Abstract
The conventional view that neuroinflammatory lesions contain strictly pro- and anti-inflammatory cytokines is being challenged. Some proinflammatory products e.g. TNF-α are crucial intermediates in axon regeneration, oligodendroglial renewal and remyelination. A more functional system of nomenclature classifies cytokines by their neuro ‘protective’ or ‘suppressive’ properties. Beyond the balance of these ‘environmental’ or ‘extrinsic’ signals, specific ‘intrinsic’ determinants of cytokine signalling appear to influence the outcome of axoglial regeneration. In this commentary, we examine the potential importance of cytokine-induced histone modification on oligodendrocyte differentiation. Neuroinflammation mediates the release of astrocytic leukaemia inhibitory factor (LIF) and erythropoietin (EPO) which potentiates oligodendrocyte differentiation and myelin production. Meanwhile, histone deacetylation strongly suppresses important inhibitors of oligodendrocyte differentiation. Given that LIF and EPO induce histone deacetylases in other systems, future studies should examine whether this mechanism significantly influences the outcome of cytokine-induced remyelination, and whether epigenetic drug targets could potentiate the effects of exogenous cytokine therapy.
Keywords: Neurotrophic cytokines, Histone modification, Myelination, Epigenetics, Therapy, Neurodegeneration, Oligodendrocytes
Background
Oligodendrocyte proliferation and differentiation is regulated by an intricate network of extrinsic signals and intrinsic genetic and epigenetic control mechanisms. These are in turn determined by the dynamic milieu of the central nervous system (CNS). Amongst an array of important extracellular signals, astrocytes release leukaemia inhibitory factor (LIF) and erythropoietin (EPO) which are neuroprotective cytokines that promote the differentiation of OLs and preserve the integrity of the myelin sheath (Ishibashi et al. 2006, 2009; Cervellini et al. 2013). In the ‘steady state’, neuronal depolarisation releases adenosine which stimulates the production of these cytokines (Ishibashi et al. 2006). This process amplifies during episodes of ‘axoglial stress’, e.g. following excitotoxicity or when TNF-α is produced by inflammatory infiltrates (Fischer et al. 2014; Moidunny et al. 2012). Previously, we discussed potential genetic elements that may influence the efficacy of exogenous cytokine therapy (Roe 2015). Surprisingly, the literature paid little attention to the interactions between cytokine signalling and mediators of epigenetic modification.
Epigenetic modifications are defined as “heritable alterations to the DNA architecture that do not change the nitrogenous base sequence”. The ‘epigenome’ describes a landscape of chemical modifications affecting the histone proteins that wrap DNA (histone acetylation and methylation), or more directly, the DNA itself (DNA methylation). Previous studies have shown that the process of (re)myelination is dependent on a variety of epigenetic remodelling mechanisms including histone modification, DNA methylation and miRNAs (Shen et al. 2005, 2008; Liu and Casaccia 2010). In this commentary, we explore the role of histone modification on oligodendrocyte differentiation, the potential for neurotrophic cytokines to induce these modifications and the scope for novel therapeutic targets to bolster remyelination in the context of neuroinflammatory disease.
Commentary
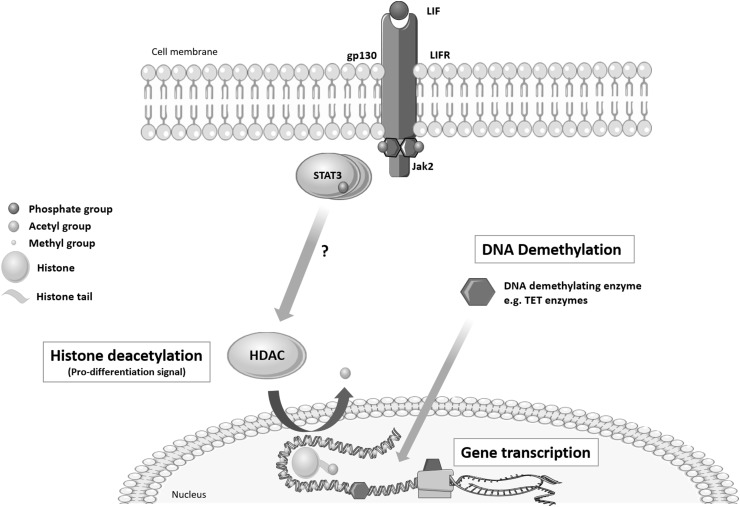
The conformational structure of chromatin determines the accessibility of genes. Acetylation of histone ‘tails’ reduces the positive charge that compresses the chromatin structure (Fig. 1). These modifications are determined by two key enzyme families; histone acetyltransferases (HATs) which acetylate histones and HDACs which remove the acetyl groups. The latter increases the electrostatic interaction to compress chromatin and hide regulatory gene elements. HDACs and HATs also (de)acetylate and physically interact with other nuclear proteins to form large transcriptional complexes that directly regulate gene expression.
Fig. 1.
Proposed schematic of LIF-induced epigenetic ‘de-repression’ of OL differentiation. As in stem cells, LIF may induce HDACs that deacetylate histones associated with gene loci that inhibit oligodendrocyte differentiation. Deacetylation leads to chromatin compaction which prevents DNA demethylation and thus the transcription of genes that inhibit myelination
In primary mouse oligodendrocyte progenitor cells, the capacity for myelination correlates directly with the activity of HDACs (Shen et al. 2008). Ye et al. found that HDAC1/HDAC2−/− mice developed severe tremors and died at postnatal day 14 (Ye et al. 2009). Oligodendrocyte progenitor-specific markers PDGFRA and OLIG2 were absent at embryonic day 15.5, and markers of differentiated oligodendrocytes were absent from postnatal day 4 (Ye et al. 2009). In addition, primary cortical oligodendrocyte precursors underwent differentiation arrest when cultured in vitro (Ye et al. 2009). As Li et al. show, HDAC1 and HDAC2 are critical for optimal oligodendrocyte specification and differentiation (Li and Richardson 2009). These HDACs were associated with transcriptional control regions believed to inhibit oligodendrocyte myelination. Furthermore, the expression of these inhibitory genes decreased in line with HDAC activity (Shen et al. 2008). Interestingly, chromatin is predominantly acetylated in primary oligodendrocyte progenitors whereas it grossly deacetylates during differentiation (Shen et al. 2005; Liu et al. 2007; Marin-Husstege et al. 2002). Indeed treatment with HDAC inhibitors arrests maturation (Shen et al. 2005; Liu et al. 2007; Marin-Husstege et al. 2002). These studies support Emery’s ‘de-repression’ theory which argues that environmental cues e.g. inflammation prompts the attenuation of inhibitors of differentiation, which in this context is open chromatin and demethylated DNA (Fig. 1) (Emery 2010).
LIF is well known for its function in maintaining the pluripotency of embryonic stem cells. This occurs through the Janus kinase/signal transducer and activator of transcription 3 (Jak/Stat3) pathway. McCool et al. showed that withdrawal of LIF leads to a global increase in histone acetylation that mimicked the effect of trichostatin A, a histone deacetylase inhibitor (McCool et al. 2007). These data could suggest that LIF-induced STAT3 regulates the action of epigenetic modifiers which control access to important genes (Fig. 1). These effects fit with the pattern of HDAC levels required to mediate (re)myelination and this may in part, explain LIF’s promyelinating effects. Yamamura et al. showed that FK228 (a HDAC inhibitor) suppresses the anti-apoptotic effects of EPO on erythroid precursor cells (Yamamura et al. 2006). The authors speculated that HDAC inhibition may block an EPO signalling pathway, indicating that EPO induces HDACs. Again, given EPO’s capacity to promote oligodendrocyte differentiation, we postulate that, as in erythrocytes, EPO may induce HDACs to attenuate inhibitors of myelin gene expression. In addition, Ye et al. showed that HDAC1 and HDAC2 stimulate oligodendrocyte differentiation by directly antagonizing the inhibitory action of Wnt signalling (Ye et al. 2009).
Conclusion
Epigenetic processes clearly influence the outcome of (re)myelination. Histone deacetylation is associated with the repression of important inhibitors of oligodendrocyte differentiation and neurotrophic cytokines such as LIF and EPO have demonstrated a potential to induce these HDACs in other systems. Future research should specifically examine the epigenetic processes that occur downstream of LIF and EPO in oligodendrocytes. This could lead to a novel therapeutic approach to demyelinating disease that incorporates epigenetic drug targets e.g. HDACs to bolster the efficacy of exogenous cytokine therapy.
Acknowledgments
Author Contribution
The corresponding author declares that he solely designed and interpreted this commentary and wrote the manuscript without external input.
Abbreviations
- TNF-α
Tumour necrosis factor-alpha
- LIF
Leukaemia inhibitory factor
- EPO
Erythropoietin
- HDACs
Histone deacetylases
- CNS
Central nervous system
- HATs
Histone acetyltransferases
- PDGFRA
Platelet derived growth factor receptor alpha
- OLIG2
Oligodendrocyte transcription factor 2
- Jak
Janus kinase
- STAT3
Signal transducer and activator of transcription 3
Compliance with Ethical Standards
Conflict of interest
The author declares no competing interests.
References
- Cervellini I, Annenkov A, Brenton T, Chernajovsky Y, Ghezzi P, Mengozzi M. Erythropoietin (EPO) increases myelin gene expression in CG4 oligodendrocyte cells through the classical EPO receptor. Mol Med. 2013;19:223–229. doi: 10.2119/molmed.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330(6005):779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Fischer R, Wajant H, Kontermann R, Pfizenmaier K, Maier O. Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia. 2014;62:272–283. doi: 10.1002/glia.22605. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Dakin KA, Stevens B, Lee PR, Kozlov SV, Stewart CL, Fields RD. Astrocytes promote myelination in response to electrical impulses. Neuron. 2006;49(6):823–832. doi: 10.1016/j.neuron.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Lee PR, Baba H, Fields RD. Leukemia inhibitory factor regulates the timing of oligodendrocyte development and myelination in the postnatal optic nerve. J Neurosci Res. 2009;87:3343–3355. doi: 10.1002/jnr.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Richardson WD. Genetics meets epigenetics: HDACs and Wnt signaling in myelin development and regeneration. Nat Neurosci. 2009;12(7):815–817. doi: 10.1038/nn0709-815. [DOI] [PubMed] [Google Scholar]
- Liu J, Casaccia P. Epigenetic regulation of oligodendrocyte identity. Trends Neurosci. 2010;33:193–201. doi: 10.1016/j.tins.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Han YR, Li J, Sun D, Ouyang M, Plummer MR, Casaccia-Bonnefil P. The glial or neuronal fate choice of oligodendrocyte progenitors is modulated by their ability to acquire an epigenetic memory. J Neurosci. 2007;27:7339–7343. doi: 10.1523/JNEUROSCI.1226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Husstege M, Muggironi M, Liu A, Casaccia-Bonnefil P. Histone deacetylase activity is necessary for oligodendrocyte lineage progression. J Neurosci. 2002;22:10333–10345. doi: 10.1523/JNEUROSCI.22-23-10333.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccool KW, Xu X, Singer DB, Murdoch FE, Fritsch MK. The role of histone acetylation in regulating early gene expression patterns during early embryonic stem cell differentiation. J Biol Chem. 2007;282:6696–6706. doi: 10.1074/jbc.M609519200. [DOI] [PubMed] [Google Scholar]
- Moidunny S, Vinet J, Wesseling E, Bijzet J, Shieh CH, Van Ijzendoorn SC, Bezzi P, Boddeke HW, Biber K. Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity. J Neuroinflamm. 2012;9:198. doi: 10.1186/1742-2094-9-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe C (2015) Can neurotrophic cytokines help to regenerate the mammalian central nervous system? Int J Neurosci. doi:10.3109/00207454.2015.1049266 [DOI] [PubMed]
- Shen S, Li J, Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura K, Ohishi K, Katayama N, Yu Z, Kato K, Masuya M, Fujieda A, Sugimoto Y, Miyata E, Shibasaki T, Heike Y, Takaue Y, Shiku H. Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Br J Haematol. 2006;135:242–253. doi: 10.1111/j.1365-2141.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- Ye F, Chen Y, Hoang T, Montgomery RL, Zhao XH, Bu H, Hu T, Taketo MM, Van Es JH, Clevers H, Hsieh J, Bassel-Duby R, Olson EN, Lu QR. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nat Neurosci. 2009;12:829–838. doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]