. Louis, MO) followed by decapitation. Eyes were rapidly dissected, the anterior segment removed, and the eyecup fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in 0.1 M phosphate buffer, pH 7.5 (PB) for 48 hr at 4°C. The fixed eyecup was washed 3 times in DEPC-treated phosphate-buffered saline, pH 7.4 (PBS), embedded in paraffin, and cut into 9 μm sections using a microtome. Retina sections were de-waxed, treated with 30 μg/ml proteinase K (Roche) in 100 mM Tris-Cl (pH 8.0), 25 mM EDTA at 37°C for 20 min, and post-fixed in 4% paraformaldehyde in 0.1 M PB for 20 min. To reduce non-specific background, sections were incubated with 0.5% acetic anhydride in 0.1M triethanolamine for 10 min. Hybridization was performed overnight with cx79.8 riboprobes at 60°C in hybridization buffer (50% deionized formamide, 10% dextran sulfate, 1x Denhardt's solution, 4x SSC (600 mM NaCl, 60 mM Na3Citrate, pH 7.0), 10mM DTT, 1 mg/ml denatured and sheared salmon sperm DNA). Sections were then washed at hybridization temperature with buffers at high stringency: 50% formamide with 2x SSC for 30 min, 1x SSC for 30 min, and 0.2x SSC for 30 min. Non-hybridized RNA probes were deactivated by treatment of 0.2 μg/ml RNase A (Sigma) at 37°C for 30 min. Sections were treated with 2% H2O2 in PBS for 10 minutes to quench endogenous peroxidase activity. The slides were then incubated with peroxidase-conjugated anti-DIG antibody (Roche) 1:400 in PBS, 0.3% Triton X-100 and TNB blocking buffer (PerkinElmer, Shelton, CT) overnight at room temperature. On the third day, color was developed with TSA plus Cy3 system (PerkinElmer, Waltham, MA) for 30 min. Sections were mounted with Vectashield mounting medium with DAPI (Vector Labs, Burlingame, CA), and imaged on a Zeiss LSM 510 confocal microscope.
Antibody development and immunostaining
We developed polyclonal antibodies against two epitopes in the C-terminus of Cx79.8. Antibody Cx79.8-345 was targeted against the sequence ELPTEEPPREGDEVDSLKT and antibody Cx79.8-645 was targeted against the sequence QEDESVKTGDLEKEEYSEESK. Peptides representing the epitopes were synthesized with an added C-terminal cysteine, linked to keyhole limpet hemocyanin via the cysteine thiol with a maleimide linker, and injected together into rabbits. Peptide synthesis, linking, immunizations and bleeds were performed by ThermoFisher (Pierce, Rockford, IL). Antibodies were affinity purified sequentially from crude antisera as previously described (Kothmann et al., 2007). Antibodies were eluted with an ethylene glycol-MgCl2 solution that minimizes antibody inactivation (Tsang and Wilkins, 1991), and were dialyzed against PBS, concentrated and stored in PBS plus 30% glycerol.
For immunofluorescence localization of Cx79.8 in adult retina, eyecups were prepared from AB strain zebrafish as previously described. The eyecup was fixed at room temperature with 4% paraformaldehyde in 0.1M PB for 30 min. For immunofluorescence localization of Cx79.8 in fry, 5 dpf AB fry were sacrificed by chilling on ice and were fixed with 4% paraformaldehyde in 0.1M PB for 30 min. Fixed tissue was cryoprotected in 30% sucrose overnight at 4°C and embedded in OCT (Sakura Finetek, Torrance, CA). Tissue was sectioned at 16 μm using a cryostat and blocked with 5% donkey serum (Jackson ImmunoResearch, West Grove, PA) in PBST (PBS with 0.3% Triton X-100) at room temperature for 30 min. Sections were incubated with primary antibodies in PBST plus 5% donkey serum overnight at room temperature. Rabbit anti-Cx79.8 antibodies were used at 1 μg/ml. Some experiments also included goat anti-cone arrestin (0.4 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA). Following extensive washing with PBS, sections were incubated with secondary antibodies including 1 μg/ml Alexa Fluor 488 donkey anti-rabbit IgG (ThermoFisher) and 1 μg/ml Cy3 donkey anti-goat IgG (Jackson) at room temperature for 3 hr. Nuclei were counterstained by mounting sections with Vectashield mounting medium with DAPI (Vector Labs). Antibodies were also tested against HeLa cells transiently transfected with Cx79.8-EGFP or EGFP alone (see Tracer Coupling section for transfection and culture conditions). Immunostaining conditions were the same as above except that anti-Cx79.8 antibodies were used at 0.25 μg/ml and detected with 2 μg/ml Cy3 donkey anti-rabbit IgG (Jackson) secondary antibodies. Samples were imaged on a Zeiss LSM 780 confocal microscope.
Tracer coupling
Functional tracer coupling was assessed in transiently transfected HeLa cells (Cat# CCL-2) obtained from American Type Culture Collection (Rockville, MD,). All media, fetal bovine serum and cell culture reagents were obtained from Invitrogen (Grand Island, NY). HeLa cells were grown in complete MEM supplemented with 10% fetal bovine serum and 1% antibiotic-antimycotic (penicillin/streptomycin/amphotericin B) and plated onto 12 mm cell culture coverslips (ThermoFisher). Cells were grown to 75% confluence overnight in 35 mm culture dishes and transiently transfected with 2 μg of plasmid DNA per dish using GenePORTER® 2 transfection reagent (Genlantis, San Diego, CA). Plasmids transfected included the Cx79.8-EGFP and Cx79.8-pcDNA constructs described above, or EGFP-N1 empty vector (control). Experiments were performed 24 hours after transfection, and two hours after replacement of culture medium with fresh medium.
Transfected HeLa cell coverslips were incubated in oxygenated Ringer's medium at 34°C for 10 min with or without PKA activator Sp-8-cpt-cAMPS (10 μM; Axxora, Farmingdale, NY), PKA inhibitor Rp-8-cpt-cAMPS (10 μM; Axxora), or PP2A inhibitor Microcystin-LR (0.5 nM; EMD Millipore, Billerica, MA). The medium was supplemented with 0.05% Neurobiotin (Vector Labs) and cells were scraped with a 26-gauge needle. Incubation with Neurobiotin was continued for 10 minutes to allow loading and diffusion. Cells were then washed three times with Ringer's medium at two minute intervals to remove extracellular Neurobiotin and fixed with 4% paraformaldehyde in 0.1 M phosphate buffer. When used, drugs were added to the oxygenated incubation medium 10 minutes before the scrape for pre-incubation and replaced with fresh drug upon Neurobiotin addition. Drugs were present throughout the 10 minute tracer diffusion period. Following fixation, cells were probed with streptavidin-Cy3 (Jackson) and photographed on a Zeiss fluorescence microscope (Axiovert 200 with 40x, 0.5 NA Hoffman Modulation Contrast objective) with a Hamamatsu C4742-95 digital camera using HCImage software (Hamamatsu, Sewickley, PA). Five images were taken of different patches of loaded cells for each experiment and treated as replicates in the data analysis.
The diffusion coefficient of Neurobiotin through the coupled network of HeLa cells was determined from fluorescence intensity data using a compartmental diffusion model (Zimmerman and Rose, 1985). The analysis utilizes a linear 25-compartment diffusion model to fit Neurobiotin concentration and diffusion distance measurements (O'Brien et al., 2004). This model has been applied to neural networks to assess gap junction coupling in the retina (Mills and Massey, 1998; O'Brien et al., 2004; Li et al., 2009). The movement of tracer between adjacent compartments is described by a series of 25 differential equations that are solved for tracer flux given the total amount of diffusion time and a diffusion coefficient k. The diffusion coefficient k represents the proportion of tracer that diffuses from the first compartment to the next per second. Optimal fitting of intensity data to the model was determined in MatLab (Mathworks, Natick, MA) by varying the diffusion coefficient k and another parameter, bo, the bolus loading rate. The parameter bo is defined as the rate of addition of tracer to the initial compartment for the loading period, which was assumed to be 1 minute in the scrape-loading experiments, and was set to zero thereafter. Data fits were determined by plotting cell intensities on a log intensity axis and determining the diffusion coefficient k that best fit the rate of decline of tracer intensity with distance from the cell of origin, and the rate of delivery, bo, that fit the overall tracer concentration (Mills and Massey, 1998; O'Brien et al., 2004; Ouyang et al., 2005). Replicate measurements were averaged to yield a single value for each treatment condition in each experiment. Diffusion coefficients were compared under different drug treatment conditions using unpaired T-tests.
RESULTS
Identification of connexins in retinal mRNA
We have previously observed that some amacrine cells in the zebrafish retina are extensively gap-junctionally coupled to neighbors, passing both the small cationic tracer Neurobiotin and the anionic dye Lucifer Yellow freely (Miller et al., 2008). The dominant connexin in the retinal inner plexiform layer, Cx35, does not pass Lucifer Yellow dye, implying that a different connexin is responsible for the extensive coupling of these cells. Teleost horizontal cells pass Lucifer Yellow, but imunostaining results with Cx55.5 and Cx52.6 are not consistent with their expression in amacrine cells (Shields et al., 2007). Cx50 of mammalian axonless horizontal cells (O'Brien et al., 2006) also passes Lucifer Yellow and teleost homologues of Cx50 might be expected to do the same. To test for the possible expression of Cx50 homologues in zebrafish retina we performed rtPCR on retinal mRNA for several connexin isoforms. The Cx50 homologue cx44.1 has previously been detected in a zebrafish retinal cDNA library, along with cx48.5, another related lens connexin gene that is homologous to Cx46 (Zoidl et al., 2008). We did not detect either of these connexins by rtPCR from zebrafish retina mRNA (fig. 1; some data not shown), but we did detect transcripts for a different Cx50 homologue annotated as cx50.5. Transcripts of this connexin were also prominently detected in the anterior portion of the eye containing the lens, while the neuronal connexin cx34.1 was detected only in the retinal portion of the eye (fig. 1).
Figure 1.
RtPCR analysis of connexin expression in zebrafish eyes. PCR products shown are derived from mRNA isolated from (A) the anterior segment of the adult eye including lens, cornea, sclera, and ciliary body, (P) the posterior portion of the adult eye including sclera, choroid, retina and pigmented epithelium, and (E) whole 5 dpf fry. B-actin was used as a control. While cx44.1 was detected only in the anterior portion of the eye containing the lens, cx50.5 was detected as well in the posterior portion of the eye containing the retina, along with the more expected cx34.1.
Cx50.5 is actually Cx79.8
Cx50.5 is annotated based upon on a partial cDNA sequence (Accession number GQ253466). The gene is mapped to linkage group 9 at 30.619 to 30.633 Mb in the Ensembl zebrafish genome version 82.10. This sequence is contained in bac DKEY-18P12 (Accession number BX571845) from the Sanger Institute Zebrafish genome sequencing project. Alignment of the cDNA and genomic sequences reveals a gene with two exons and a single open reading frame in the second exon 2127 bp in length encoding a typical connexin protein of 79.8 kDa. The gene structure and open reading frame are correctly annotated in Ensembl and other databases so the gene structure is not illustrated here. Alignment of the predicted amino acid sequence with those of zebrafish Cx44.1, mouse Cx50, and chicken Cx45.6 reveals a high degree of sequence conservation throughout the amino terminus, transmembrane domains, and extracellular loops and modest conservation in the cytoplasmic loop (fig. 2). There is far less sequence conservation in much of the C-terminus, but the final 17 amino acids of the C-terminus are highly conserved, suggesting important functional relevance. This also suggests that we have identified the correct C-terminus of Cx79.8. Alignment of the cx50.5 cDNA with cx79.8 reveals that this sequence ends within the C-terminal domain and is not shorter due to mRNA splicing. The endpoint of the cx50.5 sequence is annotated in figure 2.
Figure 2.
Alignment of predicted amino acid sequences of the zebrafish Cx50 homologues Cx79.8 and Cx44.1 with mouse Cx50 and chicken Cx45.6. Alignment symbols below the sequences indicate complete identity among all four sequences (*), high conservation of amino acid character (:), and modest conservation (.). Predicted transmembrane domains are highlighted with gray boxes and labeled M1 – M4. The end point of the cx50.5 cDNA sequence is designated by the dashed line through the alignment. Epitopes used to generate anti-Cx79.8 antibodies are outlined and labeled. Note the high degree of sequence conservation at the tip of the C-terminus.
Expression analysis of cx79.8
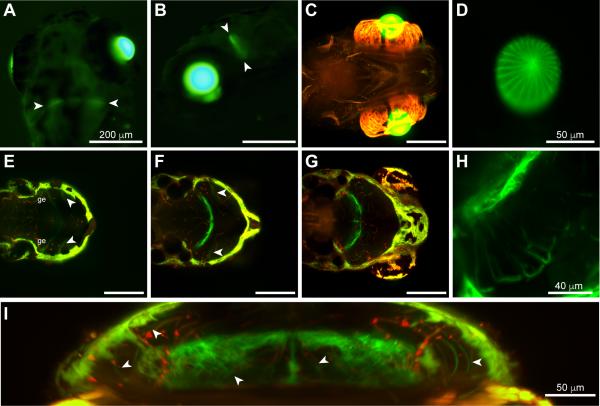
To study expression of the cx79.8 gene, we developed a GFP reporter transgene construct driven by a 5.4 kb upstream genomic fragment encompassing the presumed transcription initiation site of cx79.8. Expression of this construct (−5.4 cx79.8::EGFP) in stable transgenic animals resulted in strong GFP expression in the lens as well as weak expression in the brain in 3 day post-fertilization (dpf) zebrafish larvae (fig. 3A, B). Using high-resolution light sheet imaging (fig. 3C, D), more detail was evident in the GFP expression pattern in the lens, including outlines of fiber cells in the apical view (fig. 3D). With this imaging modality, it was evident that GFP expression in the brain was largely restricted to a series of cells along the midbrain-hindbrain boundary (fig. 3E-H). In a dorsal plane of focus (fig. 3E), weak GFP expression was visible in a sheet of cells in the granular eminence (ge) of the cerebellum. Moving ventrally to a region centered 44 μm lower (fig. 3F), dense clusters of well-labeled cells adjacent to the midline were in the valvula cerebelli, while similar clusters lateral and posterior to them may be cerebellar or may be within midbrain nuclei. In a projection centered 59 μm ventral to the previous (fig. 3G), the most lateral group of cells, originating in a midbrain nucleus, projected axons up to the optic tectum (higher magnification view in fig. 3H). In the more dorsal planes of focus, these projections could be seen spreading through the optic tectum in a single layer (arrowheads in fig. 3E, F). A z-axis projection in the region of these cells is shown in figure 3I. From this it is evident that there are interconnections within the labeled region as well as projections out of it (arrowheads). Further research will be required to identify these groups of neurons, their connections, and their projections. A group of cells adjacent to the inner ear appeared to be labeled via autofluorescence similar to surface iridopohores (fig. 3F-H).
Figure 3.
Marker gene expression driven by the cx79.8 promoter. (A, B) Conventional epifluorescence images of EGFP fluorescence in 3 dpf zebrafish fry with a high level of expression from stably-integrated −5.4 cx79.8::EGFP transgene constructs. Expression was prominent in the lens and a faint patch of fluorescence was also present at the midbrain-hindbrain boundary (arrowheads). (C-H) Light sheet images of 4 dpf zebrafish fry from the same line. (C) Ventral view showing expression in the lens. EGFP fluorescence is shown in green; the red channel shows autofluorescence detected in the Cy3 channel (shown in C and E-G). Autofluorescence is detected in both channels and appears orange to yellow, while EGFP fluorescence appears pure green. (D) Axial view of lens EGFP fluorescence at reduced gain to show detail of lens fiber cells. (E-H) Dorsal views at three planes of focus showing the web of EGFP-labeled neurons and processes at the midbrain-hindbrain boundary. The granular eminence (ge) of the cerebellum is labeled; see text for discussion of labeled cells (E) 21 μm thick projection at most dorsal level; (F) 25 μm thick projection centered 44 μm ventrally from E; (G) 84 μm thick projection centered 59 μm ventrally from F. (H) Higher magnification view of the projection neurons shown in G. (I) z-axis projection of labeled brain neurons. Some projections to the optic tectum and within the cerebellum/midbrain are marked with arrowheads. Scale bar dimensions in A are the same in B,C and E-G; other scale bars as noted.
In the retina, transient transgenic GFP expression was evident at 5 dpf diffusely in the inner and outer plexiform layers (fig. 4A), but no cell types could be resolved. To examine retinal expression more closely we examined three stable transgenic lines expressing the −5.4 cx79.8::EGFP construct. In adult zebrafish of all three lines, robust GFP expression was observed in photoreceptors morphologically consistent with cones (fig. 4B-D). Figure 4D shows adult retina from line 3 double-labeled with an antibody to cone arrestin. While this antibody appears to label only the members of the double cones in the zebrafish retina, most of these cones do express GFP in this line. Morphologically identified short single and long single cones also expressed GFP. Weak GFP expression was evident in some cells of the inner nuclear layer (INL) and ganglion cell layers (GCL) (fig. 4B, C). To test whether the transgene reporter expression was consistent with native gene expression, we performed in situ hybridization to label cx79.8 mRNA transcripts in wild type adult zebrafish. Figure 4E shows that the antisense probe labeled cells in the outer photoreceptor layer, consistent with cones, and in the INL and GCL. Sense strand labeling is shown in figure 4F. The signals produced with the antisense probe were uniformly weak, requiring long development time and resulting in relatively high background signal. However, the results indicate that cx79.8 transcripts were present in a similar set of cells as suggested by reporter gene expression.
Figure 4.
cx79.8 expression in the retina. (A) Transient EGFP expression (green) driven by the −5.4 cx79.8::EGFP construct in 5 dpf zebrafish fry. Nuclei are labeled with DAPI in blue. (B-D) Stable expression of EGFP in adult retina driven by the −5.4 cx79.8::EGFP transgene in line 1 (B), line 2 (C), and line 3 (D). Nuclei are labeled with DAPI in blue in (B) and (C), and a subset of cones is labeled with an antibody to cone arrestin in red in (D). (E, F) In situ hybridization of cx79.8 mRNA in wild type adult retina. Antisense strand probe labeling is shown in red in (E) and sense strand probe labeling is shown in (F); nuclei are labeled blue with DAPI. PR – photoreceptors; INL – inner nuclear layer; GCL – ganglion cell layer. Scale bars in C-F are the same dimensions as in B.
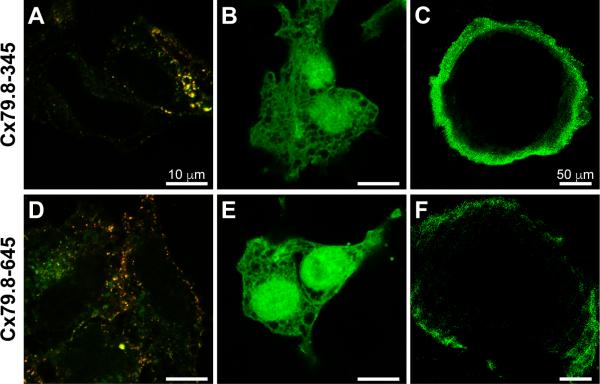
To examine localization of Cx79.8 we developed antibodies against two epitopes in the C-terminus of the protein. Both epitopes are located in regions that are not homologous between Cx79.8 and its closest homologue Cx44.1. The epitope for antibody Cx79.8-345 is located within the sequence contained in the Cx50.5 cDNA while that for antibody Cx79.8-645 is located well downstream (fig. 2). Both antibodies specifically labeled EGFP-tagged Cx79.8 expressed in HeLa cells (fig. 5A, D) but not EGFP alone (fig. 5B, E). In 5 dpf zebrafish fry both antibodies labeled abundant gap junctions in the outer layers of the lens (fig. 5C, F), as predicted by the marker gene expression analysis. Labeling was not present in the center of the lens. This could reflect proteolytic degradation of the C-termini of connexins in older lens fiber cells, as has been found for both Cx50 and Cx46 in sheep lens (Kistler et al., 1990; Lin et al., 1997), or it could represent initiation of Cx79.8 expression after formation of the lens nucleus. The fact that antibody Cx79.8-645 labeled natural gap junctions in the lens is further evidence that the open reading frame we describe coding for the 79.8 kDa protein is the natural one.
Figure 5.
Tests of anti-Cx79.8 antibodies. (A, D) Immunofluorescence labeling of Cx79.8-EGFP (green; intrinsic fluorescence) transiently transfected in HeLa cells using Cx79.8-345 antibody (red, A) or Cx79.8-645 antibody (red, D). Control labeling of EGFP empty vector transfected HeLa cells using the same labeling and imaging conditions is shown for Cx79.8-345 antibody in B and for Cx79.8-645 antibody in E. (C, F) Labeling of 5 dpf wild type zebrafish fry lens with Cx79.8-345 antibody (green, C) or Cx79.8-645 antibody (green, F). Both antibodies specifically label Cx79.8-EGFP and both label abundant gap junctions in the lens. Scale bars in A, B, D and E are of the same dimensions; scale bars in C and F are of the same dimensions.
In adult zebrafish retina, crude antiserum containing both antibodies yielded prominent clusters of signal at the level of cone inner segments that were larger than any ultrastructurally-identified gap junction from this area reported in fish (fig. 6A). These signals were not in rods, which were labeled in this sample via expression of GFP driven by a rhodopsin promoter. Fine punctate labeling more consistent with gap junctions was also present in all other layers of the retina. The separate, affinity-purified antibodies gave slightly different labeling patterns. Ab Cx79.8-345 labeled prominent clusters of objects in cone photoreceptor inner segments (fig. 6B), as well as fine punctate signals along the somata of cones, in the outer plexiform layer (OPL), and in the inner plexiform layer (IPL). The more C-terminally positioned Ab Cx79.8-645 gave abundant punctate label at the surface of cone photoreceptors largely along the inner segments, sparse punctate label in the OPL and INL, and abundant punctate label in the IPL (fig. 6C). The differences in labeling pattern within cones between the two antibodies suggests that some label in the cones may be non-specific, although the prominent expression of EGFP from the cx79.8 promoter in photoreceptors does suggest that the labeling could represent actual connexin expression. The punctate Cx79.8 immunostaining in the OPL and ONL resembled previously characterized immunostaining of photoreceptor gap junctions (e.g. (Li et al., 2009). The Cx79.8 labeling in the IPL resembled Cx35 labeling in this region (O'Brien et al., 2004; Kothmann et al., 2007), although it was weaker and sparser. This labeling could be contributed by any of the cell types that synapse in the IPL, including amacrine, bipolar, and ganglion cells. The immunostaining results suggest that several neuronal types express Cx79.8 in the zebrafish retina.
Figure 6.
Cx79.8 antibody labeling in adult retina. (A) Lableing with crude anti-Cx79.8 antiserum containing both antibodies (green) in retina of an opsin-GFP (red) transgenic zebrafish in which rod photoreceptors are labeled. Nuclei are labeled with DAPI in blue. Antibody labeling includes prominent clumps in the photoreceptor layer, but not in rods, as well as widespread punctate labeling. (B) Labeling with affinity-purified Cx79.8-345 antibody (green) in retina double-labeled with an antibody to cone arrestin (red). Note prominent clumps in some cone inner segments, fine punctate labeling along the surface of cone somata and terminals, and sparse punctate labeling in the IPL. (C) Labeling with affinity-purified Cx79.8-645 antibody (green) in retina double-labeled with an antibody to cone arrestin (red). Note fine punctate labeling along the surface of cone somata and abundant punctate labeling in the IPL. PR – photoreceptors; OPL – outer plexiform layer; IPL – inner plexiform layer. Scale bars are of the same dimensions in all panels.
Functional properties of Cx79.8
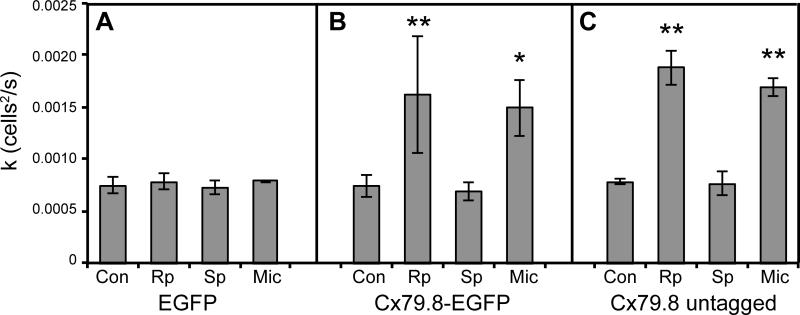
To examine functional properties of Cx79.8, we subcloned the cx79.8 open reading frame into the mammalian cell expression vectors EGFP-N1 with a C-terminal EGFP tag and pcDNA 3.1 untagged. We performed Neurobiotin tracer coupling experiments in transiently transfected HeLa cells, testing regulation of coupling by the protein kinase A (PKA) pathway. In a manner very similar to Cx35 (Ouyang et al., 2005; Patel et al., 2006), Cx79.8 showed very little coupling above the background in control conditions (fig. 7A, B), but showed a strong increase in coupling in the presence of a PKA inhibitor (Rp); activating PKA did not significantly change coupling.
Figure 7.
Tracer coupling supported by Cx79.8 in HeLa cells. Drug treatments in all panels include no treatment (Con), 10 μM PKA antagonist Rp-8-cpt-cAMPS (Rp), 10 μM PKA agonist Sp-8-cpt-cAMPS (Sp), and 0.5 nM PP2A antagonist Microcystin LR (Mic). (A) Transfection with EGFP empty vector alone; (B) transfection with Cx79.8-EGFP; (C) transfection with untagged Cx79.8. Data shown are mean diffusion coefficients for Neurobiotin transfer for 3 to 6 experiments; ** p<0.01, * p<0.05 using an unpaired T-test.
(Kothmann et al., 2009) showed that the PKA-driven uncoupling of rabbit AII amacrine cells mediated by Cx36 was a result of activation of protein phosphatase 2A (PP2A) by PKA activity. To test whether this signaling mechanism might be at work with Cx79.8 in HeLa cells, we tested the effects of 0.5 nM Microcystin LR, a selective inhibitor of PP2A. 15 minute pre-treatment with Microcystin increased coupling to a level comparable to that achieved by inhibiting PKA, suggesting that PP2A dephosphorylation was responsible for suppressing coupling in HeLa cells (fig. 7B). Finally, to test whether a C-terminal EGFP tag influenced regulation of coupling, we repeated the experiments above with untagged Cx79.8 (fig. 7C). All aspects of regulation of coupling were the same in the untagged connexin as we observed with the EGFP-tagged form.
DISCUSSION
Redundancy of lens connexin genes
Gap junction coupling is a critical feature of cellular organization in the vertebrate lens. The long-lived lens fiber cells persist throughout the life of the organism, maintaining metabolic equilibrium through solute exchange via extensive gap junction coupling in spite of the loss of most cellular organelles (Goodenough, 1992). In vertebrates that have been studied, two different connexins coexist in abundance among fiber cells. These are Gja3, e.g. Cx46 of mammals (Paul et al., 1991) and Cx56 of chicken (Rup et al., 1993), and Gja8, e.g. Cx50 of mouse (White et al., 1992) and Cx45.6 of chicken (Jiang et al., 1994). Cx43 (Gja1) is also expressed in lens, generally being restricted to the lens epithelium (Beyer et al., 1989; Musil et al., 1990). Mutations in either lens fiber cell connexin cause cataracts in humans (Mackay et al., 1997; Mackay et al., 1999), as does knockout of either connexin gene in mice (Gong et al., 1997; White et al., 1998), implying that the two connexins are not fully functionally redundant.
Homologues of all three connexins have been found in zebrafish lens (Cheng et al., 2003), with Gja3 homologue Cx48.5 and Gja8 homologue Cx44.1 present along with zebrafish Cx43. Morpholino knock-down of cx48.5 results in development of small lenses (Cheng et al., 2004), although a cataract phenotype has not been demonstrated related to a connexin in zebrafish. Our finding that a second Gja8 homologue, Cx79.8, is also expressed in zebrafish lens reveals a higher level of apparent redundancy in this species compared to mammals. The evolution of the teleost lineage includes a relatively recent whole genome duplication event that results, in general, in the presence of two genes orthologous to each gene present in mammals and most other vertebrates (Taylor et al., 2001). This situation allows for functional diversification of genes and has been proposed to have allowed for diversification of the teleost clade (Santini et al., 2009), along with many specific specializations. Duplicated orthologues of vertebrate genes are not necessarily found in the same tissues in teleosts, but they are in the case of the Gja8 pair in zebrafish lens. The extensive sequence divergence of the C-terminal domain of Gja8a (Cx79.8) and Gja8b (Cx44.1) suggests that these two connexins may have developed different properties that allow functional specialization in the lens.
A lens connexin is used in neuronal electrical synapses
While the vertebrate connexin family includes more than 20 members in mammals and nearly 40 in teleost fish (Cruciani and Mikalsen, 2006), very few are used in neurons to form electrical synapses (Meier and Dermietzel, 2006). Cx36 and its non-mammalian orthologues and paralogues are the dominant neuronal forms. However, a growing number of other connexins has been found to be expressed in limited sets of neurons, particularly in the retina. Among these, Cx50 (Gja8) has been found in axonless horizontal cells in rabbit retina (O'Brien et al., 2006), where it supports the most extensive coupling of any retinal network, and at a low expression level in mouse horizontal cells (Dorgau et al., 2015). Thus it is not entirely surprising that we have found zebrafish Cx79.8 expressed in retinal neurons and in neurons elsewhere in the central nervous system. Curiously, we did not find evidence of Cx44.1 expression in the retina, suggesting that functional specializations of the two connexins may make Cx79.8 more suitable for use as an electrical synapse.
The most surprising aspect of the expression pattern of Cx79.8 was its expression in cone photoreceptors. Cone photoreceptors in fish have already been shown to use two connexins, Cx35 and Cx34.7, which both make very small gap junctions among the photoreceptor terminals and telodendria (O'Brien et al., 2004). Labeling with anti-Cx79.8 antibodies gave some indication that there may be some small Cx79.8 gap junctions on photoreceptor terminals, but the two antibodies also produced quite unexpected labeling of protein along cone somata and inner segments. It is not clear that this represents gap junctional protein. Indeed the dense clusters labeled by Ab Cx79.8-345 appear to be within the cytoplasm of cones, and Ab Cx79.8-645 does not appear to label these clusters. Is this a result of non-specific labeling? This is possible, but the strong expression of GFP in cones driven by the cx79.8 promoter suggests that the connexin should be present in these cells. Differences in labeling by the two antibodies could be a result of proteolytic loss of the more C-terminally situated 645 epitope in some pools of protein, or perhaps masking of either epitope in some pools of protein. If this is the case, particularly the former hypothesis, the strongly labeled clusters may represent Cx79.8 in a compartment where it is degraded.
If the punctate labeling at the surface of cone somata and inner segments represents gap junctional connexins, this represents a significant pool of gap junctions that are molecularly distinct from those among the photoreceptor terminals. There is substantial evidence that gap junctions exist among the somata and inner segments of cones. These were detected by electron microscopy in the perinuclear region of catfish cones (Witkovsky et al., 1974). In amphibian retina, gap junctions are very numerous among the myoid fins that radiate from the inner segments of both rods and cones (Custer, 1973; Fain et al., 1976). In salamander, these have been shown to contain Cx35 in both rods and cones (Zhang and Wu, 2004). Thus it is conceivable that the Cx79.8 labeling represents a previously unrecognized set of gap junctions among zebrafish cone photoreceptors.
Outside of photoreceptors, punctate labeling for Cx79.8 was widespread in the inner plexiform layer of the retina. Presuming that these represent gap junctions, this connexin may be used in a wide variety of circuits. All of the presumed gap junctions were very small, with none approaching the large plaques seen in some horizontal cells. GFP reporter expression also indicates that this connexin is expressed in a limited number of neurons outside of the retina, including in the cerebellum. Additional research is required to characterize these neurons.
Plasticity of Cx79.8 electrical synapses
Our tracer coupling experiments in transfected HeLa cells show that Cx79.8 is capable of extensive functional plasticity. Inhibition of protein kinase A or protein phosphatase 2A opened up tracer coupling more than 2-fold higher than the baseline, indicating that a signaling pathway involving PKA activation of PP2A and dephosphorylation of Cx79.8 suppresses coupling. Like fish Cx35, Cx79.8 showed almost no tracer coupling above background in transiently transfected HeLa cells. HeLa cells support background tracer coupling mediated by connexin 45 expression. This coupling generally shows no significant changes with most pharmacological treatments (fig. 7A, but see Wang et al., 2015 for a very small response to PKA inhibitor). Keeping in mind that the control coupling in Cx79.8-EGFP and Cx79.8 untagged experiments is at the same level as the Cx45 background, the coupling opened up by PKA or PP2A inhibitors represents a far greater dynamic range than the two-fold indicated in the experiments, and shows that Cx79.8 channels are capable of almost complete closure when conditions suppress phosphorylation of the connexin. This property is similar to that of Cx35 and Cx36, and appears to be a useful property for an electrical synapse. Zebrafish photoreceptors support very extensive tracer coupling at night in darkness, but become almost completely uncoupled in the daytime (Li et al., 2009), showing a dynamic range of about 20-fold (Li and O'Brien, 2012). We have previously presumed that this photoreceptor coupling was supported largely by Cx35 gap junctions, but the results of this study suggest that Cx79.8 may specifically contribute to coupling among the cones.
In stark contrast to its paralogue Cx44.1, Cx79.8 has a remarkably long C-terminal domain, the longest of any currently described connexin. We believe that it is likely that this C-terminal domain is proteolytically processed in the central portion of the lens, as suggested by loss of antibody labeling in this area even in young zebrafish fry. Proteolytic cleavage of the C-terminus of mammalian Cx50 has been found to reduce its sensitivity to acidification-induced channel closure (Lin et al., 1998), and might be expected to reduce the dependence of Cx79.8 opening on phosphorylation. Such processing might stabilize the open channels needed for metabolic homeostasis in the lens. Of course, further experiments will be required to test this hypothesis. Within retinal neurons, only very small clusters that contain the full Cx79.8 protein were detected using the Cx79.8-645 antibody. It remains possible that neurons also contain proteolytically cleaved Cx79.8 in gap junctions that is not detectable by either of our antibodies.
Acknowledgements
Supported by NIH grant EY012857 (JO), core grant EY10608, the Frederic B. Asche endowment (JO), and a challenge grant to the Ruiz Department of Ophthalmology & Visual Science from Research to Prevent Blindness.
REFERENCES
- Beyer EC, Kistler J, Paul DL, Goodenough DA. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Christie T, Valdimarsson G. Expression of connexin48.5, connexin44.1, and connexin43 during zebrafish (Danio rerio) lens development. Dev Dyn. 2003;228:709–715. doi: 10.1002/dvdy.10436. [DOI] [PubMed] [Google Scholar]
- Cheng S, Shakespeare T, Mui R, White TW, Valdimarsson G. Connexin 48.5 is required for normal cardiovascular function and lens development in zebrafish embryos. J Biol Chem. 2004;279:36993–37003. doi: 10.1074/jbc.M401355200. [DOI] [PubMed] [Google Scholar]
- Cruciani V, Mikalsen SO. The vertebrate connexin family. Cell Mol Life Sci. 2006;63:1125–1140. doi: 10.1007/s00018-005-5571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti S, O'Brien J. Characteristics and plasticity of electrical synaptic transmission. BMC Cell Biol 17 Suppl. 2016;1:13. doi: 10.1186/s12860-016-0091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custer NV. Structurally specialized contacts between the photoreceptors of the retina of the axolotl. J Comp Neurol. 1973;151:35–56. doi: 10.1002/cne.901510104. [DOI] [PubMed] [Google Scholar]
- Deans MR, Paul DL. Mouse horizontal cells do not express connexin26 or connexin36. Cell Adhes Commun. 2001;8:361–366. doi: 10.3109/15419060109080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corsso C, Iglesias R, Zoidl G, Dermietzel R, Spray DC. Calmodulin dependent protein kinase increases conductance at gap junctions formed by the neuronal gap junction protein connexin36. Brain Res. 2012 doi: 10.1016/j.brainres.2012.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Kremer M, Paputsoglu G, Stang A, Skerrett IM, Gomes D, Srinivas M, Janssen-Bienhold U, Weiler R, Nicholson BJ, Bruzzone R, Spray DC. Molecular and functional diversity of neural connexins in the retina. J Neurosci. 2000;20:8331–8343. doi: 10.1523/JNEUROSCI.20-22-08331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorgau B, Herrling R, Schultz K, Greb H, Segelken J, Stroh S, Bolte P, Weiler R, Dedek K, Janssen-Bienhold U. Connexin50 couples axon terminals of mouse horizontal cells by homotypic gap junctions. J Comp Neurol. 2015;523:2062–2081. doi: 10.1002/cne.23779. [DOI] [PubMed] [Google Scholar]
- Fain GL, Gold GH, Dowling JE. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar NM, Horwitz J, Gilula NB. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–843. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3:49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- Jiang JX, White TW, Goodenough DA, Paul DL. Molecular cloning and functional characterization of chick lens fiber connexin 45.6. Mol Biol Cell. 1994;5:363–373. doi: 10.1091/mbc.5.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler J, Berriman J, Evans CW, Gruijters WT, Christie D, Corin A, Bullivant S. Molecular portrait of lens gap junction protein MP70. J Struct Biol. 1990;103:204–211. doi: 10.1016/1047-8477(90)90038-e. [DOI] [PubMed] [Google Scholar]
- Klaassen LJ, Sun Z, Steijaert MN, Bolte P, Fahrenfort I, Sjoerdsma T, Klooster J, Claassen Y, Shields CR, Ten Eikelder HM, Janssen-Bienhold U, Zoidl G, McMahon DG, Kamermans M. Synaptic transmission from horizontal cells to cones is impaired by loss of connexin hemichannels. PLoS Biol. 2011;9:e1001107. doi: 10.1371/journal.pbio.1001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Li X, Burr GS, O'Brien J. Connexin 35/36 is phosphorylated at regulatory sites in the retina. Vis Neurosci. 2007;24:363–375. doi: 10.1017/S095252380707037X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothmann WW, Massey SC, O'Brien J. Dopamine-stimulated dephosphorylation of connexin 36 mediates AII amacrine cell uncoupling. J Neurosci. 2009;29:14903–14911. doi: 10.1523/JNEUROSCI.3436-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chuang AZ, O'Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, O'Brien J. Regulation of gap junctional coupling in photoreceptors. In: Akutagawa E, Ozaki K, editors. Photoreceptors: Physiology, Types and Abnormalities. Nova Science; Hauppauge, NY: 2012. pp. 97–112. [Google Scholar]
- Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O'Brien J. Adenosine and Dopamine Receptors Coregulate Photoreceptor Coupling via Gap Junction Phosphorylation in Mouse Retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Eckert R, Kistler J, Donaldson P. Spatial differences in gap junction gating in the lens are a consequence of connexin cleavage. Eur J Cell Biol. 1998;76:246–250. doi: 10.1016/s0171-9335(98)80002-2. [DOI] [PubMed] [Google Scholar]
- Lin JS, Fitzgerald S, Dong Y, Knight C, Donaldson P, Kistler J. Processing of the gap junction protein connexin50 in the ocular lens is accomplished by calpain. Eur J Cell Biol. 1997;73:141–149. [PubMed] [Google Scholar]
- Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S, Shiels A. A new locus for dominant “zonular pulverulent” cataract, on chromosome 13. Am J Hum Genet. 1997;60:1474–1478. doi: 10.1086/515468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattacharya S. Connexin46 Mutations in Autosomal Dominant Congenital Cataract. Am J Hum Genet. 1999;64:1357–1364. doi: 10.1086/302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier C, Dermietzel R. Electrical synapses--gap junctions in the brain. Results Probl Cell Differ. 2006;43:99–128. doi: 10.1007/400_013. [DOI] [PubMed] [Google Scholar]
- Miller CS, Kothmann WW, O'Brien J. Bistratified Amacrine Cells in Zebrafish Show Lucifer Yellow Coupling. Investigative Ophthalmology & Visual Science. 2008;49:3049–3049. [Google Scholar]
- Mills SL, Massey SC. The kinetics of tracer movement through homologous gap junctions in the rabbit retina. Vis Neurosci. 1998;15:765–777. doi: 10.1017/s0952523898154159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- O'Brien J. The ever-changing electrical synapse. Curr Opin Neurobiol. 2014;29C:64–72. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Al-Ubaidi MR, Ripps H. Connexin 35: a gap-junctional protein expressed preferentially in the skate retina. Molecular Biology of the Cell. 1996;7:233–243. doi: 10.1091/mbc.7.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Bruzzone R, White TW, Al-Ubaidi MR, Ripps H. Cloning and expression of two related connexins from the perch retina define a distinct subgroup of the connexin family. J Neurosci. 1998;18:7625–7637. doi: 10.1523/JNEUROSCI.18-19-07625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J, Nguyen HB, Mills SL. Cone photoreceptors in bass retina use two connexins to mediate electrical coupling. J. Neurosci. 2004;24:5632–5642. doi: 10.1523/JNEUROSCI.1248-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. J Neurosci. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X, Winbow VM, Patel LS, Burr GS, Mitchell CK, O'Brien J. Protein kinase A mediates regulation of gap junctions containing connexin35 through a complex pathway. Brain Res Mol Brain Res. 2005;135:1–11. doi: 10.1016/j.molbrainres.2004.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Sonntag S, Skeberdis VA, Willecke K, Bukauskas FF. Gating, permselectivity and pH-dependent modulation of channels formed by connexin57, a major connexin of horizontal cells in the mouse retina. J Physiol. 2009;587:3251–3269. doi: 10.1113/jphysiol.2009.171496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Keung J, Kim IB, Snuggs MB, Mills SL, O'Brien J, Massey SC. Connexin 57 is expressed by the axon terminal network of B-type horizontal cells in the rabbit retina. J Comp Neurol. 2012;520:2256–2274. doi: 10.1002/cne.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel LS, Mitchell CK, Dubinsky WP, O'Brien J. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes. 2006;13:41–54. doi: 10.1080/15419060600631474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Bell TD, Chang BH, Czernik AJ, Nairn AC, Soderling TR, Faber DS. Ca2+/calmodulin-dependent kinase II mediates simultaneous enhancement of gapjunctional conductance and glutamatergic transmission. Proc Natl Acad Sci U S A. 1998;95:13272–13277. doi: 10.1073/pnas.95.22.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Curti S, Vanderpool KG, Kamasawa N, Nannapaneni S, Palacios-Prado N, Flores CE, Yasumura T, O'Brien J, Lynn BD, Bukauskas FF, Nagy JI, Pereda AE. Molecular and functional asymmetry at a vertebrate electrical synapse. Neuron. 2013;79:957–969. doi: 10.1016/j.neuron.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Kamasawa N, Vanderpool KG, Yasumura T, O'Brien J, Nannapaneni S, Pereda AE, Nagy JI. Heterotypic gap junctions at glutamatergic mixed synapses are abundant in goldfish brain. Neuroscience. 2015;285:166–193. doi: 10.1016/j.neuroscience.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rup DM, Veenstra RD, Wang HZ, Brink PR, Beyer EC. Chick connexin-56, a novel lens gap junction protein. Molecular cloning and functional expression. J Biol Chem. 1993;268:706–712. [PubMed] [Google Scholar]
- Santini F, Harmon LJ, Carnevale G, Alfaro ME. Did genome duplication drive the origin of teleosts? A comparative study of diversification in ray-finned fishes. BMC Evol Biol. 2009;9:194. doi: 10.1186/1471-2148-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields CR, Klooster J, Claassen Y, Ul-Hussain M, Zoidl G, Dermietzel R, Kamermans M. Retinal horizontal cell-specific promoter activity and protein expression of zebrafish connexin 52.6 and connexin 55.5. J Comp Neurol. 2007;501:765–779. doi: 10.1002/cne.21282. [DOI] [PubMed] [Google Scholar]
- Sohl G, Degen J, Teubner B, Willecke K. The murine gap junction gene connexin36 is highly expressed in mouse retina and regulated during brain development. FEBS Lett. 1998;428:27–31. doi: 10.1016/s0014-5793(98)00479-7. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol (Lond) 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas M, Rozental R, Kojima T, Dermietzel R, Mehler M, Condorelli DF, Kessler JA, Spray DC. Functional properties of channels formed by the neuronal gap junction protein connexin36. J Neurosci. 1999;19:9848–9855. doi: 10.1523/JNEUROSCI.19-22-09848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–1679. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, Guldenagel M, Bukauskas FF, Trexler EB, Verselis VK, De Zeeuw CI, Lee CG, Kozak CA, Petrasch-Parwez E, Dermietzel R, Willecke K. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang VC, Wilkins PP. Optimum dissociating condition for immunoaffinity and preferential isolation of antibodies with high specific activity. J Immunol Methods. 138:1991, 291–299. doi: 10.1016/0022-1759(91)90178-i. [DOI] [PubMed] [Google Scholar]
- Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Lin YP, Mitchell CK, Ram S, O'Brien J. Two-color fluorescent analysis of connexin 36 turnover: relationship to functional plasticity. J Cell Sci. 2015;128:3888–3897. doi: 10.1242/jcs.162586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143:815–825. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Shakib M, Ripps H. Interreceptoral junctions in the teleost retina. Invest Ophthalmol. 1974;13:996–1009. [PubMed] [Google Scholar]
- Zhang J, Wu SM. Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina. J Comp Neurol. 2004;470:1–12. doi: 10.1002/cne.10967. [DOI] [PubMed] [Google Scholar]
- Zimmerman AL, Rose B. Permeability properties of cell-to-cell channels: kinetics of fluorescent tracer diffusion through a cell junction. J Membr Biol. 1985;84:269–283. doi: 10.1007/BF01871390. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Bruzzone R, Weickert S, Kremer M, Zoidl C, Mitropoulou G, Srinivas M, Spray DC, Dermietzel R. Molecular cloning and functional expression of ZfCx52.6: A novel connexin with hemichannel-forming properties expressed in horizontal cells of the zebrafish retina. J. Biol. Chem. 2004;279:2913–2921. doi: 10.1074/jbc.M304850200. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Kremer M, Zoidl C, Bunse S, Dermietzel R. Molecular diversity of connexin and pannexin genes in the retina of the zebrafish Danio rerio. Cell Commun Adhes. 2008;15:169–183. doi: 10.1080/15419060802014081. [DOI] [PubMed] [Google Scholar]