Abstract
Dendrobium officinale (Tie Pi Shi Hu in Chinese) has been widely used to treat different diseases in China. Anticancer effect is one of the important effects of Dendrobium officinale. However, the molecular mechanism of its anticancer effect remains unclear. In the present study, gastric carcinogenesis in rats was used to evaluate the effect of Dendrobium officinale on cancer, and its pharmacological mechanism was explored. Dendrobium officinale extracts (4.8 and 2.4 g/kg) were orally administered to the rats of the gastric carcinogenesis model. Compared with the cancer model group, the high dose of Dendrobium officinale extracts significantly inhibited the rate of carcinogenesis. Further analysis revealed that Dendrobium officinale extracts could regulate the DNA damage, oxidative stress, and cytokines related with carcinogenesis and induce cell apoptosis in order to prevent gastric cancer.
1. Introduction
Gastric cancer is ranked as the fourth most common cause of cancer and as the second most frequent cause of death from cancer in the world [1]. Although the incidence of gastric cancer was decreased in the past ten years, but it was still a main cause of morbidity and mortality [2]. Inhibition, reversion, or extension of each carcinogenic stage will be helpful to prevent from the development of invasive malignancy of gastric cancer.
Chemoprevention was an important approach used to decrease cancer morbidity and mortality. The underlying theory for traditional Chinese medicine (TCM) to treat or prevent cancer is to bring the patient back to a healthy state by modifying multiple cancer-causing events [3]. Many herbal products used for traditional Chinese medicine (TCM) can exert chemopreventive effects [4], such as Ginkgo biloba extract [5], tea polyphenol [6], epigallocatechin-3-gallate (EGCG) [7], curcumin [8], Radix Curcumae [9], and lycopene [10] which had shown the interventional effect on the progression of gastric precancerous lesions.
Dendrobium officinale belongs to the Orchidaceae family, which has been used more than one thousand years in China [11]. The raw and processed Dendrobium officinale are used for healthcare products [12]. The major components of Dendrobium officinale include water soluble polysaccharides, phenanthrenes, and numerous amino acids [13]. High contents of erianin and chrysotoxene restrained the growth of liver cancer and ascites carcinoma cells [14]. D. officinale shows the anticancer effects on HeLa S3 human cervical carcinoma cells, HCT-116 cancer cells, and HepG2 liver cancer cells in vitro [15, 16]. In recent years, researchers discovered D. officinale also had the preventive effects on the formation of lung metastases and colon carcinogenesis [17, 18]. One previous study revealed that oral administration of Dendrobium officinale extraction (DOE) could reduce the incidence in MNNG-initiated rats. The results showed that DOE suppressed abnormal cell proliferation, induced apoptosis, and upregulated proapoptotic gene Bax in gastric cancer. And it could downregulate antiapoptotic gene Bcl-2 and proliferative genes in gastric cancer, such as EGF and EGFR [19]. Nevertheless, the mechanisms of antiproliferative and apoptosis-inducing activity of during MNNG-induced gastric carcinogenesis are not clearly understood. This study will explain the molecular mechanism in which Dendrobium officinale mediates the antioxidation, apoptosis, and expressions of DNA oxidative damage, as well as some cytokines related to tumorigenesis.
2. Materials and Methods
2.1. Materials and Chemicals
Dendrobium officinale (lot: SXG131208) was purchased from Zhejiang Shou Xian Gu Pharmaceutical Co., Ltd., and authenticated by Associate Professor Ke zhong Dun (Identification Department of the Jiangxi University of TCM), according to the Pharmacopoeia of the People's Republic of China (2010). The standards schaftoside (lot: 140402), isoschaftoside (lot: 141025), and vicenin 3 (150122) were provided by Weikeqi-Biotech Co. Ltd. (Chengdu, China). N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) (lot: MV340RC) was purchased from Tokyo Chemical Industry. Detection kits for the measurement of glutathione peroxidase (GSH-PX) (lot: 20141124), malondialdehyde (MDA) (lot: 20141107), and superoxide dismutase (SOD) (lot: 20141115) were purchased from Institute of Biological Engineering of NanJing JianCheng (Nanjing, China). And 8-hydroxy-2-deoxyguanosine Elisa kits (8-OHdG) (lot: 20141008) and interleukin 2 (IL-2) (lot: 20141108) were obtained from Neobioscience (Shenzhen, China). Cytokine antibody microarrays (Rat Cytokine Array C2) (lot: 115167103) were supplied by RayBiotech (USA). Antibodies against Bcl-2 (lot: G1315) and Caspase-3 (lot: D2315) were purchased from Santa Cruz Technology (Dallas, TX, USA). Antibodies against Bax (lot: GR151406-13) were purchased from Abcam Technology (Dallas, TX, USA). GAPDH (lot: D16H11) and secondary antibodies were purchased from Cell Signaling Technology. BCA Protein Assay Kit (lot: No. 00121506) was purchased from Cwbiotech Technology. Protein ladder (lot: 00275697) was supplied by Thermo Fisher Scientific.
2.2. Animals
Rats used in our trial were supplied by Beijing Wei Tong Li Hua Experimental Animal Research Center. Forty-eight male Wistar rats, 8–10 weeks old, with weights between 110–120 grams were used for this experiment. During pretrial period, rats were kept and fed in groups in the laboratory at room temperature (22°C). The animals were cared for in accordance with the Guiding Principles for the Care and Use of Animals.
2.3. Preparation and Quality Control of Plant Extracts
The fresh Dendrobium officinale was added to 20-fold volume of water and extracted three times by stirring 2 hours one time. The extraction was evaporated using a rotary evaporator and produced the power by spray drying. Dendrobium officinale extraction powders (0.1 g) were dissolved with 15% acetonitrile and extracted for 30 min ultrasonic sound. The solution was filtered through 0.45 μm Millipore membrane and the filtrate was collected as sample solution. Standards schaftoside, isoschaftoside, vicenin 3, and naringenin were dissolute in 30% methanol. The final concentrations were 100 μg/mL, 100 μg/mL, 100 μg/mL, and 200 μg/mL, respectively. The chromatographic analysis was performed on C18 column (250 mm × 4.6 mm, 5 μm) by HPLC (Shimadzu LC-20AT). The mobile phase was acetonitrile (A) and 0.1% glacial acetic acid solution (B). The flow rate was 0.8 mL/min. Column temperature was maintained at 30°C. Gradient elution was 0–25 min 14% B, 25–35 min 14%–18% B, and 35–76 min 18%–45% B. Detection wavelength of schaftoside, isoschaftoside, and vicenin 3 was set at 335 nm, and that of naringenin was set at 270 nm. Injection volume was 10 μL for each analysis.
2.4. Inhibition Effects of Dendrobium officinale on the Progression of N-Methyl-N′-nitro-N-nitrosoguanidine-Induced Gastric Carcinogenesis in Rats
Forty-eight Wistar rats were randomly divided into four groups (n = 12): normal control group (Normal), GC model but untreated group (Model), and Dendrobium officinale extraction (DOE) group (2.4 g/kg and 4.8 g/kg). Distilled water was given to rats in control group as vehicle. MNNG of 200 mg/kg was orally administered in all rat groups through a catheter after administration of DOE for one hour. MNNG administration was repeated every 10 days for three months (20-21). D. officinale was administered daily for three months, as described above. The body weights of rats were recorded weekly. After three months, all animals were sacrificed by intraperitoneal injection of thiopental after overnight fasting. The serum from the inferior vena cava was collected in a tube and centrifuged at 2000g for 10 min at 4°C. The stomach was divided into 2 parts. One was used for HE staining, and another was kept at −80°C for Western blot analysis. The protocol for these experiments was approved by the Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine.
2.5. Analysis of 8-OHdG and IL-2 in Serum
The concentrations of 8-OHdG and IL-2 in serum were measured using rat 8-OHdG and IL-2 Elisa kits. Briefly, biotinylated antibody reagent was added to 96-well plates, then supernatants of homogenized serum were added and the plates were incubated at 37°C for 2 h. After washing with phosphate buffered saline (PBS), streptavidin horseradish peroxidase (HRP) solution was added and the plate was incubated for 1 h at room temperature. The absorbance was measured at 450 nm using a microplate reader.
2.6. Analysis of SOD, MDA, and GSH-PX in Plasma
SOD activity was measured by xanthine oxidase; the MDA content was measured by the presence of thiobarbituric acid reactive substances (TBARS), and GSH-PX activity was measured by DTNB. All were determined by colorimetric method using corresponding kits.
2.7. Microarray Analysis of Cytokines
Cytokine levels in the serum were analyzed with cytokine antibody microarrays following the guideline by manufactory [20]. After development and scan for each sample, a photo of the membranes with optimal image qualities was taken and analyzed by Gel imaging system and image analysis. Finally, results recorded from each membrane were normalized. The signal densities of cytokines were analyzed using a multivariate analysis of variance with post hoc test. P values less than 0.05 were considered significant.
2.8. Protein Extraction and Western Blot Analysis in the Gastric Tissue
Total stomach tissue protein was obtained with RIPA buffer, and protein concentrations were determined with BCA Protein Assay Kit (Cwbiotech, China). For the Western blot analysis, 50 μg protein was separated by SDS-PAGE and transferred to a nitrocellulose membrane and then blocked with 5% nonfat milk and stained with Bax, Bcl-2, and Caspase-3 primary antibodies (diluted 1 : 1000). The membranes were further incubated with a horseradish peroxidase conjugated secondary antibody (diluted 1 : 10000) for 1 h at room temperature. The blots were washed three times with PBS-Tween and then developed by ECL using a Prolight HRP western blotting detection reagent (Cwbiotech, China).
2.9. Statistical Analyses
Data were expressed as mean ± SEM. Statistical differences were determined using one-way repeated measures analysis of variance (ANOVA) followed by Duncan's multiple range as post hoc test. Differences between groups were considered significantly different when P value was less than 0.05.
3. Result
3.1. Quantity Control of DOE by HPLC
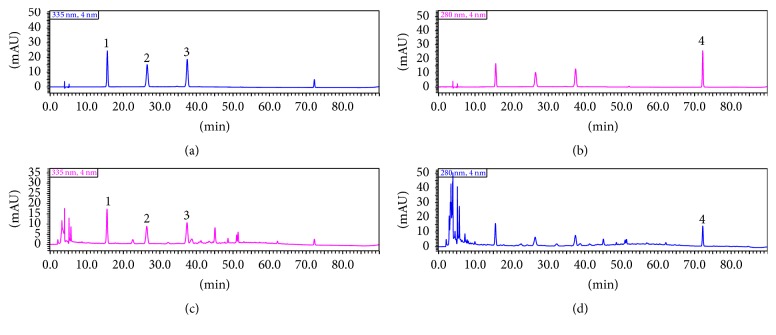
As shown in the Figure 1, the concentrations of schaftoside, isoschaftoside, vicenin 3, were in good linear relations between 5–100 μg/mL, and naringenin was 10–200 μg/mL, and the correlation coefficients were 0.9999, 0.9998, 0.9995, and 0.9997, respectively. The results of four components' methodological study demonstrated that the method satisfied the requirements of the determination. According to the method, the concentrations of schaftoside, isoschaftoside, vicenin 3, and naringenin in sample were 6.862 μg/mL, 7.5858 μg/mL, 217.7355 μg/mL, and 12.7902 μg/mL, respectively (Figure 1).
Figure 1.
The quality control of DOE by HPLC at 280 μm and 335 μm ((1) vicenin 3, (2) isoschaftoside, (3) schaftoside, and (4) naringenin. (a) standard at 335 μm, (b) standard at 280 μm, (c) sample at 335 μm, and (d) sample at 280 μm.).
3.2. Gastric Cancer Incidence and Body Changes
During the whole study period, four rats in positive group and two rats in high-dose group died from aspiration after feeding between 7 and 10 weeks. The results of autopsy showed congestion and bleeding spots in both lungs, while there was hyperkeratosis in forestomach mucosa, but no precancerous lesions or tumor was formed by histological examination. 42 rats survived till week 12 in the end. Histopathological examinations had been reported in the previous study [19], indicating that the gastric cancer incidence in the model group was 41%. DCE could reduce the incidence of cancer and accelerate tumor growth. The body weight showed no significant change between all groups during the periods (Figure 2).
Figure 2.
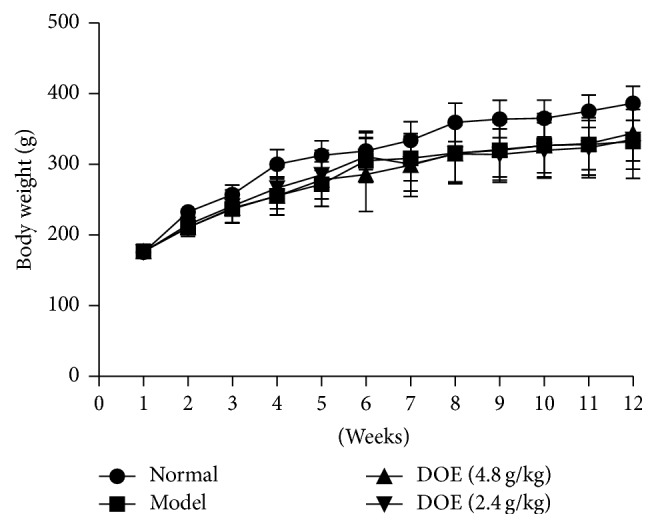
The change of body weight during the experiments (Normal means normal control group; Model means GC model but untreated group; DOE (2.4 g/kg and 4.8 g/kg) means GC model that was given Dendrobium officinale extracts (2.4 g/kg and 4.8 g/kg); n = 12).
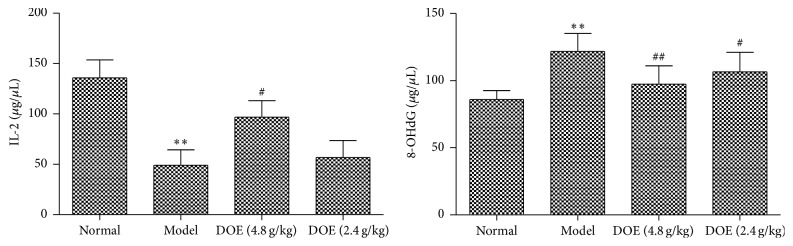
3.3. DOE Attenuated Development of Carcinogenesis Associated with 8-OHdG and IL-2 Expression
As shown in Figure 3, the expression of IL-2 and 8-OHdG was significantly different between normal and modern group. It was found that two doses of DOE could suppress 8-OHdG (P < 0.01, P < 0.05) and high-dose DOE could increase IL-2 significantly compared with the model group (P < 0.05).
Figure 3.
Effect of DOE on 8-OHdG and IL-2 in plasma of gastric cancer rats (Normal means normal control group; Model means GC model but untreated group; DOE (2.4 g/kg and 4.8 g/kg) means GC model that was given Dendrobium officinale extracts (2.4 g/kg and 4.8 g/kg). Compared with normal group, ∗∗P < 0.01; compared with model group, #P < 0.05 and ##P < 0.01; n = 12).
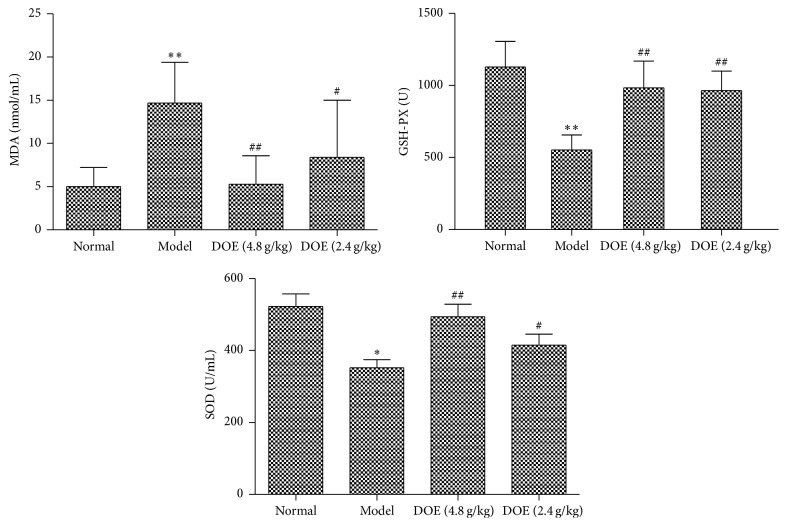
3.4. Analysis of SOD, MDA and GSH-PX in Plasma
As shown in Figure 4, compared with the normal group, GSH-PX and SOD activity in the gastric cancer groups was decreased (P < 0.01) and the MDA activity was increased markedly (P < 0.01). After 12 weeks of treatment, GSH-PX activity was greatly increased in the DOE groups (P < 0.01; P < 0.01) and also SOD (P < 0.01; P < 0.05). MDA activity in the DOE groups was decreased significantly (P < 0.01; P < 0.05).
Figure 4.
The levels of MDA, SOD, and GSH-PX in serum (Normal means normal control group; Model means GC model but untreated group; DOE (2.4 g/kg and 4.8 g/kg) means GC model that was given Dendrobium officinale extracts (2.4 g/kg and 4.8 g/kg). Compared with normal group, ∗P < 0.05 and ∗∗P < 0.01. Compared with model group, #P < 0.05 and ##P < 0.01; n = 12).
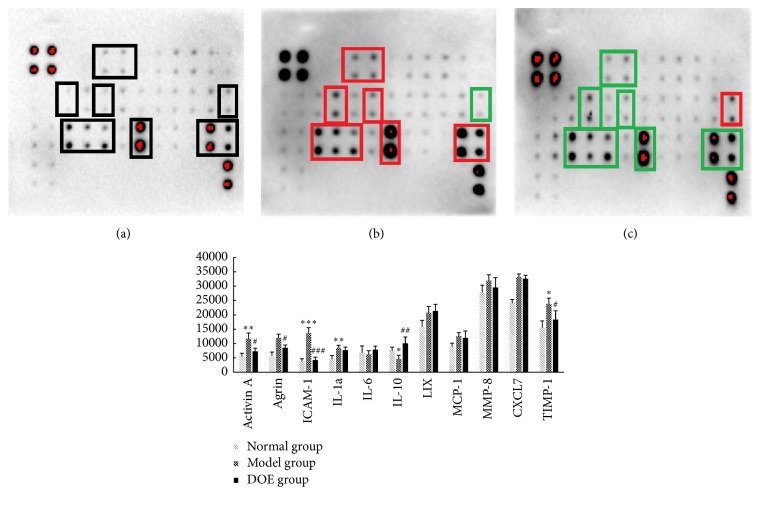
3.5. Relative Expression Levels of Cytokine in Serum Measured from the MNNG-Induced Model Group and DOE Treatment Group
The RayBio Rat Cytokine Array C2 microarrays could allow simultaneous detection of 34 inflammation-related cytokines. Comparing with the normal group, the signal densities of cytokines were upregulated in the MNNG-induced gastric cancer groups, including Activin A, Agrin, IL-1α (both P < 0.01), ICAM-1 (P < 0.001), and TIMP-1 (P < 0.05). However, the expression level of IL-10 was reduced (P < 0.05). Several other cytokines, including LIX, MCP-1, MMP-8, and CXCL7 were increased, but there was no significant difference. In the DOE treatment group, the signal densities of most inflammatory cytokines were downregulated, including Activin A, Agrin, TIMP-1 (both P < 0.01), and ICAM-1 (P < 0.001), but IL-10 was increased (P < 0.01).
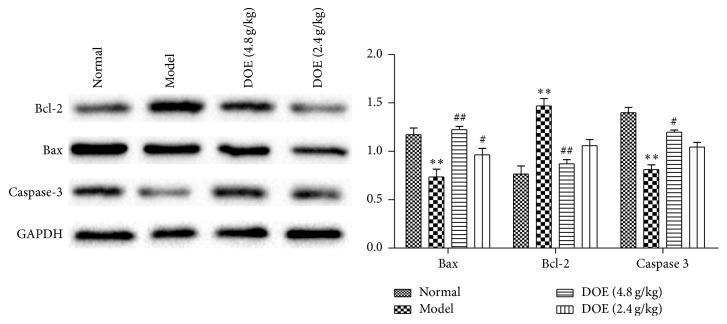
3.6. The Protein Expression of Bax, Bcl-2, and Caspase-3 in Rats' Stomach
Because Bax, Bcl-2, and caspase-3 were the key proteins involved in apoptosis, the effect of D. officinale on Bax, Bcl-2, and caspase-3 expression was measured. Compared with the model group, the content of Bcl-2 protein quickly recovered after administration of D. officinale at 4.8 and 2.4 g/kg for 12 weeks (P < 0.01; P < 0.05). Additionally, the expression of Bax and Caspase-3 was decreased in the model group. After administration of D. officinale at 4.8 and 2.4 g/kg, the contents of Bax protein (P < 0.01, P < 0.05) and Caspase-3 (P < 0.05) were significantly increased.
4. Discussion
Dendrobium officinale Kimura et Migo is ranked as “the first of the nine Chinese fairy herbs.” D. officinale possesses some important medicinal applications, which can maintain tonicity of stomach, promote the body fluid production, reduce peripheral vascular obstruction, and enhance the immune system. It has been commonly used for antitumor activity, antiaging, regulation of blood sugar, treatment of stomach disorders, etc. [21, 22]. Some quality control methods have been reported for D. officinale. Zhou et al. [23] using HPLC-DAD-ESI-MS identified eight flavone di-C-glycosides from D. officinale leaves in which apigenin and monosaccharide were connected with C-6 and C-8. Chen et al. [24] had quantified bibenzyls, phenanthrenes, and flavanones, which are used for plant identification among the Dendrobium species. Chu et al. [25] established a fast and simple method by combining normal and fluorescence microscopy to distinguish D. officinale. In our research, we established a method to simultaneously determine the concentrations of schaftoside, isoschaftoside, vicenin 3, and naringenin in D. officinale by HPLC-UV. Schaftoside, isoschaftoside, and vicenin 3 showed good linear relations between 5–100 μg/mL, and naringenin was 10–200 μg/mL. The concentrations of schaftoside, isoschaftoside, vicenin 3, and naringenin in sample were 6.862 μg/mL, 7.5858 μg/mL, 217.7355 μg/mL, and 12.7902 μg/mL, respectively (Figure 2).
The generation and increase of ROS and DNA oxidative damage are related to the damage and malignant transformation of gastric mucosa [26]. The antioxidative property of natural agents will show the therapeutic and preventive effect on the process of carcinogenesis, such as the natural antioxidants ginsenosides and curcumin [27, 28]. Our recent work has reported that D. candidum could prevent MNNG-induced gastric cancer [19]. Some researchers had reported the preventive effects on other cancers. For example, Wang et al. [18] reported that D. candidum Wall ex Lindl. exhibited preventive effects against colon carcinogenesis in mice through increasing Bax, Caspase-3, and Caspase-9 and decreasing Bcl-2.
Cytokines play an important role in inflammation cell growth and differentiation, which is involved in most disease processes, including cancer. Cytokine array microarrays could detect protein expressions in serum and urine. In our results, the expression of Activin A, Agrin, IL-1α, ICAM-1, and TIMP-1 was upregulated, but the expression of IL-10 was reduced. After administration with DOE, the signal densities of most inflammatory cytokines were downregulated, including Activin A, Agrin, TIMP-1, and ICAM-1 (P < 0.001) (Figure 5). Also IL-10 was increased. Some studies had reported Activin signal induces growth inhibition and apoptosis through SMAD-dependent pathways [29, 30]. In contrast, several articles have reported that upregulated INHBA expression may promote cancer cell proliferation in several cancers, and the level of Activin A in plasma might be associated with cancer progression in clinical [31–34], In addition, intercellular adhesion molecule 1 (ICAM-1) was upregulated in gastric cancers and cancer progression, which were associated with poor prognostic effect [35, 36]. Tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) is overexpressed in gastric cancer, and the level of TIMP-1 in serum was considered as a marker for prognosis in gastric cancer patients [37]. Therefore, decreasing TIMP activity was a useful method to modulate tumor development and metastasis [38]. In our study, serum ICAM-1 and TIMP-1 levels in MNNG-induced gastric cancer were higher than the normal group. D. officinale decreased Activin A, Agrin, TIMP-1 (both P < 0.01), and ICAM-1 (P < 0.001), and IL-10 was increased (P < 0.01). Other cytokines LIX, MCP-1, MMP-8, and CXCL7 were increased in gastric cancer model; however, D. officinale did not show the significant effect on these cytokines (Figure 5).
Figure 5.
Relative expression levels of cytokine in serum measured from the MNNG-induced model group and DCE treatment group ((a) Normal group, (b) Model group, and (c) DOE (4.8 g/kg). Normal means normal control group; Model means GC model but untreated group; DOE (2.4 g/kg and 4.8 g/kg) means GC model that was given Dendrobium officinale extracts (2.4 g/kg and 4.8 g/kg). Compared with normal group, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Compared with model group, #P < 0.05, ##P < 0.01, and ###P < 0.001. The box in (a), (b), and (c) means the difference between each group; n = 4).
Induction of apoptosis appears to be associated with their effectiveness in modulating carcinogenic processes [39, 40]. The Bcl 2 family, which includes promoters (Bax and Bid) and inhibitors (Bcl 2 and Bcl xL), is a key regulator in mitochondria mediated apoptosis. In our previous study, we have reported that the gene level of Bax was decreased, whereas Bcl 2 was increased in the model groups. Following treatment with D. officinale, Bax was increased and Bcl 2 was decreased. This paper detected the protein expressions of Bax, Bcl 2, and Caspase-3 by western blot, which further confirmed that D. officinale at 4.8 and 2.4 g/kg showed strong activity in increasing the expression of Bax (P < 0.01, P < 0.05) and Caspase-3 (P < 0.05). Also it decreased Bcl-2 (P < 0.01, P < 0.05), therefore promoting apoptosis in preventing the occurrence of gastric cancer (Figure 6).
Figure 6.
Effects of DOE on the protein expression of Bcl-2, Bax, and Caspase-3 (Normal means normal control group; Model means GC model but untreated group; DOE (2.4 g/kg and 4.8 g/kg) means GC model that was given Dendrobium officinale extracts (2.4 g/kg and 4.8 g/kg). Compared with normal group, ∗∗P < 0.01. Compared with model group, #P < 0.05 and ##P < 0.01; n = 6).
In conclusion, this study suggests that D. officinale could downregulate the expression of MDA and 8-OHdG and upregulate the activity of GSH-PX as well as IL-2 during MNNG-induced gastric tumorigenesis in rats. And D. officinale also reduced the level of Activin A, Agrin, IL-1α, ICAM-1, and TIMP-1 and upregulated the level of IL-10. Additionally, D. officinale increases the protein level of Bax and Caspase-3 and decreases the expression of Bcl-2. The results of the present study coupled with our previous findings indicate that D. officinale shows antioxidative effect, modulates some cytokines related to tumorigenesis, and induces apoptosis. These experimental results reveal that D. officinale could be developed as a chemopreventive drug for reducing the risk of gastric cancer.
Acknowledgments
This work was supported by LuoDi Project of Jiangxi Province (KJLD12086), Key projects of Natural Foundation of Jiangxi Province (20152ACB20021), the National Natural Science Foundation of China (81360509 and 81360513), and the Thousand Young Talents Program of China.
Competing Interests
The authors declare that they have no conflict of interests.
Authors' Contributions
Yi Zhao and Yan Liu contributed equally to this work. Yi Zhao conceived the design, carried out the microarray analysis, and drafted the manuscript. Hongning Liu was in charge of the study work and gave advice regarding the study design. Yan Liu participated and finished quality control of plant extracts, Western blot, and RT-PCR. Xi-Ming Lan participated in animal feeding, drug administration, and HE analysis. Guo-Liang Xu gave advice regarding quality control. You-Zhi Sun participated in manuscript writing. Fei Li gave expert advice in the study design and submission. All authors read and approved the final manuscript.
References
- 1.Ferlay J., Shin H.-R., Bray F., Forman D., Mathers C., Parkin D. M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Ford A. C. Chemoprevention for gastric cancer. Best Practice and Research: Clinical Gastroenterology. 2011;25(4-5):581–592. doi: 10.1016/j.bpg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Wang S., Penchala S., Prabhu S., Wang J., Huang Y. Molecular basis of traditional chinese medicine in cancer chemoprevention. Current Drug Discovery Technologies. 2010;7(1):67–75. doi: 10.2174/157016310791162794. [DOI] [PubMed] [Google Scholar]
- 4.Parekh H. S., Liu G., Wei M. Q. A new dawn for the use of traditional Chinese medicine in cancer therapy. Molecular Cancer. 2009;8, article 21 doi: 10.1186/1476-4598-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang X. Y., Qian L. P., Zheng X. J., Xia Y. Y., Jiang Y. B., Sun D. Y. Interventional effect of Ginkgo biloba extract on the progression of gastric precancerous lesions in rats. Journal of Digestive Diseases. 2009;10(4):293–299. doi: 10.1111/j.1751-2980.2009.00398.x. [DOI] [PubMed] [Google Scholar]
- 6.Mei Y. Y., Wei D. Zh., Liu J. W. Modulation effect of tea polyphenol toward N-methyl-N′-nitro-N-nitrosoguanidine-induced precancerous gastric lesion in rats. The Journal of Nutritional Biochemistry. 2005;16(3):172–177. doi: 10.1016/j.jnutbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Xu Q., Yang C. H., Liu Q., et al. Chemopreventive effect of epigallocatechin-3-gallate (EGCG) and folic acid on the N-methyl-N′-nitro-N-nitrosoguanidine (MNNG)-induced gastrointestinal cancer in rat model. Journal of Digestive Diseases. 2011;12(3):181–187. doi: 10.1111/j.1751-2980.2011.00494.x. [DOI] [PubMed] [Google Scholar]
- 8.Sintara K., Thong-Ngam D., Patumraj S., Klaikeaw N. Curcumin attenuates gastric cancer induced by N-methyl-N-nitrosourea and saturated sodium chloride in rats. Journal of Biomedicine and Biotechnology. 2012;2012:8. doi: 10.1155/2012/915380.915380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu B., Xu L., Yu L., Zhang L. Extract of radix curcumae prevents gastric cancer in rats. Digestion. 2008;77(2):87–91. doi: 10.1159/000122228. [DOI] [PubMed] [Google Scholar]
- 10.Velmurugan B., Bhuvaneswari V., Nagini S. Antiperoxidative effects of lycopene during N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis. Fitoterapia. 2002;73(7-8):604–611. doi: 10.1016/S0367-326X(02)00216-2. [DOI] [PubMed] [Google Scholar]
- 11.Weng D. FAAS determination of trace elements in Dendrobium candidum using suspension sampling with ultrasonic agitation. Chinese Pharmaceutical Journal. 2003;38(9):704–706. [Google Scholar]
- 12.Xiao F., Zhang J. Z., Tu Y. L. First report of Fusarium oxysporum causing wilt of Dendrobium candidum in Zhejiang province, China. Plant Disease. 2012;96(9):1377–1377. doi: 10.1094/pdis-03-12-0304-pdn. [DOI] [PubMed] [Google Scholar]
- 13.Li J., Li S. X., Huanget D., et al. Advances in the of resources, constituents and pharmacological effects of Dendrobium officinale. Science & Technology Review. 2011;18(29):74–79. [Google Scholar]
- 14.Shao H., Zhang L. Q., Li J. M., et al. Advances in research of Dendrobium officinale. Chinese Traditional and Herbal Drugs. 2004;4(1):2157–2162. [Google Scholar]
- 15.Brew K., Dinakarpandian D., Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochimica et Biophysica Acta (BBA)—Protein Structure and Molecular Enzymology. 2000;1477(1-2):267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Sun P., Qian Y., Suo H. D. candidum has in vitro anticancer effects in HCT-116 cancer cells and exerts in vivo anti-metastatic effects in mice. Nutrition Research and Practice. 2014;8(5):487–493. doi: 10.4162/nrp.2014.8.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li G., Sun P., Zhou Y., Zhao X., Chen F. Preventive effects of Dendrobium candidum Wall ex Lindl. On the formation of lung metastases in BALB/c mice injected with 26-M3.1 colon carcinoma cells. Oncology Letters. 2014;8(4):1879–1885. doi: 10.3892/ol.2014.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Sun P., Li G. J., Zhu K., Wang C., Zhao X. Inhibitory effects of Dendrobium candidum Wall ex Lindl. on azoxymethane- and dextran sulfate sodium-induced colon carcinogenesis in C57BL/6 mice. Oncology Letters. 2014;7(2):493–498. doi: 10.3892/ol.2013.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Y., Liu Y., Lan X. M., et al. Study on inhibition of Dendrobium officinale extract on gastric carcinogenesis. Chinese Traditional and Herbal Drugs. 2015;24(46):3704–3709. [Google Scholar]
- 20.Wu F., Zhang R., Shen X., Lao L. Preliminary study on pain reduction of monosodium iodoacetate-induced knee osteoarthritis in rats by carbon dioxide laser moxibustion. Evidence-Based Complementary and Alternative Medicine. 2014;2014:7. doi: 10.1155/2014/754304.754304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao M.-M., Zhang G., Zhang D.-W., Hsiao Y.-Y., Guo S.-X. ESTs analysis reveals putative genes involved in symbiotic seed germination in Dendrobium officinale. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0072705.e72705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu L. S., Jia M., Chen L., et al. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules. 2016;21, article 14 doi: 10.3390/molecules21010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou G. F., Lu G. Y. Study on eight flavone c-glycosides in dendrobium officinale leaves and their fragmentation pattern by HPLC-DAD-ESI-MS. Chinese Pharmaceutical Journal. 2012;1(47):13–18. [Google Scholar]
- 24.Chen X., Wang F., Wang Y., et al. Discrimination of the rare medicinal plant Dendrobium officinale based on naringenin, bibenzyl, and polysaccharides. Science China Life Sciences. 2012;55(12):1092–1099. doi: 10.1007/s11427-012-4419-3. [DOI] [PubMed] [Google Scholar]
- 25.Chu C., Yin H., Xia L., Cheng D., Yan J., Zhu L. Discrimination of dendrobium officinale and its common adulterants by combination of normal light and fluorescence microscopy. Molecules. 2014;19(3):3718–3730. doi: 10.3390/molecules19033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ni J., Mei M., Sun L. Oxidative DNA damage and repair in chronic atrophic gastritis and gastric cancer. Hepato-Gastroenterology. 2012;59(115):671–675. doi: 10.5754/hge12177. [DOI] [PubMed] [Google Scholar]
- 27.Strimpakos A. S., Sharma R. A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling. 2008;10(3):511–545. doi: 10.1089/ars.2007.1769. [DOI] [PubMed] [Google Scholar]
- 28.Choi J.-S., Chun K.-S., Kundu J., Kundu J. K. Biochemical basis of cancer chemoprevention and/or chemotherapy with ginsenosides. International Journal of Molecular Medicine. 2013;32(6):1227–1238. doi: 10.3892/ijmm.2013.1519. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran A., Marshall E. S., Love D. R., Baguley B. C., Shelling A. N. Activin is a potent growth suppressor of epithelial ovarian cancer cells. Cancer Letters. 2009;285(2):157–165. doi: 10.1016/j.canlet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Bauer J., Sporn J. C., Cabral J., Gomez J., Jung B. Effects of Activin and TGFβ on p21 in Colon cancer. PLoS ONE. 2012;7(6) doi: 10.1371/journal.pone.0039381.e39381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanagihara K., Takigahira M., Tanaka H., et al. Establishment and molecular profiling of a novel human pancreatic cancer panel for 5-FU. Cancer Science. 2008;99(9):1859–1864. doi: 10.1111/j.1349-7006.2008.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arao T., Fukumoto H., Takeda M., Tamura T., Saijo N., Nishio K. Small in-frame deletion in the epidermal growth factor receptor as a target for ZD6474. Cancer Research. 2004;64(24):9101–9104. doi: 10.1158/0008-5472.CAN-04-2360. [DOI] [PubMed] [Google Scholar]
- 33.Mu Y., Gudey S. K., Landström M. Non-Smad signaling pathways. Cell & Tissue Research. 2012;347(1):11–20. doi: 10.1007/s00441-011-1201-y. [DOI] [PubMed] [Google Scholar]
- 34.Togashi Y., Kogita A., Sakamoto H., et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Bioorganic & Medicinal Chemistry Letters. 2005;24(15):5397–5401. doi: 10.1016/j.canlet.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Fujihara T., Yashiro M., Inoue T., et al. Decrease in ICAM-1 expression on gastric cancer cells is correlated with lymph node metastasis. Gastric Cancer. 1999;2(4):221–225. doi: 10.1007/s101200050067. [DOI] [PubMed] [Google Scholar]
- 36.Jung W.-C., Jang Y.-J., Kim J.-H., et al. Expression of intercellular adhesion molecule-1 and E-selectin in gastric cancer and their clinical significance. Journal of Gastric Cancer. 2012;12(3):140–148. doi: 10.5230/jgc.2012.12.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joo Y. E., Seo K. S., Kim H. S., Rew J. S., Park C. S., Kim S. J. Expression of tissue inhibitors of metalloproteinases (TIMPs) in gastric cancer. Digestive Diseases & Sciences. 2000;45(1):114–121. doi: 10.1023/a:1005421713137. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S., Li L., Lin J.-Y., Lin H. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World Journal of Gastroenterology. 2003;9(5):899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun S.-Y., Hail N., Jr., Lotan R. Apoptosis as a novel target for cancer chemoprevention. Journal of the National Cancer Institute. 2004;96(9):662–672. doi: 10.1093/jnci/djh123. [DOI] [PubMed] [Google Scholar]
- 40.Kuno T., Tsukamoto T., Hara A., Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. Journal of Biophysical Chemistry. 2012;3(2):156–173. doi: 10.4236/jbpc.2012.32018. [DOI] [Google Scholar]