Abstract
Accruing patients in a timely manner represents a significant challenge to early phase cancer clinical trials. The NCI Cancer Therapy Evaluation Program analyzed 19 months of corrective action plans (CAPs) received for slow-accruing Phase 1 and 2 trials to identify slow accrual reasons, evaluate whether proposed corrective actions matched these reasons, and assess the CAP impact on trial accrual, duration, and likelihood of meeting primary scientific objectives. Of the 135 CAPs analyzed, 69 were for Phase 1 trials and 66 for Phase 2 trials. Primary reasons cited for slow accrual were safety/toxicity (Phase 1: 48%), design/protocol concerns (Phase 1: 42%, Phase 2: 33%), and eligibility criteria (Phase 1: 41%, Phase 2: 35%). The most commonly proposed corrective actions were adding institutions (Phase 1: 43%, Phase 2: 85%) and amending the trial to change eligibility or design (Phase 1: 55%, Phase 2: 44%). Only 40% of CAPs provided proposed corrective actions that matched the reasons given for slow accrual. Seventy percent of trials were closed to accrual at time of analysis (Phase 1=48; Phase 2=46). Of these, 67% of Phase 1 and 70% of Phase 2 trials met their primary objectives, but they were active three times longer than projected. Among closed trials, 24% had an accrual rate increase associated with a greater likelihood of meeting their primary scientific objectives. Ultimately, trials receiving CAPs saw improved accrual rates. Future trials may benefit from implementing CAPs early in trial lifecycles, but it may be more beneficial to invest in earlier accrual planning.
INTRODUCTION
In the October 2015 CCR Focus, Bates et al examined numerous new and emerging opportunities and challenges in our drug development system and noted that “perhaps most challenging and important of all are the difficulties with clinical trial accrual that can prevent study completion (1).” Inadequate accrual hampers our ability to complete important scientific trials in a timely fashion (2), and potentially diverts both patients and limited financial resources to studies that are unable to meet their primary scientific objective (3, 4). Working to ensure trials accrue an adequate number of patients in a timely manner is a priority in cancer research (5, 6), and particularly in early phase trials (7). This paper presents the findings from a review of slow accruing, early phase National Cancer Institute (NCI) clinical trials (Phases 1 and 2) to identify reasons for slow accrual and proposed actions to improve their accrual rate.
Accrual planning has been a persistent challenge to the modern clinical trials system (8, 9). Despite acknowledging a lack of sufficient planning almost 30 years ago, Kost et al reported in 2014 a continued lack of routine pre-study planning and ongoing monitoring to ensure adequate accrual (10, 11). At a 2014 NCI two-day meeting focused on improving accrual to late phase cancer clinical trials, several potential barriers were highlighted (see Box 1); among these it was noted that a major systems-level challenge to accrual planning in cancer clinical trials may be unrealistic optimism, in that investigators assume their idea is scientifically important, and hence the clinical trial will successfully accrue (12). As a result, investigators may overestimate the availability of eligible patients and underestimate the challenges in actually accruing those patients. A recent analysis found that greater accrual competition, in the form of more trials in the same disease setting, and fewer available patients were significant predictors of low accrual in NCI group trials (13). A more feasible method of estimating the number of patients eligible, able, and potentially willing to participate in any given trial is needed.
Box 1. Potential barriers to accrual at the patient, provider, site, and system Level.
Patient Level Barriers
Awareness of trials as an option
Understanding of what trial participation involves
Concerns around placebos, randomization, side effects
Fear or distrust (e.g. being treated as a guinea pig) instead of trust in physician recommendation
History of discrimination in medical research
Inconvenience of trial logistics or procedures, including number, timing, and travel required
Financial burden
Provider Level Barriers
Awareness of available trials
Time and resources required to discuss trials with patients
Fear of “losing” patients referred for trials
Concerns around suitability of trial for patient (e.g. tolerability, possible benefit)
Belief that patient should not be offered trial (e.g. would not adhere, is not competent)
Comfort with and style of communicating trials to patients
Site Level Barriers
Staffing (e.g. research nurses and support staff)
Redundant review processes and slow trial activation
Lack of standard processes to screen patients for trials
System Level Barriers
Overly optimistic accrual goals and/or limited accrual feasibility assessment prior to activation
Inadequate recruitment planning during trial development process
Limited funding and incentives for site and provider involvement (to conduct and offer trials)
Trial availability and eligibility for incident patients (e.g. exclusions for comorbidities, prior treatment)
Recent analyses of ClinicalTrials.gov data have found that slow accrual is a challenge for both industry-funded and publicly funded trials (3, 14–16). However, our understanding of early phase trials’ accrual is still limited by the fact that Phase 1 trials are not required to register on ClinicalTrials.gov (17). More in-depth analyses of cross-trial accrual have been possible for NCI trials, although most have looked at later-phase trials (18, 18). A review of NCI non-pediatric phase 3 cancer treatment trials activated from 2000 to 2007 found that 26.7% had less than 90% of projected accrual because of inadequate accrual rates and 2.0% of all patients (n=2,930) accrued in that time frame were on trials that had inadequate accrual (20).
Research exploring accrual challenges, specifically in early phase trials, has been limited and largely focused on the experience of trials accruing at a single site. However, a common theme is the challenge of administrative processes around early phase trials, which have been linked to lower patient referral (21) and higher cost per enrollment when evaluating patients for Phase 1 trials (22). Another challenge facing early phase trial accrual is the determination of the eligibility criteria, which require balancing the need to maximize safety in vulnerable patient populations without jeopardizing trial success by being overly selective (23–25). More broadly, when researchers compared early and late phase trials in ClinicalTrials.gov, they found that Phase 2 trials were significantly more likely than Phase 3 trials to have inadequate accrual (3); furthermore, single-center trials were significantly more likely than multi-center trials to fail to complete (15).
Within NCI, much work has been done in the past decade to streamline its early phase clinical trial systems and processes with the goal of improved access to innovative therapies, reduced study activation time, improved accrual rates, and timely completion of trials. These efforts have included the implementation of timelines for clinical trial development (26) and the creation of the Experimental Therapeutics Clinical Trials Network (ETCTN) in 2014 (27). One NCI effort focused explicitly on early phase trials led to the implementation of Corrective Action Plans (CAPs) in certain early phase trials.
In 2011, NCI began requesting CAPs for all CTEP-held Investigational New Drug (IND) Phase 1 and 2 trials that are deemed poor accruing six months after activation. Trials slow to accrue early in their lifecycle are more likely to fail overall (2). CTEP believed that requesting a CAP could improve the accrual rate and reduce the likelihood that the trial will fail overall. To initiate a CAP, CTEP conducts quarterly reviews of its Phase 1 and 2 trials where CTEP is the holder of the Investigational New Drug (IND) and identifies trials that are accruing less than 50% of their projected accrual rate after Quarter 2 (Phase 1) and Quarter 3 (Phase 2). Study chairs are notified via a letter from CTEP that provides an accrual summary and requests a CAP within 10 business days that describes the reasons for slow accrual and the proposed steps to be taken to improve accrual and achieve the trial’s enrollment objectives.
The remainder of this article presents the findings from a mixed-methods content analysis conducted on CAPs requested by CTEP between August 2011 and February 2013. The objectives of the content analysis were to: 1) categorize the reasons given by investigators for slow accrual and their proposed corrective actions; 2) identify potential patterns of accrual across the CAPs (trial duration and monthly accrual rates); and 3) assess the impact of CAP receipt on the attainment of trials’ primary scientific objectives. Previous analyses of accrual barriers have predominantly considered late phase trials (16, 20) and pre-activation barriers, but this analysis focuses on early phase trials and considers post-activation barriers to trial completion.
METHODS
Sample
Of the 327 CTEP-held IND Phase 1 and 2 studies active between August 2011 and February 2013, CAP requests were made for 150 (46%) due to poor accrual. Fifteen studies were excluded due to trial closures or corrections for reasons other than inadequate accrual, resulting in 135 (41%) studies in the final analyses. For the analysis, Phase 1/2 trials (n=25; 18.5%) were coded as either a Phase 1 or 2 trial depending upon which phase the trial was in when the CAP was received; pilot trials were categorized as Phase 1 trials.
Content Analysis
CAPs were qualitative letters from study chair principal investigators (PIs) describing their perceived reasons for the trial’s slow accrual and their planned action steps to increase, or correct, the accrual rate. A content analysis design was used to systematically quantify the CAP content into standardized variables (28) using a coding sheet developed after a review of 10 randomly selected CAPs. Three trained coders independently coded the CAPs, with each CAP coded twice (intercoder reliability of 78%); post-coding deliberations among the coders yielded a 100% consensus on any discrepancies.
Primary Scientific Objective
All CAP trials that were permanently closed to accrual at the time of analysis were coded as to whether or not they met their stated primary scientific objectives. For each, the CTEP lead medical officer reviewed the trial’s protocol and final CTEP documentation and reports to assess, retrospectively, whether or not the CAP trial met its primary scientific objective.
Slow Accrual Variables
The final coding sheet had eight high-level category options to comprehensively describe a CAPs reasons for slow accrual; each option had up to eight sub-categories to provide a more granular reason. Coders were asked to code to the sub-category level to describe reasons for slow accrual.
Accrual Action Variables
Five high-level codes, with corresponding sub-categories, were used to categorize proposed action steps described in the CAPs. Three additional codes were available: “other” (if none of the action steps were sufficient); “Proposed element not considered an action item” (if the CAP provided a narrative, but no clear action step); and, “No action provided” (if the CAP omitted the section describing proposed actions). Similarly, coders were asked to code to the sub-category level to describe proposed action steps.
Comparison of Reasons with Actions
Crosstabs were run to compare slow accrual reasons with their respective proposed corrective actions. Each cell was then coded to reflect how well the reason and action were aligned (i.e., could a proposed action possibly affect the slow accrual reason). Three categories were assigned: A proposed action is well matched; a proposed action has potential (to influence slow accrual); and no action matches.
Trial-Specific Data
For each trial receiving a CAP, the CTEP trial database was used to report its duration (projected trial duration, number of months active before and after a CAP receipt, and total number of months active); and accrual (projected monthly accrual, actual total accrual, and actual monthly accrual rate at time of CAP receipt and post CAP). Duration and accrual rates were adjusted to exclude any time for when a trial may have been temporarily closed to accrual.
Mixed-Methods Analysis
A triangulation mixed-methods approach (29) was utilized. This involved triangulating qualitative and quantitative data from the CAPS letters, as well as quantitative data regarding the accrual of CAPS trials, so that each data source informed and enhanced understanding of the overall results.
RESULTS
The objectives of the content analysis described in this paper were to categorize the reasons for slow accrual to NCI’s early phase clinical trials and proposed corrective actions, identify potential patterns across the CAPs regarding trial duration, monthly accrual rates, and assess the impact of CAP receipt on accrual and the attainment of primary objectives.
Description of CAP Trials
Of the 135 CAPs analyzed (“CAP trials”), 69 (51%) were Phase 1 and 66 (49%) were Phase 2 trials; 119 (88%) were trials involving adults, with 11 (8%) pediatric-focused trials, and the remaining 5 (4%) for both adults and children. At the time of analysis (2/24/14), 94 (70%) were closed to accrual. The median trial activation date was October 15, 2010, and CAP trials were open a median of 30 months, with a median time open of 14 months pre-CAP and 16 months post-CAP. For Phase 1 trials the CAP was received at approximately the midpoint of their active duration (at 15 of 29 active months); CAPs were received earlier in the trial life cycle for Phase 2 trials (at 12 of 31 active months).
Reasons for Slow Accrual
Table 1 provides a detailed summary of slow accrual reasons by category and trial phase for the 135 studies analyzed. For 56% of Phase 1 trials, PIs reported two or more reasons for slow accrual, with the most common reasons being safety/toxicity issues (48%), design/protocol concerns (42%), eligibility criteria (41%), delays due to administrative problems at the site level (30%), and extended Institutional Review Board (IRB) or site activation delays (16%). If safety/toxicity reasons are removed, 74% of Phase 1 trials still have at least one other slow accruing reason listed. For Phase 2 trials, PIs listed two or more slow accrual reasons for 40% of the trials, with top reasons being: eligibility criteria (35%); design/protocol concerns (33%); extended IRB or site activation delays (29%); institutional or administrative delays (23%); and, safety/toxicity issues (20%). No reasons for slow accrual were given by PIs for 6% of Phase 1 trials and 12% of Phase 2 trials.
Table 1.
Percent of trials (N=135) with reported reasons for slow accrual, by phase of trial*
| Categories and Reasons for Slow Accrual | Phase 1 (N=69) | Phase 2 (N=66) |
|---|---|---|
| Safety/Toxicity | 48% | 20% |
| Enrollment slowed/halted due to safety/toxicity but not a DLT | 19% | 6% |
| Dose Limiting Toxicity (DLT) leading to dose reduction | 16% | 2% |
| Condense Adverse Event Protocol Reporting (CAEPR) update required/RRA/Action letter | 9% | 11% |
| FDA holds or amendments | 3% | 0% |
| Safety-other | 1% | 2% |
| Study Design/Protocol | 42% | 33% |
| Longer than expected pre-specified halt/change to enrollment due to interim analyses, stopping rules, data review by DMC | 20% | 12% |
| Safety observation periods/safety run-in taking longer than planned and slowing accrual (but enrollment not stopped) | 10% | 9% |
| Treatment schedule, procedures, or requirements causing undue patient burden leading to slow enrollment | 6% | 5% |
| Design/protocol-other | 6% | 3% |
| Trial complexity | 0% | 5% |
| Eligibility | 41% | 35% |
| Strict eligibility criteria | 23% | 14% |
| High rate of screen failures | 12% | 9% |
| Rare disease | 4% | 11% |
| Eligibility-other | 1% | 2% |
| Institutional/PI/Administrative | 30% | 23% |
| Delays due to staffing/management/operational issues | 14% | 9% |
| Site initiated amendments | 6% | 5% |
| Competing trials within institution | 4% | 3% |
| Low rate of internal referrals | 4% | 2% |
| Institution/admin-other | 1% | 2% |
| Scientific review internally | 0% | 2% |
| IRB/Site Activation | 16% | 29% |
| Extended delays for IRB approval and/or site activation at planned sites | 7% | 15% |
| Planned site(s) have not yet activated trial/were removed | 4% | 12% |
| Trial open at one or a limited number of institutions limiting population access | 4% | 2% |
| CTEP Administrative Issues | 7% | 4% |
| CTEP requests for amendments (not related to safety) | 3% | 2% |
| CTEP-other | 4% | 2% |
| Environment/External Issues | 6% | 8% |
| Newly approved drugs for disease/changes in standard of care | 6% | 6% |
| Environ/external-other | 0% | 2% |
| Drug Supply | 5% | 4% |
| Lack of available drug supply causing accrual delays/discontinued | 4% | 2% |
| Company not willing to supply drug/withdrew support | 1% | 0% |
| Drug-other | 0% | 2% |
| Budget/Insurance Issues | 1% | 11% |
| Loss of study funding | 0% | 5% |
| Study procedures or drug not being covered by insurance/reimbursed | 1% | 3% |
| Low reimbursement rate negatively impacting enrollment | 0% | 3% |
| No Reason Given | 6% | 12% |
Sorted in descending order by Phase 1 trial slow accruing reasons
Corrective Actions for Slow Accrual
PIs provided a median of one corrective action per Phase 1 trials, and a median of two corrective actions for Phase 2 trials. Table 2 provides a detailed summary of the proposed corrective actions, by category and phase of trial. The most commonly listed corrective actions for Phase 1 trials were: amend trial (to change eligibility or design) (55%); add institutions (43%); promote the trial either internally or externally (36%); and make administrative or procedural changes at their institution (29%). Thirteen percent of Phase 1 trials failed to provide a corrective action in their CAP. For Phase 2 trials, adding institutions was the top action step provided (85%), followed by amending the trial (44%); promoting the trial (42%); and making administrative or procedural changes at their institution (26%). Only 2% of Phase 2 trials provided no corrective action in their CAP.
Table 2.
Percent of trials with reported corrective actions to address slow accrual, by phase of trial*
| Categories and Actions to Address Slow Accrual | Phase 1 (n=69) | Phase 2 (n=66) |
|---|---|---|
| Amend the Trial | 55% | 44% |
| Broaden eligibility criteria | 33% | 27% |
| Amend the treatment design (decrease toxicity, statistical design, revise schema) | 20% | 14% |
| Amend the trial-other | 1% | 3% |
| Add Institutions | 43% | 85% |
| Open group-wide/group endorsement | 1% | 5% |
| Add international collaborators | 0% | 3% |
| Place on CTSU | 0% | 5% |
| Activation/IRB approval at planned participating sites | 6% | 27% |
| Add new institutions beyond those originally planned | 36% | 45% |
| Promotion of Trial | 36% | 42% |
| External (reach out to other communities for additional referral base) | 14% | 12% |
| Internal (within own group/CTSU/Cooperative Group system) | 19% | 27% |
| Increase awareness through advocacy groups and professional societies | 3% | 2% |
| Promotion-other | 0% | 2% |
| Administrative/Institutional Processes | 29% | 26% |
| Broaden patient screening activities (by study team) to identify eligible patients | 6% | 6% |
| Increase study team review processes, meetings, conference calls around trial | 7% | 5% |
| Increase/change staffing | 4% | 5% |
| Change administrative processes (streamlining, increase efficiencies) | 4% | 3% |
| Close competing trials internally | 4% | 3% |
| Administrative-other | 3% | 5% |
| Funding | 6% | 11% |
| Increase per case funding | 0% | 5% |
| Obtain additional funding | 6% | 6% |
| Proposed element not considered an action | 32% | 23% |
| No action provided | 13% | 2% |
Sorted in descending order for Phase 1 trial corrective actions
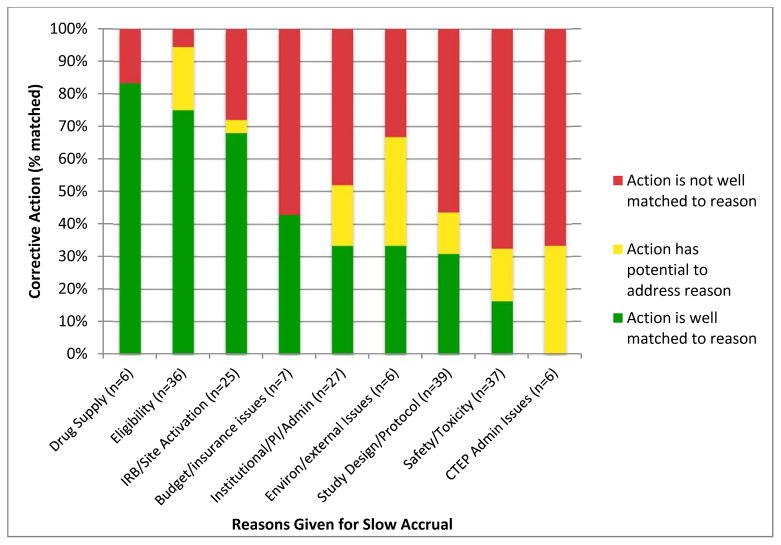
There were 189 separate reasons given for slow accrual for the 135 trials with CAPs. Forty percent of these separate reasons were well matched by a proposed action in the CAP. Another 14% of these reasons were addressed by a proposed action that could potentially influence the slow accrual reason, while the remaining 46% were identified as poorly matched by any of the proposed actions in the CAP. Table 3 provides examples of each category of matching, and Figure 1 shows the percent matched for the nine high-level slow accrual reasons. The best matched reasons were drug supply (83% matched), eligibility (75%) and IRB/site activation concerns (68%). Safety/toxicity (16%) and CTEP administrative reasons (0%) were the most poorly matched of the slow accrual reasons.
Table 3.
Examples of coded reasons with matching and non-matching actions
| Slow Accrual Reason | Proposed Action | Action Matches Reason? |
|---|---|---|
| Strict eligibility criteria | Broaden eligibility criteria | Well matched |
| Extended delays for IRB/site activation | Activation/IRB approval at planned participating site | Well matched |
| High rate of screen failures | Promotion of trial – internal | Potential to influence |
| Longer than expected pre-specified halt/change to enrollment due to interim analyses, stopping rules, data review by DMC | Add new institutions | Does not match |
| Longer than expected pre-specified halt/change to enrollment due to interim analyses, stopping rules, data review by DMC | No action provided | Does not match |
Figure 1.
Reasons given for slow accrual and percentage of proposed corrective actions that match the slow accrual reasons (N=189).
Monthly Accrual Rate Change
Trials’ monthly accrual rates were compared for the months before and after a CAP receipt. For 74% (n=100) of all trials, the monthly accrual rate increased post-CAP; 47% (n=63) of all trials increasing accrual by at least 0.5 patients per month. For Phase 1 trials, 30% (n=21) showed no improvement in their monthly accrual rate post-CAP; 39% (n=27) increased their monthly accrual rate by less than 0.5 patients; and the remaining 30% (n=20) increasing their rate by 0.5 to 3 patients post-CAP. For Phase 2 trials, 21% (n=14) showed no improvement post-CAP; 15% (n=10) had an increase of less than 0.5 patients per month; 47% (n=31) had an increase between 0.5 and 3 patients per month, and 17% (n=11) increased more than 3 patients per month post-CAP.
Percent of Closed Trials Meeting Primary Scientific Objectives
Seventy percent (n=94) of the trials were closed at time of analysis. Of the 48 closed Phase 1 trials, 67% (n=32) were reported to meet their primary scientific objectives. Of the 46 closed Phase 2 trials, 70% (n=32) were reported to meet their primary objectives. No differences were noted by phase of trial. Regardless of whether or not a trial met its objectives, actual accrual exceeded the estimated time to accrual by a factor of three: Trials meeting their primary objectives were projected to take a median of nine months to reach minimum accrual, but required a median of 31 months; while trials not meeting their primary objectives were projected to take a median of eight months but were active a median of 26 months before closure. Of trials that met their primary objectives, Phase 1 trials exceeded their estimated median accrual time by a factor of five (projected seven months vs. requiring 34 months). Phase 2 trials exceed their estimated median accrual time by a factor of three (projected 10 months vs. requiring 31 months).
CAP Impact on Scientific Objectives
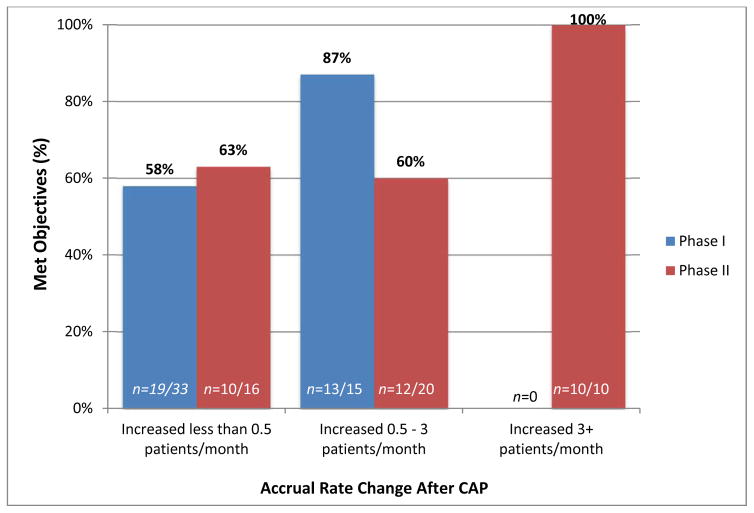
The percent of closed trials reported as meeting their primary scientific objectives was categorized by phase of trial and the degree of increase in monthly accrual rate post-CAP. Figure 2 presents these comparisons. Of the closed Phase 1 trials in the study (N=48), 69% (n=33) had a post-CAP monthly accrual rate increase of less than 0.5 patients; among these, 58% (n=19) were reported as having met their primary scientific objectives. The remaining closed Phase I trials (31%; n=15) had a post-CAP monthly increase between 0.5 and 3 patients; among these, 87% (n=13) were reported as having met their primary objectives.
Figure 2.
The percent of closed CAP trials (N=94) that met primary scientific objectives, by rate change and phase of trial. Of the 48 closed Phase 1 trials, 33 had an accrual increase of less than 0.5 patient/month, and 15 increased 0.5–3 patients/month. Of the 46 closed Phase 2 trials, 16 had an accrual increase of less than 0.5 patient/month, 20 increased 0.5–3 patients/month, and 10 increased 3+ patients/month. Of all closed trials, 24% had an accrual rate increase associated with a greater likelihood of meeting their primary scientific objectives (13 Phase 1 and 10 Phase 2 trials).
Of the closed Phase 2 trials (N=46), 35% (n=16) had a post-CAP monthly accrual rate increase of less than 0.5 patients; among these, 63% (n=10) were reported as having met their primary scientific objectives. An additional 44% (n=20) of closed Phase 2 trials had a post-CAP monthly increase between 0.5 and 3 patients; among these, 60% (n=12) were reported as having met their primary objectives. Of the closed Phase 2 trials that increased their post-CAP monthly accrual by greater than 3 patients (n=10), all (100%) were reported as having met their primary scientific objectives.
Of the 94 closed trials, 13 Phase 1 studies and 10 Phase 2 studies had an accrual rate increase of > 0.5/month and 3+ patients/month, respectively, that were associated with increased levels of meeting clinical trial objectives above the basal rate of 60 – 63%. Thus, 23 of 94 (24%) of trials had greater accrual associated with a greater likelihood of meeting clinical trial objectives.
DISCUSSION
This paper provides the first analysis of the use of corrective action plans (CAPs) to improve accrual to early phase NCI clinical trials. The trials that required CAPs in this study underperformed: Of the two-thirds of closed trials that ultimately met their primary scientific objectives, actual accrual time exceeded the estimated time by a factor of three for Phase 2 trials and a factor of five for Phase 1 trials. This speaks to the need for both enhanced accrual planning and realistic accrual projections.
CAPs ask study PIs to provide an open-ended assessment of their trials’ accrual barriers and proposed solutions. For Phase 1 trials, safety and toxicity dominated reasons given by PIs for slow accruing trials, but strict eligibility and protocol design challenges also posed substantial accrual concerns. Phase 2 trials also encountered eligibility and protocol design barriers, but extended IRB or site activation delays were an additional challenge. Both Phase 1 and 2 trials reported slow accrual due to institutional or administrative delays at the site level. Though many corrective actions were proposed by investigators to improve accrual, almost half of these actions were poorly aligned with the reasons given for the slow accrual, in part because some of the reasons may have been difficult to address.
Overall, however, implementing the CAPs was associated with improving the accrual rate for about three-quarters of the trials, with just under half of all trials recording an increase of at least 0.5 patients per month. More notably, 24% of all closed trials had an accrual rate increase associated with a greater likelihood of meeting their primary scientific objectives, with only modest increases of 0.5 to 3 patients required for Phase 1 trials, and increases of more than 3 patients required for Phase 2 trials. Thus, the utility of requesting a CAP for slow accruing trials was seen in increased monthly accrual rates for many trials and a greater likelihood of achieving their scientific primary objectives for fewer trials.
A content analysis is a purely descriptive method to measure observed patterns and therefore is subject to increased error due to independent interpretations of the coders. The high inter-coder reliability and two-step process to reach 100% agreement for this study, however, lends support to the quality of this data collection method. An additional strength of this method is that investigators were able to describe the accrual barriers they perceived and propose responses without restrictions. Many of the accrual barriers described in the CAPs (Table 1) were not among those commonly cited by other sources (Box 1), perhaps suggesting that barriers to accrual in early phase trials may be substantively different than the barriers seen in later phase trials, which have been more widely studied.
Our understanding of the qualitative analysis in this study was further enhanced by the incorporation of quantitative data on trial accrual. This study is limited in that comparison data are not available for Phase 1 and 2 trials that did not receive a CAP during this same time frame. Through recent consolidation of its data collection processes, however, NCI now has the ability to track the performance of non-CAP trials and thus provide comparisons in future years to address this limitation of this study. Also, the determination of whether closed trials had met their primary scientific objectives was performed retrospectively. To ensure accuracy, CTEP’s lead medical officers were provided sufficient archived trial documentation and reports on which to make a determination.
Though CAPs were intended to be sent to trials that met less than 50% of their projected accrual rate by their sixth (Phase 1) or ninth month (Phase 2) after activation, the median CAP request was made late in the second year of trials, thereby allowing slow accruing trials to linger longer without a deliberate review of trial challenges and potential corrective actions. Since conducting this research, NCI further tightened the CAP guidelines and enforcement, announcing in March 2015 that both Phase 1 and 2 studies will be reviewed at the six-month period and that any trial receiving a CAP request will be reassessed six months post-CAP. CAP trials will need to increase their projected quarterly accrual rate to at least 75% of their projected accrual or risk receiving another CAP request or be administratively closed due to poor accrual. In addition, NCI has since created a standardized CAP form for PIs to use when responding to a CAP request (see Supplementary Figure S1). The form provides a checklist of slow accrual reasons from which PIs can select, as well as a respective checklist of corrective actions to be taken (including ‘other’ write-in options if needed). These checklists were developed based on the findings of the described analysis.
The CAP program is centered on the need to seek solutions when a trial is already underperforming. To use a health metaphor, CAPs are called in to treat an already sick patient, but strategies to prevent the illness may be more effective. As noted earlier, accrual planning typically involves defining the population of eligible participants but rarely takes other variables into account or describes proactive measures to enhance accrual (10). The CAP analysis is based on whether or not trials are meeting their target accrual rates; perhaps, as mentioned earlier, investigators are overly optimistic during the planning phase of a trial with respect to how many eligible patients they will encounter monthly, and the criteria of how target rates are determined should be re-evaluated.
Unfortunately, PIs and trial sponsors often do not incorporate planning to increase awareness and interest in their trial and identify trial barriers and potential intervening action steps before a trial is activated. Investigators may also be hampered by a lack of evidence-based strategies to increase accrual (30). However, planning in advance to support trial accrual may reduce the need for later action in the form of a CAP or other mechanism (8, 9). Recently, the Clinical Trials Transformation Initiative (CTTI), a public-private partnership of more than 60 organizations from across the clinical trial enterprise, released a set of recommendations to guide study teams on how to efficiently and effectively plan for clinical trial recruitment (31). Attention should also be paid to enhancing accrual for minority and underserved patients who may otherwise be underrepresented, particularly in early phase trials (32, 33). Although more research is needed to further develop the evidence base for accrual, a number of resources and a curated library of relevant journal articles are available at NCI’s AccrualNet website (34, 35).
Related to the need for advanced planning is the need for PIs to thoughtfully plan their corrective actions in response to a CAP request (36). Most PIs who provide a CAP response back to NCI have done so in a thorough, introspective manner, giving a clear picture of the challenges impeding accrual to their trial. That just under half of the actionable slow accrual reasons had a relevant matching corrective action proposed by PIs is probably reflective of the inherent difficulty in addressing some of the reasons. Also, PIs may need additional support (and, possibly, more than the allotted 10 days) in thinking through the potential available solutions. It is likely that both pre-study planning and a CAP program with enforceable consequences are both necessary to improve accrual to the early phase treatment trials conducted by CTEP.
Many of the slow accrual reasons identified in this study require changes at a more systemic level and are out of the control of the PIs overseeing the trials, such as slow responses from IRBs, difficulty opening the trial at other institutions, or access to rare patient populations. As described earlier, that NCI completely overhauled its early phase clinical trial program into its new ETCTN. The ETCTN support an experimental therapeutics clinical trials program through the creation of a consolidated, integrated, network of early phase investigators and organizations around North America. Through this network, PIs can easily open their trials at other network sites using a central infrastructure and NCI’s central IRB, where NCI is the sole IRB of record. The ETCTN provide PIs an efficient, accessible clinical trial network system as well as access to far greater number patients beyond single institutions.
Overall, this research highlights various program recommendations moving forward. Future trials are likely to benefit from implementing an accrual plan strategy early in trial development to limit the need for CAPs. Implementing and enforcing CAPs early in a trial’s lifecycle is encouraged to allow earlier corrective actions and to better align corrective actions with any issues related to slow accrual. NCI’s new ETCTN is well poised to address many of the slow accrual reasons that surfaced in the CAPs analysis, such as reducing IRB delays, increasing site activation, and applying a team approach that involves more support with trial design, should also be addressed.
Supplementary Material
Acknowledgments
The authors acknowledge the support of the National Cancer Institute at the National Institutes of Health.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interests: No potential conflicts of interest were disclosed.
References
- 1.Bates SE, Berry DA, Balasubramaniam S, Bailey S, LoRusso PM, Rubin EH. Advancing clinical trials to streamline drug development. Clin Cancer Res. 2015;21:4527–35. doi: 10.1158/1078-0432.CCR-15-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng SK, Dietrich MS, Dilts DM. Predicting accrual achievement: monitoring accrual milestones of NCI-CTEP–sponsored clinical trials. Clin Cancer Res. 2011;17:1947–55. doi: 10.1158/1078-0432.CCR-10-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: An empirical analysis of recently closed trials. Clin Trials. 2015;12:77–83. doi: 10.1177/1740774514558307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med. 2011;86:1360–66. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doroshow JH. Timely completion of scientifically rigorous cancer clinical trials: an unfulfilled priority. J Clin Oncol. 2013;31:3312–14. doi: 10.1200/JCO.2013.51.3192. [DOI] [PubMed] [Google Scholar]
- 6.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9:267–76. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, Levit LA, Adamson PC, Bruinooge S, Burris HA, Carducci MA, et al. American Society of Clinical Oncology policy statement update: the critical role of phase I trials in cancer research and treatment. J Clin Oncol. 2015;33:278–84. doi: 10.1200/JCO.2014.58.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beasley D. Perfect harmony. Applied Clinical Trials. 2004 Nov 1; [cited 2016 Mar 10]. Available from: http://www.appliedclinicaltrialsonline.com/perfect-harmony.
- 9.White C. Engaging physicians, staff and patients for oncology trials. Applied Clinical Trials. 2015 Nov 11; [cited 2016 Mar 10]. Available from: http://www.appliedclinicaltrialsonline.com/engaging-physicians-staff-and-patients-oncology-trials.
- 10.Hunninghake DB, Darby CA, Probstfield JL. Recruitment experience in clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1987;8:6S–30S. doi: 10.1016/0197-2456(87)90004-3. [DOI] [PubMed] [Google Scholar]
- 11.Kost RG, Mervin-Blake S, Hallarn R, Rathmann C, Kolb R, Himmelfarb CD, et al. Accrual and recruitment practices at Clinical and Translational Science Award (CTSA) institutions: a call for expectations, expertise, and evaluation. Acad Med. 2014;89:1180–9. doi: 10.1097/ACM.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denicoff A, Massett HA, Mishkin G, Bangs R, Berlin J, Bischoff MB, et al. Creating a national collaborative strategy to enhance trial accrual in NCI’s National Clinical Trials Network (NCTN) in the era of precision medicine. J Clin Oncol. 2015;33(suppl) abstr 6589. [Google Scholar]
- 13.Bennette CS, Ramsey SD, McDermott CL, Carlson JJ, Basu A, Veenstra DL. Predicting low accrual in National Cancer Institute’s Cooperative Group Clinical Trials. J Natl Cancer Inst. 2016;108:1–7. doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams RJ, Tse T, DiPiazza K, Zarin D. Terminated trials in the ClinicalTrials. gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS ONE. 2015;10:e0127242. doi: 10.1371/journal.pone.0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stensland KD, McBride RB, Latif A, Wisnivesky J, Hendricks R, Roper N. Adult cancer clinical trials that fail to complete: an epidemic? J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju229. pii: dju229. [DOI] [PubMed] [Google Scholar]
- 16.Haddad RI, Chan ATC, Vermorken JB. Barriers to clinical trial recruitment in head and neck cancer. Oral Oncol. 2015;51:203–11. doi: 10.1016/j.oraloncology.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 17.FDAAA 801 Requirements [about 6 screens] [cited 2016 Mar 9]. Available from. https://clinicaltrials.gov/ct2/manage-recs/fdaaa.
- 18.Shroen AT, Petroni GR, Wang H, Gray R, Wang XF, Cronin W, et al. Preliminary evaluation of factors associated with premature trial closure and feasibility of accrual benchmarks in phase III oncology trials. Clin Trials. 2010;7:312–321. doi: 10.1177/1740774510374973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng SK, Dietrich M, Dilts DM. A sense of urgency: evaluating the link between clinical trial development time and the accrual performance of Cancer Therapy Evaluation Program (NCI-CTEP) sponsored studies. Clin Cancer Res. 2010;16:5557–63. doi: 10.1158/1078-0432.CCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korn EL, Freidlin B, Mooney M, Abrams JS. Accrual experience of National Cancer Institute Cooperative Group phase III trials activated from 2000 to 2007. J Clin Oncol. 2010;28:5197–201. doi: 10.1200/JCO.2010.31.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez AG, Chalela P, Suarez L, Muñoz E, Pollock BH, Weitman SD, et al. Early phase clinical trials: referral barriers and promoters among physicians. J Community Med Health Educ. 2012;2(8) pii: 1000173. [PMC free article] [PubMed] [Google Scholar]
- 22.Penberthy LT, Dahman BA, Petkov VI, DeShazo JP. Effort required in eligibility screening for clinical trials. J Oncol Pract. 2012;8:365–70. doi: 10.1200/JOP.2012.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinato DJ, Stavraka C, Flynn MJ, Forster MD, O’Cathail SM, Secki MJ, et al. An inflammation based score can optimize the selection of patients with advanced cancer considered for early phase clinical trials. PLoS One. 2014;9:e83279. doi: 10.1371/journal.pone.0083279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavraka C, Pinato DJ, Tumbull SJ, Flynn MJ, Forster MD, O’Cathail SM. Developing an objective marker to optimize patient selection and predict survival benefit in early-phase cancer trials. Cancer. 2014;120:262–70. doi: 10.1002/cncr.28381. [DOI] [PubMed] [Google Scholar]
- 25.Hyman DM, Eaton AA, Gounder MM, Smith GL, Pamer EG, Hensley ML. Nomogram to predict cycle-one serious drug-related toxicity in phase I oncology trials. J Clin Oncol. 2014;32:519–26. doi: 10.1200/JCO.2013.49.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Report of the Operational Efficiency Working Group of the Clinical Trials and Translational Research Advisory Committee: compressing the timeline for cancer clinical trial activation [PDF on the Internet] Bethesda (MD): National Cancer Institute; 2010. [cited 2016 Mar 9]. Available from: http://deainfo.nci.nih.gov/advisory/ctac/workgroup/OEWG-Report.pdf. [Google Scholar]
- 27.National Cancer Institute Experimental Therapeutics Clinical Trials Network program guidelines [PDF on the Internet] Bethesda (MD): National Cancer Institute; 2014. [cited 2016 Mar 9]. Available from: http://ctep.cancer.gov/initiativesPrograms/docs/ETCTN_Program_Guidelines.pdf. [Google Scholar]
- 28.Krippendorff K. Content analysis. In: Barnouw E, Gerbner G, Schramm W, Worth TL, Gross L, editors. International encyclopedia of communication. New York: Oxford University Press; 1989. pp. 403–7. [Google Scholar]
- 29.Creswell JW, Clark VLP, Gutmann ML, Hanson WE. Advanced mixed methods research designs. In: Tashakkori A, Teddlie C, editors. Handbook of mixed methods in social & behavioral research. Thousand Oaks (CA): Sage; 2003. pp. 209–40. [Google Scholar]
- 30.Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrom M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3:e002360. doi: 10.1136/bmjopen-2012-002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CTTI recommendations on efficient and effective clinical trial recruitment planning [PDF on the Internet] Durham (NC): Clinical Trials Transformation Initiative; 2016. [cited 2016 May 24]. Available from: http://www.ctti-clinicaltrials.org/files/Recruitment_Retention/Recruitment-Recs-full.pdf. [Google Scholar]
- 32.Wallington SF, Dash C, Sheppard VB, Goode TD, Oppong BA, Dodson EE. Enrolling minority and underserved populations in cancer clinical research. Am J Prev Med. 2016;50:111–117. doi: 10.1016/j.amepre.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noor AM, Sarker D, Vizor S, McLennan B, Hunter S, Suder A. Effect of patient socioeconomic status on access to early-phase cancer trials. J Clin Oncol. 2013;31:224–30. doi: 10.1200/JCO.2012.45.0999. [DOI] [PubMed] [Google Scholar]
- 34.AccrualNet. Strategies, Tools and Resource to Support Accrual to Clinical Trials [database on the Internet] Bethesda (MD): National Cancer Institute; 2010. [cited Feb 2016]. Available from: https://accrualnet.cancer.gov/ [Google Scholar]
- 35.Massett HA, Parreco LK, Padberg RM, Richmond ES, Rienzo ME, Leonard CE, et al. AccrualNet: addressing low accrual via a knowledge-based, community of practice platform. J Oncol Pract. 2011;7:e32–9. doi: 10.1200/JOP.2011.000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter RE, Sonne SC, Brady KT. Practical considerations for estimating clinical trial accrual periods: application to a multi-center effectiveness study. BMC Med Res Methodol. 2005;5:11. doi: 10.1186/1471-2288-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.