Pseudoloma neurophilia, a microsporidium, causes subclinical infections in laboratory zebrafish (Danio rerio). It preferentially infects tissues of the central nervous system (Spagnoli, Xue, Murray, Chow, & Kent 2015). Transmission occurs vertically and via consumption of spores in carcasses, eggs, and contaminated water (Sanders, Watral, & Kent 2012; Sanders, Watral, & Kent 2013)In 2006, 75% of all laboratories submitting fish to the Zebrafish International Resource Center (ZIRC, Eugene, Oregon) diagnostic service were contaminated (Murray 2015). Although most facilities ship live fish as embryonated eggs rather than adults, P. neurophilia transmission is still a risk because it forms environmentally resistant spores (Sanders, Watral, Clarkson, & Kent 2013; Murray, Dreska, Nasiadka, Rinne, Matthews, Carmichael, Bauer, Varga & Westerfield 2011). Spores survive bleaching at 25–50ppm, which is standard for egg sterilization (Ferguson, Watral, Schwindt, & Kent 2007) and intra-ovum spores are completely shielded from disinfectants (Sanders et al. 2013). Clinical signs, when present, are nonspecific (Ramsay, Watral, Schreck, & Kent 2009) and most positive cases are subclinical, so diagnosis is difficult without robust screening. This results in a high prevalence of cryptic infections among facilities (Spagnoli et al. 2015).
The zebrafish is currently used in nearly every experimental field formerly reserved for rodents including neurology and behavior (Phillips & Westerfield 2014; Bugel, Tanguay, & Planchart 2014). Zebrafish are used to study the neurobehavioral effects of various pharmaceuticals, genetic manipulations, and situational disturbances such as acute and chronic stressors (Maximino, de Brito, Silva Batista, Hurculano, Morato & Gouveia Jr 2010; Morris 2009; Pittman & Lott 2014; Stewart, Gaikwad, Kyzar, Green, Roth, & Kalueff 2012; Stewart, Wong, Cachat, Gaikwad, Kyzar, Wu, Hart, Piet, Utterback, Elegante, Tien, & Kalueff 2011; Stewart, Nguyen, Wong, Poudel, & Kalueff 2014; Willemsen, Hasselaar, van der Linde, & Bonifati 2008). Zebrafish have been proposed as models for a wide range of human disorders including autism, schizophrenia, epilepsy, anxiety, depression, and post-traumatic stress disorder (Maximino et al. 2010; Morris 2009; Pittman & Lott 2014; Stewart et al. 2012; Stewart et al. 2011; Stewart et al. 2014; Willemsen et al. 2008; Caramillo, Khan, Collier, & Echevarria 2015). One advantage of the zebrafish as a model for behavior in man is the fact that both are essentially social species (Miller & Gerlai 2011; Miller & Gerlai 2012; Oliveira 2013; Engeszer, Da Barbiano, Ryan, & Parichy 2007;). Zebrafish are used extensively to study the effects of human pharmaceutical compounds such as ethanol, LSD, ketamine, and mescaline using shoaling endpoints as indicators of social behavior (Buske & Gerlai 2012; Riehl, Kyzar, Allain, Green, Hook, Monnig, Rhymes, Roth, Pham, Razavi, DiLeo, Gaikwad, Hart, & Kalueff 2011; Grossman, Utterback, Stewart, Gaikwad, Chung, Suciu, Wong, Elegante, Elkhayat, Tan, Gilder, Wu, DiLeo, Cachat, & Kalueff 2010; Kyzar, Collins, Gaikwad, Green, Roth, Monnig, El-Ounsi, Davis, Freeman, Capezio, Stewart, & Kalueff 2012; Pham, Raymond, Hestr, Kyzar, Gaikwad, Bruce, Fryar, Chanin, Enriquez, Bagwandoss, Zapolsky, Green, Stewart, Robinson, & Kalueff 2012). Behavioral studies are inherently burdened with high inter-subject variability and the addition of an occult neural parasite poses the risk of increased non-protocol induced variation (Kent, Harper, & Wolf 2015).
We showed that P. neurophilia is associated with altered host behavior in a previous study that investigated startle response habituation (Spagnoli, Xue, & Kent 2015), wherein infected fish habituated significantly less overall than both exposed negative and control fish. In the current study, we conducted a shoaling assay to evaluate the effects of P. neurophilia on social behavior. Here, we used mean interfish distance (MID) as an indicator of shoaling based on Pham et al. (2012). The procedures we used were modeled after Pham et al. (2012) and modified to apply to chronic exposure. Zebrafish of the 5D strain were obtained from the Sinnhubber Aquatic Resource Center, a SPF Pseudoloma-free facility (Kent et al. 2012). 140 fish were netted from the stock tank and separated into 12 tanks containing 10 fish each and a sentinel tank containing 20 fish. Fish acclimated for seven days in individual 2.8L tanks to make the pre-and post- exposure assays as similar as possible.
On the day of pre-exposure testing, fish were netted from their holding tanks into a beaker. They were then transported to the staging area and poured into a novel container. The 2.8L testing tank measured 35x5x19cm and was filled to a height of 16cm with system water. Fish were digitally filmed for 10 minutes, beginning when the investigator left the room. After filming, fish were returned to their home tanks. The order of testing was randomized and noted so that post-exposure testing could be performed in the same order. Tanks were individually identified and labeled. Testing occurred during daylight activity hours.
Following pre-exposure filming, tanks were divided into six control and six exposure tanks, with the addition of one sentinel tank holding 20 fish, which was used to evaluate the progression of infection. Two tanks containing 70 adult zebrafish each were used as water donor tanks. One tank was confirmed negative and the other positive for P. neurophilia via histopathology of resident fish. To mimic transmission conditions present in most facilities, 100mL of water from the contaminated donor tank was added to each exposure shoal once per day, five days per week, for 123 days. 100mL of water from the uncontaminated tank was added to each control shoal on an identical schedule. Water quality parameters were within acceptable ranges and identical between donor tanks.
Five sentinel fish were sampled for histopathology at 4 months post-exposure. All of these fish were infected and post-exposure testing was performed at this time. Fish were netted from their home tanks into a beaker and transferred into a novel container—the same used in pre-exposure testing. Fish were recorded using previously described methods. Following filming, fish were euthanized via ice water in accordance with IACUC approval. Fish were fixed in Dietrich’s solution, decalcified, bisected sagitally, processed, sectioned, placed onto glass slides, and stained with H&E in the standard manner. All fish from all exposed shoals and five fish from each control shoal were evaluated. None of the control fish were infected, while the infection prevalence among exposed shoals was high, ranging from 80–100% between tanks.
As detailed in Pham et al. (2012), eight screen captures were taken per tank per recording session. With time 0 at the start of filming and following a three minute rest period, frames were analyzed at 3:20, 3:40, 4:00, 4:20, 4:40, 5:00, 5:20, and 5:40 using Image J analysis software (Rasband 2015). Interfish distances were measured manually from the center of each fish as suggested by Pham et al. (2012).
Statistical analyses were performed using the statistical programming environment R version 3.1.2 (R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. ). MID was calculated from measurements between all fish in all 8 screenshots per tank as has been previously described (Pham et al. 2012). As the measurement data were not normally distributed, the nonparametric Wilcoxon-Mann-Whitney U- test was used to compare the MID measured between the two treatment groups at pre-treatment and post-treatment time points.
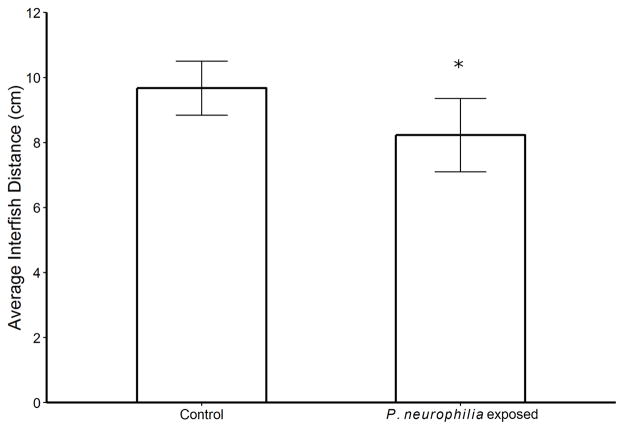
There was no significant difference in MID between cohorts in pretreatment measurements (p = 0.81, U-test). After the four-month exposure period, the P. neurophilia exposure fish exhibited significantly less (about 15%) MID than controls p = 0.026, U-test (Figure 1).
Figure 1.
Effects of Pseudoloma neurophilia exposure on shoaling behavior of zebrafish. Exposure to P. neurophilia significantly decreased the average inter-fish distance with 12 shoals (tanks), 6 exposed and 6 controls. * p = 0.026, U-test. Error bars = 1 standard deviation.
Here, we show a causative link between the presence of P. neurophilia and altered shoaling behavior, which is supported by the fact that there were no significant differences in MID between cohorts in the pre-exposure period. Following the exposure period, during which the only difference between cohorts was exposure to spore-laden versus uninfected water, a significant difference in MID developed, suggesting that infection was responsible. Strikingly, the percent reduction in MID associated with P. neurophilia was greater than that observed when fish were exposed to an alarm substance in another study (Green et al. 2012). Increased shoaling is thought to be an indicator of stress, which, along with the findings of our tap test study, could indicate that neural microsporidiosis causes the development of a high stress or anxious behavioral phenotype (Pham et al. 2012; Spagnoli et al. 2015)
The fact that infection causes changes in shoaling behavior has wide-ranging implications for neurobehavioral research. Altered social interactions are associated with a wide range of neurobehavioral disorders in man including schizophrenia, Asperger’s disease, and social anxiety (Maximino et al. 2010; Morris 2009; Pittman & Lott 2014; Stewart et al. 2012; Stewart et al. 2011; Stewart et al. 2014; Willemsen et al. 2008). A laboratory examining the treatment of social disorders using various pharmaceuticals could be adversely affected by the presence of Pseudoloma-contaminated fish, particularly because intertank infection statuses and rates can vary wildly within a facility (Spagnoli et al. 2015). With zebrafish becoming a popular neurobehavioral model, it is imperative that facilities introduce robust biosecurity and screening protocols to maintain parasite-free experimental populations.
Acknowledgments
This study was funded in part by NIH ORIP 2R24OD010998 to M. Kent and fish were provided by the Sinnhuber Aquatic Resource Center. Environmental Health Sciences Center. NIH NIEHS P30 ES000210.
Literature Cited
- Bugel SM, Tanguay RL, Planchart A. Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Current Environmental Health Report. 2014;1:341–352. doi: 10.1007/s40572-014-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dompaminergic and seratoninergic systems in adult zebrafish. Neurotoxicology and Teratology. 2012;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramillo EM, Khan KM, Collier AD, Echevarria DJ. P.T.S.D. Modeling in the zebrafish: are we there yet? Behavioural Brain Research. 2015;276:151–160. doi: 10.1016/j.bbr.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Engeszer RE, Da Barbiano LA, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behavior in the zebrafish Danio rerio. Animal Behavior. 2007;74 doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J, Watral V, Schwindt A, Kent ML. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomola) are highly resistant to chlorine. Diseases of Aquatic Organisms. 2007;76:205–214. doi: 10.3354/dao076205. [DOI] [PubMed] [Google Scholar]
- Grossman L, Utterback E, Stewart A, Gaikwad S, Chung KM, Suciu C, Wong K, Elegante M, Elkhayat S, Tan J, Gilder T, Wu N, DiLeo J, Cachat J, Kalueff AV. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behavioural Brain Research. 2010;214:277–284. doi: 10.1016/j.bbr.2010.05.039. [DOI] [PubMed] [Google Scholar]
- Kent ML, Harper C, Wolf JC. Documented and potential research impacts of subclinical diseases in zebrafish. ILAR Journal. 2012;53:126–134. doi: 10.1093/ilar.53.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Collins C, Gaikwad S, Green J, Roth A, Monnig L, El-Ounsi M, Davis A, Freeman A, Capezio N, Stewart AM, Kalueff AV. Effects of hallucinogenic agents mescaline and phencyclidine on zebrafish behavior and physiology. Progress in Neuropsychopharmacology and Biological Psychiatry. 2012;37:194–202. doi: 10.1016/j.pnpbp.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, de Brito TM, Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: a critical review. Behavioural Brain Research. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Reviews in Neuroscience. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio) PLoS one. 2012 doi: 10.1371/journal.pone.0048865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA. Zebrafish as a model system to examine the neurodevelopmental basis of schizophrenia. Progress in Brain Research. 2009;179:97–106. doi: 10.1016/S0079-6123(09)17911-6. [DOI] [PubMed] [Google Scholar]
- Murray K. Summary of submissions to the ZIRC Diagnostic Service on The Zebrafish International Resource Center webpage. 2015 http://zebrafish.org/health/diseaseManual.php#SUMMARY OF SUBMISSIONS TO THE ZIRC DIAGNOSTIC SERVICE.
- Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Bauer J, Varga ZM, Westerfield M. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comparative Medicine. 2011;61:322–329. [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF. Mind the fish: zebrafish as a model in cognitive social neuroscience. Frontiers in Neural Circuits. 2013;8:131. doi: 10.3389/fncir.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, Fryar C, Chanin S, Enriquez J, Bagawandoss S, Zapolsky I, Green J, Stewart AM, Robison BD, Kalueff AV. Assessing social behavior phenotypes in adult zebrafish: Shoaling, social preference, and mirror biting tests. In: Kalueff AV, Stewart AM, editors. Zebrafish Protocols for Neurobehavioral Research. Springer; New York: 2012. pp. 231–246. [Google Scholar]
- Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Disease Models and Mechanisms. 2014;7:739–743. doi: 10.1242/dmm.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JT, Lott CS. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiology of Behavior. 2014;123:174–179. doi: 10.1016/j.physbeh.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Ramsay JM, Watral V, Schreck CB, Kent ML. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Diseases of Aquatic Organisms. 2009;88:69–84. doi: 10.3354/dao02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2015. http://imagej.nih.gov/ij/ [Google Scholar]
- Riehl R, Kyzar E, Allain A, Green J, Hook M, Monnig L, Rhymes K, Roth A, Pham M, Razavi R, DiLeo J, Gaikwad S, Hart P, Kalueff AV. Behavioral and physiological effects of acute ketamine exposure in adult zebrafish. Neurotoxicology and Teratology. 2011;33:658–667. doi: 10.1016/j.ntt.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Watral V, Kent ML. Microsporidiosis in zebrafish researchfacilities colonies. ILAR Journal. 2012;53:106–113. doi: 10.1093/ilar.53.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Watral V, Clarkson K, Kent ML. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish Danio rerio. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli S, Xue L, Murray KM, Chow F, Kent ML. Pseudoloma neurophilia: a retrospective and descriptive study of nervous system and muscle infections with new implications for pathogenesis and behavioral phenotypes. Zebrafish. 2015;12:189–201. doi: 10.1089/zeb.2014.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli S, Xue L, Kent ML. The common neural parasite Pseudoloma neurophilia is associated with altered startle response habituation in adult zebrafish (Danio rerio): Implications for the zebrafish as a model organism. Behavioural Brain Research. 2015;291:351–360. doi: 10.1016/j.bbr.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Wong K, Cachat J, Gaikwad S, Kyzar E, Wu N, Hart P, Piet V, Utterback E, Elegante M, Tien D, Kalueff AV. Zebrafish models to study drug abuse-related phenotypes. Reviews in Neuroscience. 2011;22:95–105. doi: 10.1515/RNS.2011.011. [DOI] [PubMed] [Google Scholar]
- Stewart A, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology. 2012;62:135–143. doi: 10.1016/j.neuropharm.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Nguyen M, Wong K, Poudel KW, Kalueff AV. Developing zebrafish models of autism spectrum disorder (asd) Progress in Neuropsychopharmacology and Biological Psychiatry. 2014;50:27–36. doi: 10.1016/j.pnpbp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Willemsen R, Hasselaar W, van der Linde H, Bonifati V. Zebrafish as a new model organism for Parkinson’s disease. Proceedings of Measuring Behavior. 2008;5:0–1. [Google Scholar]
- Wulliman MF, Barbara R, Reichert H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhaüser; Boston: 1996. [Google Scholar]