Abstract
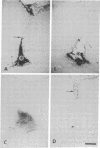
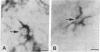
Deposition of the beta-amyloid protein in senile plaques is a pathologic hallmark of Alzheimer disease (AD). Focal deposition of beta amyloid in the adult rat cerebral cortex caused profound neurodegenerative changes, including neuronal loss and degenerating neurons and neurites. Chronic induction of the Alz-50 antigen appeared in neurons around focal cortical deposits of beta amyloid. Immunoblot analysis showed that beta amyloid induced Alz-50-immunoreactive proteins in rat cerebral cortex that were very similar to the proteins induced in human cerebral cortex from patients with AD. The neuropeptide substance P prevented beta-amyloid-induced neuronal loss and expression of Alz-50 proteins when coadministered into the cerebral cortex. Systemic administration of substance P also provided protection against the effects of intracerebral beta amyloid. Thus, beta amyloid is a potent neurotoxin in the adult brain in vivo, and its effects can be blocked by substance P.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks W. A., Kastin A. J. Peptides and the blood-brain barrier: lipophilicity as a predictor of permeability. Brain Res Bull. 1985 Sep;15(3):287–292. doi: 10.1016/0361-9230(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Mazurek M. F. Substance P-like immunoreactivity is reduced in Alzheimer's disease cerebral cortex. Neurology. 1987 Jul;37(7):1205–1209. doi: 10.1212/wnl.37.7.1205. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Swartz K. J., Finn S. F., Mazurek M. F., Kowall N. W. Neurochemical characterization of excitotoxin lesions in the cerebral cortex. J Neurosci. 1991 Jan;11(1):147–158. doi: 10.1523/JNEUROSCI.11-01-00147.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C., Vallet P. G., Hof P. R., Charnay Y., Golaz J., Constantinidis J. Substance P immunoreactivity in Alzheimer disease: a study in cases presenting symmetric or asymmetric cortical atrophy. Alzheimer Dis Assoc Disord. 1990;4(1):24–34. [PubMed] [Google Scholar]
- Crystal H. A., Davies P. Cortical substance P-like immunoreactivity in cases of Alzheimer's disease and senile dementia of the Alzheimer type. J Neurochem. 1982 Jun;38(6):1781–1784. doi: 10.1111/j.1471-4159.1982.tb06665.x. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C., Delaère P., Poulain V., Brion J. P., Hauw J. J. Does amyloid precede paired helical filaments in the senile plaque? A study of 15 cases with graded intellectual status in aging and Alzheimer disease. Neurosci Lett. 1988 Sep 12;91(3):354–359. doi: 10.1016/0304-3940(88)90706-9. [DOI] [PubMed] [Google Scholar]
- Gallyas F., Güldner F. H., Zoltay G., Wolff J. R. Golgi-like demonstration of "dark" neurons with an argyrophil III method for experimental neuropathology. Acta Neuropathol. 1990;79(6):620–628. doi: 10.1007/BF00294239. [DOI] [PubMed] [Google Scholar]
- Giaccone G., Tagliavini F., Linoli G., Bouras C., Frigerio L., Frangione B., Bugiani O. Down patients: extracellular preamyloid deposits precede neuritic degeneration and senile plaques. Neurosci Lett. 1989 Feb 13;97(1-2):232–238. doi: 10.1016/0304-3940(89)90169-9. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Wong C. W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984 May 16;120(3):885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Wolozin B. L., Davies P., Kromer L. J., Damasio A. R. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 1988 Apr;23(4):371–379. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Mori H., Selkoe D. J. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature. 1989 Sep 21;341(6239):226–230. doi: 10.1038/341226a0. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kidd M., Allsop D., Landon M. Senile plaque amyloid, paired helical filaments, and cerebrovascular amyloid in Alzheimer's disease are all deposits of the same protein. Lancet. 1985 Feb 2;1(8423):278–278. doi: 10.1016/s0140-6736(85)91054-2. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Mann D. M., Jones D., Prinja D., Purkiss M. S. The prevalence of amyloid (A4) protein deposits within the cerebral and cerebellar cortex in Down's syndrome and Alzheimer's disease. Acta Neuropathol. 1990;80(3):318–327. doi: 10.1007/BF00294651. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Multhaup G., Simms G., Pottgiesser J., Martins R. N., Beyreuther K. Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J. 1985 Nov;4(11):2757–2763. doi: 10.1002/j.1460-2075.1985.tb04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley B. J., Jr, Kowall N. W. Substance P-like immunoreactive neurons are depleted in Alzheimer's disease cerebral cortex. Neuroscience. 1991;41(1):41–60. doi: 10.1016/0306-4522(91)90199-x. [DOI] [PubMed] [Google Scholar]
- Rumble B., Retallack R., Hilbich C., Simms G., Multhaup G., Martins R., Hockey A., Montgomery P., Beyreuther K., Masters C. L. Amyloid A4 protein and its precursor in Down's syndrome and Alzheimer's disease. N Engl J Med. 1989 Jun 1;320(22):1446–1452. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Tagliavini F., Giaccone G., Frangione B., Bugiani O. Preamyloid deposits in the cerebral cortex of patients with Alzheimer's disease and nondemented individuals. Neurosci Lett. 1988 Nov 11;93(2-3):191–196. doi: 10.1016/0304-3940(88)90080-8. [DOI] [PubMed] [Google Scholar]
- Uéda K., Masliah E., Saitoh T., Bakalis S. L., Scoble H., Kosik K. S. Alz-50 recognizes a phosphorylated epitope of tau protein. J Neurosci. 1990 Oct;10(10):3295–3304. doi: 10.1523/JNEUROSCI.10-10-03295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson J. S., Selkoe D. J., Cotman C. W. Amyloid beta protein enhances the survival of hippocampal neurons in vitro. Science. 1989 Mar 17;243(4897):1488–1490. doi: 10.1126/science.2928783. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P. A neuronal antigen in the brains of Alzheimer patients. Science. 1986 May 2;232(4750):648–650. doi: 10.1126/science.3083509. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Davies P. Alzheimer-related neuronal protein A68: specificity and distribution. Ann Neurol. 1987 Oct;22(4):521–526. doi: 10.1002/ana.410220412. [DOI] [PubMed] [Google Scholar]
- Wolozin B., Scicutella A., Davies P. Reexpression of a developmentally regulated antigen in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6202–6206. doi: 10.1073/pnas.85.16.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Caceres A., Duffy L. K. Nerve growth factor potentiates the neurotoxicity of beta amyloid. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9020–9023. doi: 10.1073/pnas.87.22.9020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner B. A., Dawes L. R., Fisher S., Villa-Komaroff L., Oster-Granite M. L., Neve R. L. Neurotoxicity of a fragment of the amyloid precursor associated with Alzheimer's disease. Science. 1989 Jul 28;245(4916):417–420. doi: 10.1126/science.2474201. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]