Abstract
BACKGROUND
There is increasing popularity of high power lasers for surgical debridement and antimicrobial therapy in management of peri-implantitis and periodontal therapy. Removal of the noxious foci would naturally promote tissue healing directly. But there are also anecdotal reports of better healing around routine high power laser procedures. The precise mechanisms mediating these effects remain to be fully elucidated. This work examines these low dose laser bystander effects on oral human epithelial and fibroblasts particularly focusing on the role for Human β defensin-2 (HBD-2 or DEFB4A), a potent factor capable of anti-microbial effects and promoting wound healing.
METHODS
Laser treatments were performed using a near-infrared laser (810nm diode) at low doses. Normal human oral keratinocytes and fibroblast cells were used and HBD-2 mRNA and protein expression was assessed with real time PCR, western blotting, and immunostaining. Role of TGF-β1 signaling in this process was dissected using pathway-specific small molecule inhibitors.
RESULTS
We observed laser treatments robustly induced HBD-2 expression in an oral fibroblast cell line compared to a keratinocyte cell line. Low dose laser treatments results in activation of the TGF-β1 pathway that mediated HBD-2 expression. The two arms of TGF-β1 signaling, Smad and non-Smad are involved in laser-mediated HBD-2 expression.
CONCLUSIONS
Laser activated TGF-β1 signaling and induced expression of HBD-2, both of which are individually capable of promoting healing in tissues adjacent to high power surgical laser applications. Moreover, the use of low dose laser therapy itself can provide additional therapeutic benefits for effective clinical management of periodontal or peri-implant disease.
Keywords: Photobiomodulation therapy, Low level light/laser therapy, Periodontitis, Peri-implantitis, Defensins, lasers
INTRODUCTION
Progress in clinical management of periodontal disease spans a wide range from more effective surgical debridement to antimicrobial agents, immunomodulation, and regenerative stem cell approaches. While the microbial etiopathogenesis in periodontal disease predominates these approaches, restoring lost periodontal tissues prosthetically with implants has become a standard of care with superior aesthesis and functions. There are few therapeutic procedures in dentistry, or clinical medicine, that offer such an impressive clinical success rate (1). Recent innovations in implant surface topology are capable of promoting beneficial biological responses in terms of both osseointegration as well as ligament-like tissue differentiation continue to push the frontiers of regenerative dentistry (2). However, there remain a few persistent clinical issues with implants such as peri-implant mucositis (soft tissue) and peri-implantitis (bone-implant interface) (3). The former includes the inflammatory reaction of the soft tissue surrounding the implant site, but without any bone loss around the implant. The latter process involves peri-implant bone loss usually accompanied by soft tissue inflammation and pocket formation. Both these complications of dental implants result from complex polymicrobial infection of the implant interface.
The major clinical management protocols here are similar to periodontal disease where the goal is to decontaminate and facilitate good hygiene at diseased sites to promote osseous regeneration (4, 5). Given the complexity of the anatomical niche in periodontal defects and around implants, many approaches have been attempted to overcome the limitations of mechanical instrumentation. The use of high power lasers as precision surgical tools allows thorough curettage of inflamed or necrotic tissues. Moreover, they can be used along with an exogenous dye to most effectively decontaminate complex microbial microenvironments, a technique termed antimicrobial photodynamic therapy (aPDT) (or Photoactivated disinfection, PAD) (6, 7). During both treatments, high power laser illuminates the targeted zones but also exposed peri-treatment zones with lower doses generating bystander effects.
Interestingly, low dose laser treatment has been shown to reduce inflammation and improve wound healing, including promoting osseointegration in a process termed Low Level Light/Laser Therapy (LLLT) or more aptly Photobiomodulation (PBM) therapy (8). This latter process refers to the use of non-ionizing biophotonics devices for a non-thermal form of light therapy utilizing endogenous chromophores to promote therapeutic biological responses (9). Despite a vast amount of basic and clinical research studies, the clinical benefits of this therapy remains equivocal due to a lack of complete understanding of its molecular mechanisms. While most emphasis has been placed on the intracellular effects of visible and near-infrared light on mitochondrial Cytochrome C Oxidase, our recent demonstration of an extracellular target, TGF-β1 offers significant new opportunities. TGF-β1 is a central player in various pathophysiological processes such as development, wound healing, immunomodulation and. microbial infections (10).
Antimicrobial peptides are charged, small molecules (12–50 amino acids) demonstrating broad spectrum effects against microbes. Among them, Defensins were first described in 1966 by Zeya and Sitznagel as cationic antimicrobial peptides (11). Human beta-defensin-2 (HBD-2) has been shown to have potent antimicrobial activity against gram-negative and gram-positive bacteria, fungi, and viruses (12, 13). HBD-2 can also induce chemotaxis of a wide range of immune cells including T cells, dendritic cells, neutrophils and mononuclear cells via the chemokine receptor CCR6 (14, 15). The purpose of this study is to examine role of laser-activated TGF-β1 on antimicrobial peptide, HBD-2 in clinical management of periodontal and peri-implant disease.
MATERIALS AND METHODS
Cell Lines
Normal human oral keratinocytes (NOKSI) and fibroblast (HOF) cells were maintained in DMEM (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (Atlas Biologicals, USA) along with 100 units/mL penicillin and 100 μg/mL streptomycin (Life Technologies, USA). Cells were grown at 37° C in a humidified chamber with 5% CO2.
Laser treatments in cell culture
Laser treatments were performed using an 810 nm diode (GaAlAs, AMD Lasers, USA) for 5 minutes in continuous wave mode at a distance of 14.5 cm in six-well plates containing 2 ml media. Power and irradiances (W/cm2) were varied to assess dose dependent effects.
RNA extraction and PCR analyses
Cells were lysed in RLT buffer (Qiagen, USA) with β-mercaptoethanol and total RNA was isolated using a Qiagen RNA isolation kit (Qiagen, USA) according to manufacturer’s protocol. For cDNA synthesis, 1 μg of RNA was reverse transcribed using ABI cDNA kit (Applied Biosystems, USA) and real-time PCR quantitation was performed with Dynamo™ SYBERgreen mix (Finnzymes, Finland) in triplicate reactions for HBD-2 (F-5′AGACTCAGCTCCTGGTGAAGC3′ and R-5′AGGCAGGTAACAGGATCGC3′; 59.5° C and 131bp), RPL35A (F-5′AAGGGAGCACACAGCTCTTC3′ and R-5′CTGGTTTTGTTTGGTTTGCC3′; Tm 59.5°C, 141bp) was performed in ABI StepOne Plus (Applied Biosystems, USA). Differential expression was determined by the formula: δCT=CT gene-CT RPL; δδCT=δCT treated- δCT untreated; Fold Change= 2−δδCT.
Western Blot Analysis
Cells were treated in 6 well plates and washed briefly with DPBS (Gibco, USA) and lysed in RIPA lysis buffer containing protease inhibitor cocktail (both, Sigma Aldrich, USA). Total protein was determined using the BCA reagent (Pierce, USA). Equal amount of total protein were resolved on SDS-PAGE gels, transferred to polyvinylidene difluoride (PVDF) membrane and immunoblotted. To block nonspecific binding sites, blots were incubated in blocking buffer (Licor, USA) for 1h followed by overnight incubation in primary antibodies (Phospho-Smad2, Phospho-AKT from Cell Signaling Technology, human defensin 2 from Abcam and β-Actin, Tubulin from Sigma) at 4°C. Next day, blots were washed in tris-buffered saline thrice (10 min each), followed by incubation with secondary antibody (anti-rabbit or anti-mouse IRDye 800 or 680 CW, Licor, USA 1:10000) for 1hr. Following washes, bands were visualized using Odyssey Imaging Systems (Licor, USA) and quantitated using AlphaImager software (ProteinSimple, USA).
Immunocytochemistry
Cells were plated in chamber slides (Nunc, USA) and treated with specified conditions. Following incubation for 24hours, cells were fixed and permeabilized in chilled methanol for 5 min at −20°C. Following blocking, incubation with primary antibody (human defensin 2 from Abcam, 1:100) incubated over night at 4 °C. The following day, cells were washed and incubated with secondary (anti-rabbit 488, Jackson Immunologicals, USA) for 1 hr,, washed and mounted with DAPI aqueous mount (Life Technologies, USA) and slides were imaged with a fluorescent microscope (Zeiss, USA).
Statistical Analyses
Statistical analyses were performed in GraphPad prism software (GraphPad Software, Inc., USA). Significance was assessed using the non-parametric Mann-Whitney or Kruskal-Wallis test. All treatments were compared to untreated control and p < 0.05 was considered significant.
RESULTS
Laser induces HBD-2 expression
We first assessed the ability of low power laser treatments to induce HBD-2 isoforms using quantitative RT-PCR analysis. We observed that laser treatments robustly induced HBD-2 expression in oral fibroblast cell line compared to milder expression in the keratinocyte cell line (Figure 1A). We further varied laser irradiance and noted a dose dependent induction of HBD-2 expression with optimum induction at a total fluence of 4 J/cm2 for 300 seconds followed by decrease at higher doses (Figure 1B). We further validated these observations by immunoblotting and immunostaining for HBD-2 (Figure 1C and D). These observations indicate that low dose laser treatment was able to induce a potent antimicrobial peptide, HBD-2, in oral fibroblasts.
Figure 1.
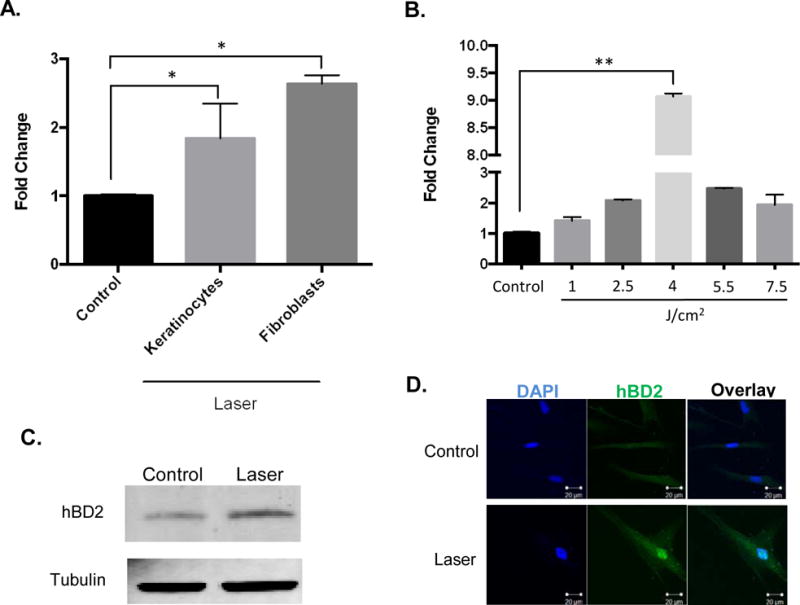
(A) HBD-2 mRNA expression in human oral keratinocytes (NOKSI) and fibroblasts (HOF) following laser treatments assessed by quantitative real time PCR at 24hrs. (B) HBD-2 expression following dose dependent laser treatment in human oral fibroblasts. HBD-2 protein expressions following laser treatments by (C) Western blotting and (D) immunofluorescence. Statistical significance was determined using Mann-Whitney test or Kruskal-Wallis tests and denoted by *p<0.05 and **p<0.005.
Induction of HBD-2 by laser treatment is via TGF-β1
TGF-β1 has been known to induce HBD-2 expression in wide range of cells types in the oral cavity including keratinocytes, fibroblasts, neutrophils and endothelial cells (16–18). We first inquired if TGF-β1 is able to induce HBD-2 expression in our cell lines and noted robust induction in oral fibroblasts (Figure 2A). We had recently reported the ability of low power laser-generated reactive oxygen species (ROS) to activate latent TGF-β1 (19). Therefore, we next asked if the laser-induced HBD-2 expression was mediated via TGF-β. Pre-incubation of oral fibroblasts with a TGF-β receptor 1 (Alk5) inhibitor prior to laser treatments resulted in abrogation of the increased HBD-2 expression (Figure 2B). To further investigate laser activation of the TGF-β pathway, we assessed expression of another downstream target named TGF-β Induced (TGFBI). We observed similar upregulation of TGFBI expression (Figure 2C) indicating that the laser activated TGF-β induces multiple downstream gene expression. These results suggest that low dose laser treatments results in activation of the TGF-β1 pathway that mediates HBD-2 expression.
Figure 2.
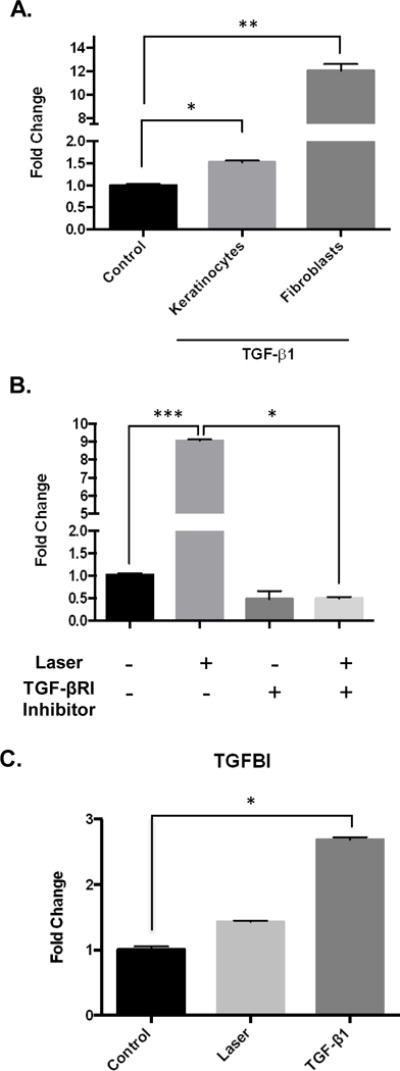
(A) TGF-β1 treatments induce HBD-2 expression assessed by quantitative real time PCR at 24hrs. (B) Pretreatment with TGF-βR1 inhibitor (SB431542) was performed prior to laser treatment and HBD-2 expression was assessed. (3) To assess other TGF-β1 downstream targets, TGFβ-Induced (TGFBI) expression was also assessed by quantitative real time PCR at 24hrs. Statistical significance was determined using Mann-Whitney test and denoted by *p<0.05, **p<0.05 and ***p<0.005.
Lasers activates both canonical and non-canonical TGF-β signal transduction
The TGF-β ligand binds to its transmembrane serine-threonine receptor (TGF-βRII) that forms a complex with another receptor, the TGF-βRI or ALK5, leading to intracellular downstream signaling. The cytoplasmic signaling intermediates recruited to the activated receptor complex are broadly categorized as the canonical Smads or the non-cannonical arm including Akt, MAPKs (JNK, p38 or ERK), TAK among others (Figure 3A) (20–23). Crosstalk between the two arms, along with individual cell lineage transcriptional profile, appear to contribute to a broad range of TGF-β responses (24, 25). Therefore, we next examined the activation of the canonical and non-canonical signaling pathways by laser treatments. We noted that laser treatments of oral fibroblasts results in increased phospho-smad2 and phospho-AKT levels (Figure 3B). This indicates both arms of TGF-β signal transduction pathways are activated by low dose laser treatments.
Figure 3.
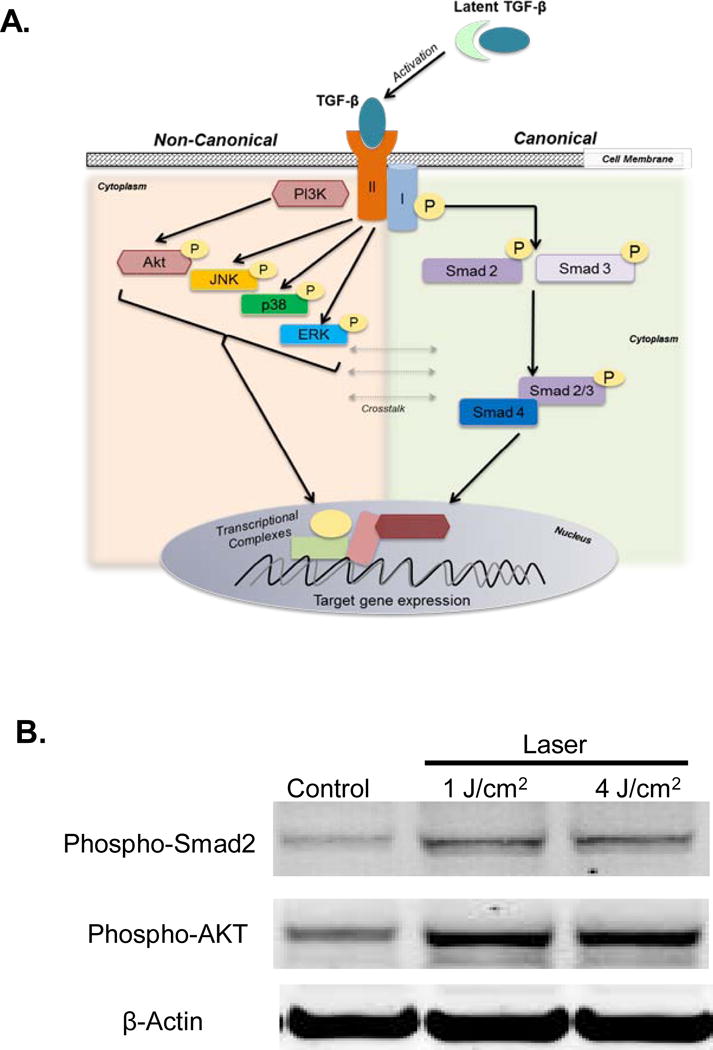
(A) Outline of distinct canonical and non-canonical arms of TGF-β signal transduction. (B) Activation of both arms following laser treatment was assessed by western blotting for phosphorylated Smad2 and Akt levels. B-Actin was used as loading control.
HBD-2 expression utilizes concerted TGF-β signaling
To further dissect the contribution of these individual signaling arms to HBD-2 expression, we first verified the specificity of these inhibitors to block respective signaling arms (Figure 4A). Then, we pretreated the oral fibroblast cell lines with either TGF-βRI or AKT inhibitor followed by laser treatments. We noted both inhibitors were able to block the laser-induced HBD-2 expression (Figure 4B). This suggests that laser-activated TGF-β1 induces HBD-2 expression via both Smad (canonical) and non-Smad (non-canonical) TGF-β signaling pathways.
Figure 4.
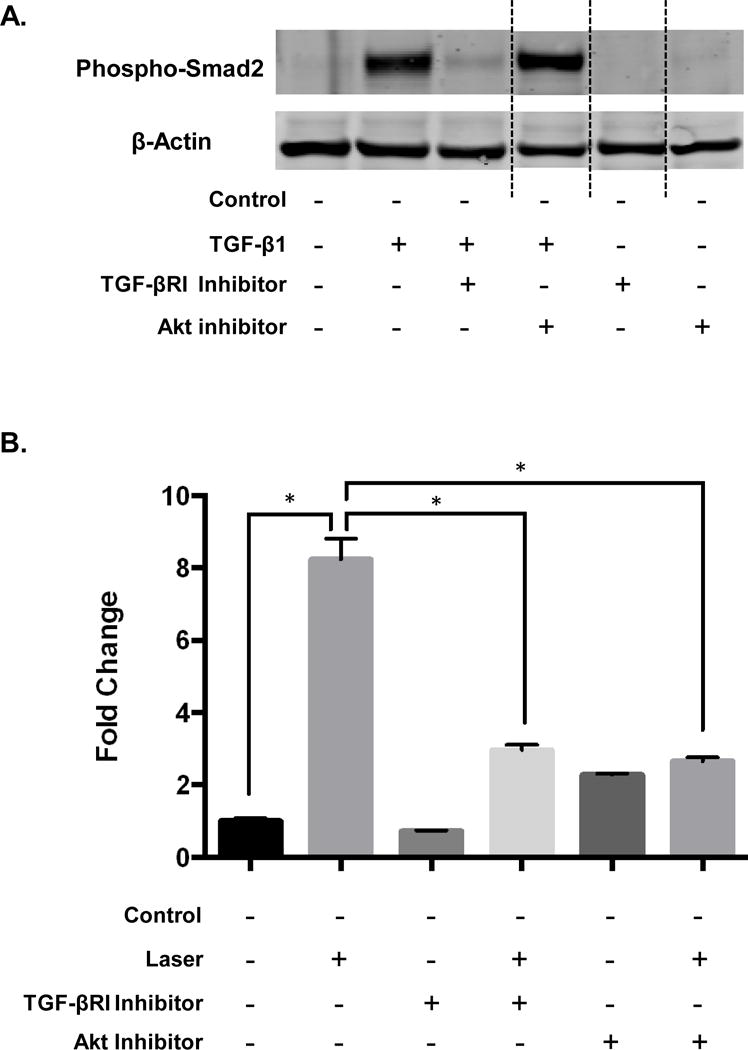
(A) Distinct TGF-β signaling arms were specifically targeted with small molecule inhibitors against Alk5 (TGF-βR1) and Akt and assessed by western blots for phospho-smad2. (B) Pretreatment with TGF-βR1 or Akt inhibitors were performed 2hrs prior to laser treatments and HBD-2 expression was assessed at 24hrs using quantitative real time PCR. Statistical significance was determined using Mann-Whitney test and denoted by *p<0.05.
DISCUSSION
Clinical success with oral implants is based on a large number of factors but its biological foundation predominantly hinges on robust integration of implant-tissue interface without clinical signs and symptoms of infection or inflammation. The process of osseointegration involves a sequence of well-orchestrated biological responses initiated following implant placement involving immediate, rapid deposition of blood and interstitial fluid followed by amorphous deposition of proteoglycans and randomly aligned collagen and finally, direct bone apposition (26). The intricate complexity of cell signaling networks involving growth factors (eg; bone morphogenetic protein (BMP), Hedgehog, fibroblast growth factor (FGF), Notch and Wnt) and transcriptional factors (eg; Sox9, Runx2 and Osterix) have all been shown to have pivotal roles in driving peri-implant tissue healing and regeneration gene expression signatures that are being investigated to enable better, predictive clinical responsiveness (27, 28). There is increased recognition of the predominant foreign body reaction that implants represent within the jaw bones characterized by a prominent inflammatory cytokine expression as well as discrete transcriptional responses such as those involving PAS (Per-ARNT-Sim) proteins and Per2 (circadian rhythm) expression (29, 30). The initial inflammation-immune processes are critical in determining the success of the biological sequela at implant interface. However, successful osseointegration and long term clinical success necessitates eventual resolution of these acute processes and robust healing.
Current prevalence of peri-implant disease appears to vary from 28–56% but the severity and extent of tissue destruction varies greatly due to multitude of factors (31). Persistent inflammation due to differences in physical characteristics of the metal implant interface, non-ideal clinical implant placement techniques and presence of pathogenic poly-microbial infections (32, 33). All of these factors appear to act in concert and can be clinically detrimental in implant success. Supporting implant therapy, besides initial implant cost, is a major determinant of clinical cost effectiveness (33). Despite some difference in the microbiome and histopathology between Periodontitis and Peri-implantitis, clinical management strategies have similar objectives which are to reduce infection causing microbiota, improve self-cleansing anatomy, and promote tissue healing and regeneration (34, 35).
Lasers are currently used for a wide range of applications in clinical dentistry (36, 37). The rationale for their use in management of periodontal and peri-implant disease has been proposed to be three fold; firstly to reduce damaged or inflamed tissues via surgical curettage of granulation tissues; second, antimicrobial effects on disease biofilms in conjunction with an exogenous dye (aPDT/PAD) and thirdly, using low dose lasers to reduce inflammation and promote tissue regeneration (PBM) (38, 39). This study demonstrates one of the plausible mechanisms involved in the use lasers in these clinical scenarios (Figure 5). The directed use of high laser energy, with or without exogenous dyes, can clearly be detrimental to microorganisms. There are some clinical protocols that currently advocate the use of low dose laser treatments after surgical debridement. However, the potential benefits and mechanisms of these bystander, low dose laser effects in these contexts have not yet been carefully investigated.
Figure 5.
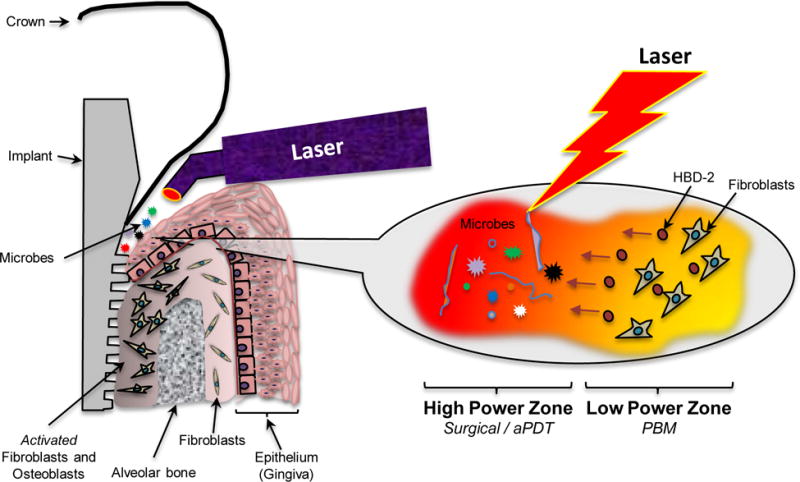
Outline of proposed PDT and PBM synergistic effects using lasers for peri-implant or periodontal disease management.
We examined these low dose laser effects with the rationale that the tissues adjacent to high power laser treatment zones would evoke potent bystander biological responses. We had recently noted the ability of low power lasers to activate endogenous latent TGF-β1 (19). TGF-βs have a central role in mediating a diverse range of biological functions in wound healing such as cell migration, proliferation, secretion, differentiation and functions of a broad range of cell types (40, 41). However, TGF-β appears to have distinct effects on individual cell populations in a context dependent manner. While TGF-β promotes re-epithelization of wounds by promoting keratinocyte migration, it also stimulates extracellular matrix deposition and wound contraction by myofibroblasts (42, 43). Further, it has potent immunomodulatory roles, both stimulating and inhibiting T-cells depending on the cellular milieu (44). A distinct immunosuppressive role for TGF-β includes its ability to drive development of regulatory T cells via expression of the transcription factor Foxp3.
TGF-β1 has multiple roles in bone pathophysiology and is a key player in osseointegration (45, 46). Laser-activated endogenous TGF-β could potentially harness many of its immunomodulation and wound healing responses in resolving peri-implantitis and promoting osseointegration. Given the potent immunomodulatory roles of TGF-βs, this study investigated one of the downstream targets, human β Defensins-2 (HBD-2) that would have direct antimicrobial effects as well as indirectly modulate inflammation and promote tissue regeneration in the peri-implant or periodontal tissues. β-Defensins are small, cysteine-rich, positively charged peptides that have broad antimicrobial activity (16). Of the four HBD isoforms described, three have been reported to be expressed in the oral mucosa. While HBD-1 is constitutively expressed, the other two isoforms, HBD-2 and HBD-3, can be induced by microbial components, such as lipopolysaccharide, and inflammatory mediators such as IL-1α, IL-1β, TNF-α and TGF-β (47–49). Prior to this report with near-infrared lasers, the only form of electromagnetic radiation capable of inducing HBD-2 expression was high energy, ionizing ultraviolet (UV) radiation (50). Interestingly, it has been noted that UV treatments downregulates HBD-2 expression in psoriasis and scleroderma while cells from distinct anatomical sites do not respond, similarly suggesting HBD-2 expression could be context dependent irrespective of the stimuli.
A major limitations of this study was the use of spontaneously immortalized cell lines that were readily available for these analyses. Examining the effects of laser treatments of HBD-2 expression in primary cells as well as in vivo analyses, in the appropriate clinical contexts, would be ideal areas of future investigations. The lack of responsiveness of the keratinocyte cell line can perhaps to be attributed to the specific cell line rather than a feature of epithelial responsiveness and requires further exploration. In conclusion, this study demonstrates the ability of laser treatments to induce expression of a potent antimicrobial peptide HBD-2. This provides a mechanistic rationale for optimization of the use of lasers in effective clinical management strategies for peri-implant and periodontal disease.
Acknowledgments
We thank Sharon Wahl for helpful discussions and the Gutkind lab for providing the NOKSI and HOF cell lines, James Russell and Vincent Schram for technical imaging assistance from the microscope and imaging core, NICHD. This research was supported by the intramural research program, NIDCR, National Institutes of Health.
Footnotes
CONFLICTS OF INTEREST
The authors declare they have no conflicts to declare with this work.
AUTHOR CONTRIBUTIONS
EGT and PRA conceived the project; EGT, IK and PRA performed experiments and analyzed data. EGT and IK performed statistical analyses. EGT, IK, SA and PRA wrote the manuscript.
References
- 1.Thalji G, Cooper LF. Molecular assessment of osseointegration in vitro: a review of current literature. Int J Oral Maxillofac Implants. 2014;29:e171–199. doi: 10.11607/jomi.te55. [DOI] [PubMed] [Google Scholar]
- 2.Oshima M, Inoue K, Nakajima K, et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep. 2014;4:6044. doi: 10.1038/srep06044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindhe J, Meyle J, Group DoEWoP Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:282–285. doi: 10.1111/j.1600-051X.2008.01283.x. [DOI] [PubMed] [Google Scholar]
- 4.Zander HA, Polson AM, Heijl LC. Goals of periodontal therapy. J Periodontol. 1976;47:261–266. doi: 10.1902/jop.1976.47.5.261. [DOI] [PubMed] [Google Scholar]
- 5.Teles RP, Haffajee AD, Socransky SS. Microbiological goals of periodontal therapy. Periodontol 2000. 2006;42:180–218. doi: 10.1111/j.1600-0757.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dental Res. 2007;86:694–707. doi: 10.1177/154405910708600803. [DOI] [PubMed] [Google Scholar]
- 8.Tang E, Arany P. Photobiomodulation and implants: implications for dentistry. J Periodontal Implant Sci. 2013;43:262–268. doi: 10.5051/jpis.2013.43.6.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33:183–184. doi: 10.1089/pho.2015.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reed SG. TGF-beta in infections and infectious diseases. Microbes Infect. 1999;1:1313–1325. doi: 10.1016/s1286-4579(99)00252-x. [DOI] [PubMed] [Google Scholar]
- 11.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 12.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–450. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 13.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 14.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Specific binding and chemotactic activity of mBD4 and its functional orthologue hBD2 to CCR6-expressing cells. J Biol Chem. 2010;285:7028–7034. doi: 10.1074/jbc.M109.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 16.Ganz T, Selsted ME, Szklarek D, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Inves. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawsar HI, Ghosh SK, Hirsch SA, Koon HB, Weinberg A, Jin G. Expression of human beta-defensin-2 in intratumoral vascular endothelium and in endothelial cells induced by transforming growth factor beta. Peptides. 2010;31:195–201. doi: 10.1016/j.peptides.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 19.Arany PR, Cho A, Hunt TD, et al. Photoactivation of endogenous latent transforming growth factor-beta1 directs dental stem cell differentiation for regeneration. Sci Translat Med. 2014;6:238ra269. doi: 10.1126/scitranslmed.3008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- 22.Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genetics Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zhou F, ten Dijke P. Signaling interplay between transforming growth factor-beta receptor and PI3K/AKT pathways in cancer. Trends in Biochem Sci. 2013;38:612–620. doi: 10.1016/j.tibs.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Arany PR, Flanders KC, DeGraff W, Cook J, Mitchell JB, Roberts AB. Absence of Smad3 confers radioprotection through modulation of ERK-MAPK in primary dermal fibroblasts. J Dermatol Sci. 2007;48:35–42. doi: 10.1016/j.jdermsci.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-beta: implications for carcinogenesis. Oncogene. 2005;24:5742–5750. doi: 10.1038/sj.onc.1208928. [DOI] [PubMed] [Google Scholar]
- 26.Branemark PI. Osseointegration and its experimental background. J Prosthetic Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura I. Genetic networks in osseointegration. J Dent Res. 2013;92:109S–118S. doi: 10.1177/0022034513504928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thalji G, Cooper LF. Molecular assessment of osseointegration in vivo: a review of the current literature. Int J Oral Maxillofac Implants. 2013;28:e521–534. doi: 10.11607/jomi.te33. [DOI] [PubMed] [Google Scholar]
- 29.Mengatto CM, Mussano F, Honda Y, Colwell CS, Nishimura I. Circadian rhythm and cartilage extracellular matrix genes in osseointegration: a genome-wide screening of implant failure by vitamin D deficiency. PLoS One. 2011;6:e15848. doi: 10.1371/journal.pone.0015848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moglich A, Ayers RA, Moffat K. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure. 2009;17:1282–1294. doi: 10.1016/j.str.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 32.Gittens RA, Olivares-Navarrete R, Schwartz Z, Boyan BD. Implant osseointegration and the role of microroughness and nanostructures: lessons for spine implants. Acta Biomater. 2014;10:3363–3371. doi: 10.1016/j.actbio.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heuer W, Kettenring A, Stumpp SN, et al. Metagenomic analysis of the peri-implant and periodontal microflora in patients with clinical signs of gingivitis or mucositis. Clin Oral Investig. 2012;16:843–850. doi: 10.1007/s00784-011-0561-8. [DOI] [PubMed] [Google Scholar]
- 34.Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):325–345. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- 35.Berglundh T, Zitzmann NU, Donati M. Are peri-implantitis lesions different from periodontitis lesions? J Clin Periodontol. 2011;38(Suppl 11):188–202. doi: 10.1111/j.1600-051X.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 36.Walsh LJ. The use of lasers in implantology: an overview. J Oral Implantol. 1992;18:335–340. [PubMed] [Google Scholar]
- 37.Sun G, Tuner J. Low-level laser therapy in dentistry. Den Clin North America. 2004;48:1061–1076, viii. doi: 10.1016/j.cden.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz F, Bieling K, Bonsmann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10:279–288. doi: 10.1007/s00784-006-0070-3. [DOI] [PubMed] [Google Scholar]
- 39.Aykol G, Baser U, Maden I, et al. The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol. 2011;82:481–488. doi: 10.1902/jop.2010.100195. [DOI] [PubMed] [Google Scholar]
- 40.Sporn MB, Roberts AB. TGF-beta: problems and prospects. Cell Regul. 1990;1:875–882. doi: 10.1091/mbc.1.12.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finnson KW, Arany PR, Philip A. Transforming Growth Factor Beta Signaling in Cutaneous Wound Healing: Lessons Learned from Animal Studies. Adv Wound Care. 2013;2:225–237. doi: 10.1089/wound.2012.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts AB. Transforming growth factor-beta: activity and efficacy in animal models of wound healing. Wound Repair Regen. 1995;3:408–418. doi: 10.1046/j.1524-475X.1995.30405.x. [DOI] [PubMed] [Google Scholar]
- 43.Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-beta regulation and wound healing. Int J Mol Sci. 2014;15:16257–16269. doi: 10.3390/ijms150916257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahl SM, Chen W. Transforming growth factor-beta-induced regulatory T cells referee inflammatory and autoimmune diseases. Arthritis Res Ther. 2005;7:62–68. doi: 10.1186/ar1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonewald LF, Mundy GR. Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res. 1990:261–276. [PubMed] [Google Scholar]
- 46.Smith RA. The effect on TGF-beta 1 on osseointegration. J Calif Dent Assoc. 1995;23:49–53. [PubMed] [Google Scholar]
- 47.Ouhara K, Komatsuzawa H, Shiba H, et al. Actinobacillus actinomycetemcomitans outer membrane protein 100 triggers innate immunity and production of beta-defensin and the 18-kilodalton cationic antimicrobial protein through the fibronectin-integrin pathway in human gingival epithelial cells. Infec Immun. 2006;74:5211–5220. doi: 10.1128/IAI.00056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawaki K, Mizukawa N, Yamaai T, Fukunaga J, Sugahara T. Immunohistochemical study on expression of alpha-defensin and beta-defensin-2 in human buccal epithelia with candidiasis. Oral Dis. 2002;8:37–41. doi: 10.1034/j.1601-0825.2002.1o770.x. [DOI] [PubMed] [Google Scholar]
- 49.Liu AY, Destoumieux D, Wong AV, et al. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Investig Derm. 2002;118:275–281. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 50.Glaser R, Navid F, Schuller W, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]


