Abstract
Background
Recent studies have demonstrated the superiority of endovascular therapy (EVT) for emergent large vessel occlusion.
Objective
To determine the effectiveness of EVT in nonagenarians, for whom data are limited.
Methods
We retrospectively reviewed clinical and imaging data of all patients who underwent EVT at two stroke centers between January 2012 and August 2014. The 90-day functional outcome (modified Rankin Scale (mRS) score) was compared between younger patients (age 18–89 years; n=175) and nonagenarians (n=18). The relationship between pre-stroke and 90-day post-stroke mRS was analyzed in these two groups. Multivariable analysis of age, recanalization grade, and admission National Institutes of Health Stroke Scale (NIHSS) for predicting outcome was performed.
Results
Age ≥90 years was associated with a poor (mRS >2) 90-day outcome relative to those under 90 (89% vs 52%, OR=8, 95% CI 1.7 to 35.0; p=0.0081). Nonagenarians had a higher pre-stroke mRS score (0.77; 95% CI 0.44 to 1.30) than younger patients (0.24; 95% CI 0.17 to 0.35; p=0.005). No difference was observed between nonagenarians and younger patients in the rate of mRS change from pre-stroke to 90 days (p=0.540). On multivariable regression, age (OR=1.05, 95% CI 1.03 to 1.08; p<0.0001), recanalization grade (OR=0.62 95% CI 0.42 to 0.91; p=0.015), and admission NIHSS (OR=1.07 95% CI 1.02 to 1.13; p=0.01) were associated with a poor 90-day outcome.
Conclusions
Nonagenarians are at a substantially higher risk of a poor 90-day outcome after EVT than younger patients. However, a small subset of nonagenarians may benefit from EVT, particularly if they have a good pre-stroke functional status. Further research is needed to identify factors associated with favorable outcome in this age cohort.
Keywords: acute ischemic stroke, endovascular therapy, nonagenarians, outcome
Introduction
The incidence of stroke doubles in octogenarians and mortality triples for patients above the age of 85 in comparison with younger patients with stroke.1, 2 The very elderly population is growing rapidly worldwide. In the USA the number of people aged >85 years, who currently constitute 2% of the total population, is projected to double by 2060.3 This growth of the aging population combined with their higher incidence of stroke is expected to significantly increase the number of very old patients presenting with a hyperacute stroke.
Recently, five randomized controlled trials established the efficacy of endovascular therapy (EVT) in patients with emergent large vessel occlusion (ELVO).4–9 Subgroup analyses in these trials indicated the efficacy of treatment in the seventh and eighth decade of life.4, 5, 7, 8, 10 However, substantial uncertainty exists about treatment efficacy for those in the ninth decade of life and no studies have systematically assessed the efficacy of EVT in nonagenarians. Earlier studies compared outcomes after IV thrombolysis in nonagenarians versus younger patients.11, 12 In light of the overall aging population, it is expected that an increasing number of nonagenarians with ELVO will meet the criteria for EVT.4–9 Therefore, it is important to better understand the efficacy of EVT in this age group and to define factors that might help to identify patients likely to benefit from therapy.
We sought to determine functional outcomes in nonagenarians after EVT for ELVO. We hypothesize that nonagenarians frequently have a poor 90-day functional outcome after EVT as compared with younger patients. In addition, we hypothesized that nonagenarians have a sharper decline in modified Rankin Scale (mRS) score (worse 90-day outcome relative to their pre-stroke function) than younger patients.
Methods
Study Sample
We retrospectively analyzed consecutive, prospectively collected patients with ELVO who were treated with EVT at two academic stroke centers between January 2012 and August 2014. All patients underwent head CT and CT angiography at admission. Patient demographics, National Institutes of Health Stroke Scale (NIHSS) score, laboratory data, comorbidities, pre-admission medications, and stroke etiology (using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification)13 were collected for all patients. Functional disability was assigned a mRS score pre-stroke and 90 days post-stroke. This study was reviewed and approved by institutional review boards at Lifespan Hospital System and the University of Massachusetts, Worcester, Massachusetts, USA.
Digital Subtraction Angiography
All endovascular procedures were performed by senior interventional neuroradiologists and radiologists using biplane angiography unit with 3D rotational angiography capability (Allura Xper FD20/20; Philips Medical Systems, Best, the Netherlands) with patients under conscious sedation or general anesthesia. Endovascular access was obtained via a standard transfemoral approach. Final recanalization was assessed according to the Thrombolysis In Cerebral Infarction (TICI) classification, as previously defined.14 Good recanalization was defined as TICI 2b and TICI 3.14 Hemorrhagic transformation on follow-up imaging was determined according to the European Cooperative Acute Stroke Study (ECASS) definition.15
Statistics
Demographic, clinical, and angiographic variables were compared between nonagenarians and patients younger than 90 years of age. Continuous variables are reported as mean±SD or as median (IQR).
All analyses were conducted using SAS Software V.9.4 (SAS Inc, Cary, North Carolina, USA) and all modeling was accomplished using the GLIMMIX procedure. Because the mRS score is not a continuous variable, it was examined using a generalized linear model assuming a binomial distribution (ie, scores 0–6) and a logistic regression model when mRS was dichotomized as favorable versus poor. We defined a favorable outcome as mRS 0–3 because of frequent pre-existing functional deficits in the oldest old16 and because people of advanced age identify the ability to walk (mRS 3) as a key marker of functional independence.17–19 Nevertheless, we conducted additional analyses dichotomizing the outcome to mRS 0–2 versus 3–6 as this definition is more often used in clinical studies. Predictors of interest included age, admission NIHSS, recanalization grade, and age dichotomized to 18–89 years versus ≥90 years. When multivariable models were examined, multicollinearity was assessed and found to have a negligible effect on model coefficient estimates and their significance in comparison with single variable models. In addition, the change within and between patients on pre-stroke and 90-day mRS was examined using a generalized mixed multivariate model assuming a binomial distribution. As a conservative effort, sandwich estimation was used to correct any covariance model misspecification. Differences between octogenarians and nonagenarians, before and after stroke, were estimated using least squares estimation, and relationships with binary outcomes were assessed using ORs. Categorical differences between nonagenarians and younger patients were assessed with Fisher’s exact test using the FREQ procedure. Multiple comparisons were evaluated using the Tukey method. Significance was established at the 0.05 level (two-sided) and all interval estimates were calculated for 95% confidence.
Results
A total of 210 patients underwent EVT for ELVO at the two centers during the study period. Of these, 193 patients had complete datasets available for inclusion in the study. Eighteen (9.3%) patients were nonagenarians and 175 (90.7%) were aged 18–89 years (including 45 octogenarians). Baseline clinical and radiologic characteristics of the nonagenarian and younger patients are summarized in table 1. Mean pretreatment NIHSS score was 18 for nonagenarians and 17 for younger patients. Mean pre-stroke mRS was higher in nonagenarians (0.77) than in younger patients (0.24) (p=0.005). The frequency of good recanalization (TICI 2b or 3) was not significantly different in nonagenarians and younger patients (67% vs 62%, p=0.999). Further, the incidence of parenchymal hemorrhage (PH type I and type II) (33.3% vs 26.3% p=0.58) and infarct volume (130 vs 84 mL, p=0.08) did not differ between nonagenarians and younger patients.
Table 1.
Baseline characteristics of the cohort as stratified by age
| Variable | Age < 90 (n=175) | Age > 90 (n= 18) | p=value |
|---|---|---|---|
| Age, y, mean (SD) | 68.4 (±13.7) | 91.9 (±2.3) | |
| Female | 75 (43%) | 11(61%) | 0.212 |
| Hypertension | 139 (79.4%) | 15 (83.3%) | 0.999 |
| Hyperlipidemia | 101 (57.7%) | 10 (55.5%) | 0.999 |
| Atrial Fibrillation | 63 (36%) | 13 (72.2%) | 0.004 |
| IV tPA | 84 (48%) | 12 (66.6%) | 0.146 |
| NIHSS, mean (SD) | 16.92(6.27) | 18.28 (5.44) | 0.379 |
| Pre stroke mRS (CI) | 0.24 (0.17–0.35) | 0.77 (0.44–1.28) | 0.005 |
| mRS 0 | 130 | 5 | |
| mRS 1 | 24 | 8 | |
| mRS 2 | 9 | 4 | |
| mRS 3 | 12 | 1 | |
| Location of arterial occlusion | |||
| Internal carotid artery | 51 (29.1%) | 6 (33.3%) | 0.920 |
| Middle cerebral artery | 109 (62.3%) | 12 (66.7%) | 0.910 |
| Basilar artery | 8 (4.6%) | 0 (0.0%) | 0.999 |
| Others | 7 (1.7%) | 0 (0.0%) | 0.999 |
| Complete recanalizationa | 109 (62.3%) | 12 (66.6%) | 0.999 |
| Infarct Volume, mL (CI) | 84 [68–104] | 130 [83–203] | 0.0832 |
| Parenchymal Hemorrhage | 46 (26.3%) | 6 (33.3%) | 0.579 |
| mRS 3–6 at 90 days | 91 (52%) | 16 (88.9%) | 0.0027 |
| mRS 4–6 at 90 days | 73 (41%) | 14 (77%) | 0.006 |
Data are n (%) or mean (± standard deviation SD). CI=95% confidence interval; IV=intravenous; mRS=modified Rankin Scale; NIHSS=National Institutes of Health Stroke Scale; PH=Parenchymal Hemorrhage Type 1 (PH1) and 2 (PH2); tPA=recombinant tissue plasminogen activator
Defined as Thrombolysis In Cerebral Infarction (TICI) 2b and 3
Defined as PH1 and PH2
No significant interaction effects were found among patient age, recanalization, and admission NIHSS and dichotomized mRS. The odds of a poor outcome (mRS>3) increased 5% with every 1 year increase in age, decreased 40% with each 1 grade increase in recanalization, and increased 5% with every 1 point increase in admission NIHSS (though only approached significance), respectively (table 2). Similarly, the odds of a poor outcome defined as mRS >2 increased 5.1% with every 1 year increase in age, decreased 38% with each 1 grade increase in recanalization, and increased 7% with every 1 point increase in admission NIHSS, respectively (table 3).
Table 2.
Multivariable predictors of a poor outcome (mRS>3) at 3 months with age as a continuous variable
| Independent variable | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age | 1.049 (1.024–1.075) | 0.0001 |
| Admission NIHSS | 1.051 (0.999–1.106) | 0.0526 |
| Recanalization | 0.603 (0.424–0.858) | 0.0052 |
NIHSS=National Institutes of Health Stroke Scale.
Age, recanalization grade and NIHSS were independently associated with 90 day outcome.
Table 3.
Multivariable predictors of a poor outcome (mRS>2) at 3 months with age as a continuous variable
| Independent variable | Odds Ratio (95% CI) | P-value |
|---|---|---|
| Age | 1.051 (1.027–1.076) | <0.0001 |
| Admission NIHSS | 1.07 (1.017–1.126) | 0.0098 |
| Recanalization | 0.622 (0.424–0.912) | 0.0153 |
NIHSS=National Institutes of Health Stroke Scale.
Age, recanalization grade and NIHSS were independently associated with 90 day outcome.
An inverse association between recanalization grade and mRS was found on linear trend analysis (p=0.014)—that is, more complete recanalization (higher TICI score) resulted in better functional outcomes (lower 90-day mRS). Specifically, least squares estimates were used to examine mRS scores for each recanalization grade (mean (95% CI)): TICI 0 (4.4 (3.4 to 5.2)), TICI 1 (4.8 (3.5 to 5.8)), TICI 2a (3.9 (3.3 to 4.6)), TICI 2b (3.1 (2.7 to 3.6)), TICI 3 (3.4 (2.1 to 4.6)).
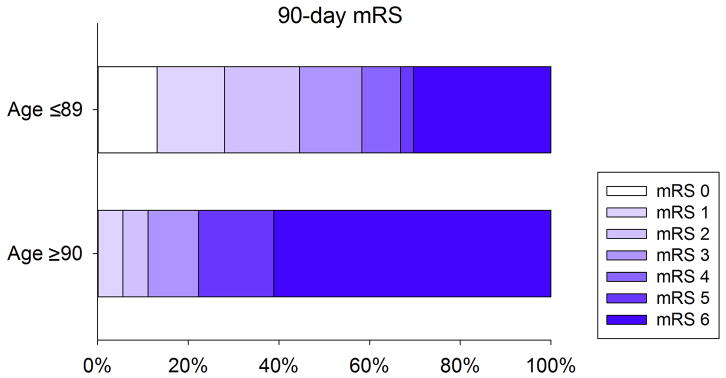
The odds of a poor outcome (mRS>3) were five times greater for nonagenarians than for younger patients (OR=5.0, 95% CI 1.6 to 16.2; p=0.006, figure 1). When we used the more frequently used definition of a poor outcome defined as mRS >2, the odds of a poor outcome were eight times greater for nonagenarians than for younger patients (OR=7.7, 95% CI 1.7 to 35.0; p=0.0081).
Figure 1. Distribution of 90 day Modified Rankin Scale in nonagenarians and younger patients.
Nonagenarians had worse 90 day mRS as compared to younger patients with a high mortality after endovascular therapy (P=0.006).
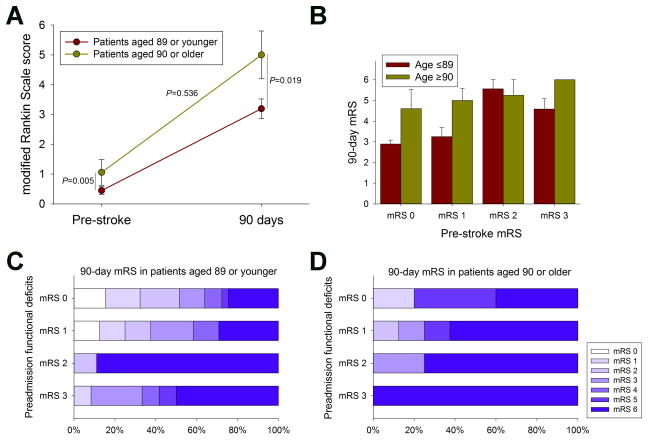
Overall, nonagenarians had higher pre-stroke mRS scores (0.77; 95% CI 0.4 to 1.3) than younger patients (0.24; 95% CI 0.2 to 0.4; p=0.005; table 1). To better understand the association of the pre-stroke mRS with the 90-day outcome we conducted several additional analyses. First, we examined the changes within and between patients on the mean pre-stroke and 90-day mRS, respectively. Consistent with the multivariable analysis, 90-day mean mRS scores were higher (5.3; 95% CI 4.3 to 5.7) in nonagenarians than in younger patients (3.5; 95% CI 3.0 to 4.0) p=0.019 (figure 2A). Interestingly, however, there was no difference in the rate (delta) of mRS worsening from baseline to 90 days between the two age groups (p=0.536; figure 2A). When we stratified analyses according to the pre-stroke mRS, the 90-day mRS was similar in nonagenarians and younger patients in each pre-stroke mRS category (figure 2B). Lastly, when we analyzed the 90-day mRS score distribution by the pre-stroke mRS score categories, we found that nonagenarians essentially achieved a favorable outcome only when they had no or only mild pre-stroke functional deficits (figure 2C, D). Together, these data indicate that the poor outcome among nonagenarians subjected to EVT is largely driven by the severity of pre-existing functional deficits rather than being a phenomenon of the chronological age itself. Lastly, we analyzed our cohort for outcomes in two elderly groups (octogenarians vs nonagenarians) to evaluate any rationale for excluding nonagenarians from EVT for ELVO. We found no significant difference between the 90-day mRS scores for octogenarians and nonagenarians (p=0.740; figure 3).
Figure 2. Association between pre-stroke and 90 day modified Rankin Scale (mRS) scores of nonagenarians and younger patients.
(A) Nonagenarians had higher pre-stroke as well as 90 day mRS scores as compared to younger patient. However, there was no difference in the rate of magnitude of mRS worsening between the two groups (data are mean ± 95% confidence intervals). (B) Nonagenarians had an overall similar 90 day mRS compared to younger patients when stratified by the pre stroke mRS category (P>0.05; data are mean±s.e.m.). Analysis of the 90 day mRS score distribution according to the pre stroke mRS score categories shows that (C) patients under the age of ninety achieved a favorable outcome even when having a pre stroke mRS of 3. (D) Conversely, among nonagenarians essentially only those achieved a favorable outcome who had no or only mild pre stroke functional deficits.
Figure 3. Comparison of 90 day modified Rankin Scale score between octogenarians and nonagenarians.
Octogenarians and nonagenarians had similar 90 day mRS after endovascular therapy.
Discussion
The most important finding of our study is that although nonagenarians have a fivefold higher risk of having an mRS >3 by 90 days, the outcome was largely related to the severity of pre-stroke functional deficits. Examination of the rate of functional decline in nonagenarians from pre-stroke to 90 days, as well as stratification of the 90-day functional outcome by the pre-stroke mRS, indicated that baseline function is a key determinant of the final outcome in nonagenarians. In our cohort, almost all nonagenarians with a good functional outcome had no pre-stroke disability. This is an important finding as it suggests that nonagenarians with a good pre-stroke functional status are most likely to benefit from EVT for ELVO. It highlights the need to consider overall functional status rather than the chronological age itself when making a decision about EVT in the oldest old. Specifically, nonagenarians with an excellent pre-stroke functional status (mRS 0–1) have a reasonable chance of a favorable outcome with EVT. Importantly, when we reanalyzed our data defining good outcome as mRS 0–2, overall results were similar, suggesting that our result extrapolate to results gained from previous clinical EVT studies.
Previous studies showed that advanced age is a strong predictor of poor outcome after EVT.20, 21 However, subgroup analyses from the recent positive endovascular stroke trials indicated that this might not hold true when the latest generation of recanalization devices are used.10 Yet, whether this also applies to nonagenarians remains to be shown. Our study found that nonagenarians have a higher likelihood than younger patients of having a poor functional status at 90 days after undergoing EVT for ELVO. We hypothesized that nonagenarians would experience a sharper functional decline after stroke than younger patients as measured by the mRS. However, although nonagenarians had a poorer functional status before and after stroke relative to younger patients, there was no difference in the rate of decline between the two groups. Further analysis of ongoing epidemiological studies of aging populations is needed to understand the predictors of outcome after severe stroke in this group.22
Various factors have been shown to modulate functional outcomes in patients of advanced age undergoing EVT. PH rates are reported to be higher in older patients, leading to higher morbidity and mortality.20, 21 This high rate of PH may be related to a higher incidence of cardioembolic stroke, use of anticoagulants, hypertension, and leukoarariosis.23, 24 We did not find a significantly higher rate of PH in nonagenarians, which is consistent with earlier studies, which did not show a higher rate of PH in older patients,25–27 highlighting the relative safety of EVT in nonagenarians. However, we did not determine the incidence of symptomatic hemorrhage in our cohort.
Recanalization is an important determinant of outcome in patients with ELVO.28, 29 Indeed, high recanalization rates are considered the main reason for the remarkable functional outcomes seen in the recent EVT trials.30, 31 Importantly, recanalization was a predictor of outcome independent of age and stroke severity in our cohort, which is consistent with earlier studies.29, 32, 33 The rates were similar in the two study age groups. Previous studies have also shown that elderly patients have similar recanalization rates to those of younger patients.15, 19, 28, 29 However, some studies have shown a trend towards lower recanalization rates in the elderly.16, 20, 21 Advanced age is associated with an increased tortuosity of the cerebral vasculature, which may increase the technical difficulty of endovascular procedures and result in less complete recanalization.21, 34 In addition, collateral blood supply may be worse in older patients, leading to earlier tissue loss and therefore worse functional outcomes than in younger patients. An important predictor of symptomatic intracranial hemorrhage (sICH) in patients undergoing EVT is leukoaraiosis, which increases with age.35 It has been noticed that leukoaraiosis is an independent predictor of outcome regardless of sICH in patients undergoing EVT for ELVO.36
Elderly patients tend to have a higher rate of medical comorbidities.37, 38 The high prevalence of medical comorbidities might affect functional outcome after stroke. Pre-existing disability is more common in the elderly.39, 40 Consistent with these prior observations, we found a higher prevalence of pre-existing disability in nonagenarians than in younger patients. However, despite this, the trajectory of functional worsening was similar to that of the younger age group. This is consistent with earlier studies of IV thrombolysis which showed that despite pre-existing disability, patients had a potential for recovery back to their pre-stroke functional level.39, 40
Two studies have compared these two age groups in IV thrombolysis, with conflicting results. Sarikaya et al11 reported worse outcomes in nonagenarians than in octogenarians, whereas Mateen et al12 showed similar outcomes. Our study provides a comparison of these two age groups receiving EVT. We found that nonagenarians and octogenarians had similar functional outcomes and therefore should be offered EVT when presenting with ELVO. Thus our observations add to the notion that chronological age cut-off points should not be used in isolation to make decisions about hyperacute recanalization therapies. Instead, markers of overall functional status and biological brain age and health may be better for making such decisions.41
The 90+ section of the population is projected to grow significantly in the next few decades.16 Higher incidence of stroke in the elderly, combined with an increasing incidence of atrial fibrillation in this age group, leads to ELVO stroke.1, 42 A significant number of nonagenarians are functionally independent.16 Therefore, exclusion of these patients from mechanical thrombectomy based on age alone is not a pragmatic approach.
The strengths of our study relate to the analysis of a large and well-defined multicenter patient population. The clinical and radiological variables were prospectively collected and standardized assessment of 90-day outcomes is provided in all patients. A robust statistical analysis using pre-stroke mRS to gauge the magnitude of the functional outcome provides a pragmatic understanding of problems related to stroke care in nonagenarians.
Our study has some limitations related to its retrospective design and modest sample size. We did not gather specific information about door-to-puncture and door-to-recanalization times or use of general anesthesia. Further investigations should clarify whether these factors are related to a poor outcome in the oldest old. A further limitation is that patients were not treated randomly. Given the lack of evidence for the efficacy of EVT in nonagenarians as well as the frequent presence of pre-existing functional deficits and multimorbidity, there may be a selection bias in our study. However, it is important to recognize that our cohort reflects contemporary clinical practice, with probably a relatively greater inclusion of patients with expected favorable outcome. This would have biased our results towards the null hypothesis (no difference between nonagenarians and younger patients). Nevertheless, nonagenarians still had a worse outcome. Hence, our observations are important and strongly suggest that when considering EVT for nonagenarians the pre-stroke functional status needs to be carefully considered. Future studies comparing nonagenarians treated with IV recombinant tissue-type plasminogen activator alone versus EVT could help to clarify the question as to whether or not nonagenarians truly benefit from EVT.
In conclusion, EVT for ELVO should be carefully considered for nonagenarians on a case-by-case basis, particularly if they have a good pre-stroke functional status.
Acknowledgments
We gratefully acknowledge Derek Merck and Scott Collins, HIRES for expert assistance provided for image processing.
Funding: NH is supported by grant K08NS091499 from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health.
Footnotes
| Muhib A. Khan | Study concept and design, data acquisition, statistical analysis, interpretation of data and drafting the article |
| Grayson Baird | Statistical analysis, interpretation of data, critical revision of the manuscript for important intellectual content |
| David Miller | Data acquisition, critical revision of the manuscript for important intellectual content |
| Anand Patel | Data acquisition, critical revision of the manuscript for important intellectual content |
| Shawn Tsekhan | Data acquisition, critical revision of the manuscript for important intellectual content |
| Shadi Yaghi | Interpretation of data, drafting the article and critical revision of the manuscript for important intellectual content |
| Ajit Puri | Critical revision of the manuscript for important intellectual content |
| Mahesh Jayaraman | Critical revision of the manuscript for important intellectual content |
| Nils Henninger | Study concept, interpretation of data, drafting the article, critical revision of the manuscript for important intellectual content |
| Brian Silver | Study concept, interpretation of data, drafting the article, critical revision of the manuscript for important intellectual content |
Disclosures
The authors report no conflicts of interest.
Contributors: MAK: study concept and design, data acquisition, statistical analysis, interpretation of data, and drafting of the article. GLB: statistical analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. DM, AP, and ST: data acquisition and critical revision of the manuscript for important intellectual content. SY: interpretation of the data, drafting of the article, and critical revision of the manuscript for important intellectual content. AP and MJ: critical revision of the manuscript for important intellectual content. NH and BS: study concept, interpretation of data, drafting of the article, and critical revision of the manuscript for important intellectual content.
Competing interests: None declared.
Ethics approval: Institutional review boards at Rhode Island Hospital and University of Massachusetts.
References
- 1.Mozaffarian D, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Kammersgaard LP, et al. Short- and long-term prognosis for very old stroke patients. The Copenhagen Stroke Study. Age Ageing. 2004;33:149–154. doi: 10.1093/ageing/afh052. [DOI] [PubMed] [Google Scholar]
- 3.Colby SL, Ortman JM. Current Population Reports. U.S. Census Bureau; Washington, DC: 2014. pp. 25–1141. [Google Scholar]
- 4.Berkhemer OA, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BCV, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 8.Jovin TG, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 9.Jayaraman MV, et al. Embolectomy for stroke with emergent large vessel occlusion (ELVO): report of the Standards and Guidelines Committee of the Society of NeuroInterventional Surgery: Table 1. J NeuroInterventional Surg. 2015;7:316–321. doi: 10.1136/neurintsurg-2015-011717. [DOI] [PubMed] [Google Scholar]
- 10.Goyal M, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet Lond Engl. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 11.Sarikaya H, et al. Intravenous thrombolysis in nonagenarians with ischemic stroke. Stroke J Cereb Circ. 2011;42:1967–1970. doi: 10.1161/STROKEAHA.110.601252. [DOI] [PubMed] [Google Scholar]
- 12.Mateen FJ, Buchan AM, Hill MD CASES Investigators. Outcomes of thrombolysis for acute ischemic stroke in octogenarians versus nonagenarians. Stroke J Cereb Circ. 2010;41:1833–1835. doi: 10.1161/STROKEAHA.110.586438. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke J Cereb Circ. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Higashida RT, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke J Cereb Circ. 2003;34:e109–137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 16.Kawas CH. The oldest old and the 90+ Study. Alzheimers Dement J Alzheimers Assoc. 2008;4:S56–59. doi: 10.1016/j.jalz.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mack R, Salmoni A, Viverais-Dressler G, Porter E, Garg R. Perceived risks to independent living: the views of older, community-dwelling adults. The Gerontologist. 1997;37:729–736. doi: 10.1093/geront/37.6.729. [DOI] [PubMed] [Google Scholar]
- 18.Iezzoni LI, McCarthy EP, Davis RB, Siebens H. Mobility problems and perceptions of disability by self-respondents and proxy respondents. Med Care. 2000;38:1051–1057. doi: 10.1097/00005650-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Rantakokko M, et al. Perceived environmental barriers to outdoor mobility and changes in sense of autonomy in participation outdoors among older people: a prospective two-year cohort study. Aging Ment Health. 2016:1–5. doi: 10.1080/13607863.2016.1159281. [DOI] [PubMed] [Google Scholar]
- 20.Zeevi N, Kuchel GA, Lee NS, Staff I, McCullough LD. Interventional stroke therapies in the elderly: are we helping? AJNR Am J Neuroradiol. 2012;33:638–642. doi: 10.3174/ajnr.A2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffis EJ, He W, Prestigiacomo CJ, Gandhi CD. Endovascular treatment for acute ischemic stroke in octogenarians compared with younger patients: a meta-analysis. Int J Stroke Off J Int Stroke Soc. 2014;9:308–312. doi: 10.1111/ijs.12098. [DOI] [PubMed] [Google Scholar]
- 22.Murabito JM, Yuan R, Lunetta KL. The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J Gerontol A Biol Sci Med Sci. 2012;67:470–479. doi: 10.1093/gerona/gls089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paciaroni M, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke J Cereb Circ. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakaran S, et al. Symptomatic intracerebral hemorrhage among eligible warfarin-treated patients receiving intravenous tissue plasminogen activator for acute ischemic stroke. Arch Neurol. 2010;67:559–563. doi: 10.1001/archneurol.2010.25. [DOI] [PubMed] [Google Scholar]
- 25.Pundik S, et al. Older age does not increase risk of hemorrhagic complications after intravenous and/or intra-arterial thrombolysis for acute stroke. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc. 2008;17:266–272. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi AI, Suri MFK, Georgiadis AL, Vazquez G, Janjua NA. Intra-arterial recanalization techniques for patients 80 years or older with acute ischemic stroke: pooled analysis from 4 prospective studies. AJNR Am J Neuroradiol. 2009;30:1184–1189. doi: 10.3174/ajnr.A1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arkadir D, et al. Multimodal reperfusion therapy for large hemispheric infarcts in octogenarians: is good outcome a realistic goal? AJNR Am J Neuroradiol. 2012;33:1167–1169. doi: 10.3174/ajnr.A2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke J Cereb Circ. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 29.Cheang MY, et al. Recanalisation success is associated with good clinical outcome despite advanced age and stroke severity in patients treated with the Solitaire stentriever. J Clin Neurosci Off J Neurosurg Soc Australas. 2014;21:401–405. doi: 10.1016/j.jocn.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Alberts MJ, Shang T, Magadan A. Endovascular Therapy for Acute Ischemic Stroke: Dawn of a New Era. JAMA Neurol. 2015;72:1101. doi: 10.1001/jamaneurol.2015.1743. [DOI] [PubMed] [Google Scholar]
- 31.Sardar P, et al. Endovascular therapy for acute ischaemic stroke: a systematic review and meta-analysis of randomized trials. Eur Heart J. 2015;36:2373–2380. doi: 10.1093/eurheartj/ehv270. [DOI] [PubMed] [Google Scholar]
- 32.Maingard J, et al. Recanalisation success is independent of ASPECTS in predicting outcomes after intra-arterial therapy for acute ischaemic stroke. J Clin Neurosci Off J Neurosurg Soc Australas. 2014;21:1344–1348. doi: 10.1016/j.jocn.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Soize S, et al. Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology. 2013;55:977–987. doi: 10.1007/s00234-013-1191-4. [DOI] [PubMed] [Google Scholar]
- 34.Linfante I, Andreone V, Akkawi N, Wakhloo AK. Internal carotid artery stenting in patients over 80 years of age: single-center experience and review of the literature. J Neuroimaging Off J Am Soc Neuroimaging. 2009;19:158–163. doi: 10.1111/j.1552-6569.2008.00269.x. [DOI] [PubMed] [Google Scholar]
- 35.Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J. 2012;88:79–87. doi: 10.1136/postgradmedj-2011-130307. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Puri AS, Khan MA, Goddeau RP, Henninger N. Leukoaraiosis predicts a poor 90-day outcome after endovascular stroke therapy. AJNR Am J Neuroradiol. 2014;35:2070–2075. doi: 10.3174/ajnr.A4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batchelor WB, et al. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol. 2000;36:723–730. doi: 10.1016/s0735-1097(00)00777-4. [DOI] [PubMed] [Google Scholar]
- 38.Feldman DN, et al. Comparison of outcomes of percutaneous coronary interventions in patients of three age groups (<60, 60 to 80, and >80 years) (from the New York State Angioplasty Registry) Am J Cardiol. 2006;98:1334–1339. doi: 10.1016/j.amjcard.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Karlinski M, et al. Role of preexisting disability in patients treated with intravenous thrombolysis for ischemic stroke. Stroke J Cereb Circ. 2014;45:770–775. doi: 10.1161/STROKEAHA.113.003744. [DOI] [PubMed] [Google Scholar]
- 40.Foell RBT, et al. Effects of thrombolysis for acute stroke in patients with pre-existing disability. CMAJ Can Med Assoc J J Assoc Medicale Can. 2003;169:193–197. [PMC free article] [PubMed] [Google Scholar]
- 41.Onteddu SR, Goddeau RP, Minaeian A, Henninger N. Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J Neurol Sci. 2015;359:418–423. doi: 10.1016/j.jns.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Yiin GSC, et al. Age-specific incidence, outcome, cost, and projected future burden of atrial fibrillation-related embolic vascular events: a population-based study. Circulation. 2014;130:1236–1244. doi: 10.1161/CIRCULATIONAHA.114.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]