Abstract
Background
Accurate diagnosis is critical to both therapeutic decisions and prognostication in interstitial lung diseases (ILD). However, surgical lung biopsies carry high complication rates. Fibred confocal fluorescence microscopy (FCFM) offers an alternative as it can visualize lung tissue in vivo at the cellular level with minimal adverse events. We wanted to investigate the diagnostic utility, and safety of using FCFM for patients with ILD.
Methods
In patients with suspected ILD, FCFM images were obtained from multiple bronchopulmonary segments using a miniprobe inserted through the working channel of a flexible bronchoscope. The procedure was performed under moderate sedation in an outpatient setting. Morphometric measurements and fibre pattern analyses were co-related with computed tomography (CT) findings and patients’ final diagnoses based on multi-disciplinary consensus.
Results
One hundred and eighty four segments were imaged in 27 patients (18 males) with a median age of 67 years (range, 24–79 years). They were grouped into chronic fibrosing interstitial pneumonia (16 patients) and other ILDs. Six distinct FCFM patterns were observed: normal, increased fibres, densely packed fibres, hypercellular, thickened fibres and others/non-specific. The pattern resembling densely packed fibres was seen in at least one segment in 68.8% patients with chronic fibrosing interstitial pneumonia, but only 36.4% in other ILD (P=0.097). An association between inflammatory patterns on CT and a hypercellular pattern on FCFM was also found (P<0.001).
Conclusions
Our study shows the potential of FCFM in classifying ILD, but its role in further diagnosis remains limited.
Keywords: Fluorescence, idiopathic pulmonary fibrosis (IPF), lung disease, interstitial, microscopy, confocal
Introduction
Interstitial lung diseases (ILD) are a heterogeneous group of lung disorders presenting with bilateral pulmonary infiltrates on chest imaging and abnormal pulmonary function tests. They are also described as diffuse parenchymal lung diseases (DPLD), with more than 300 entities that differ based on aetiology, pathophysiology and prognosis (1-5). Idiopathic interstitial pneumonias can be divided into chronic fibrosing, acute/subacute, and smoking-related categories (3,4). Chronic fibrosing interstitial pneumonia is further sub-divided into idiopathic pulmonary fibrosis (IPF), and nonspecific interstitial pneumonia (NSIP).
Lung biopsies are needed to make a definitive diagnosis if the radiological pattern on high-resolution computed tomography (HRCT) is not characteristic of IPF (6). However, mortality and complication rates of open-lung biopsies and thoracoscopy make the procedure risky (7). Furthermore, a surgical biopsy can precipitate a life-threatening acute exacerbation of IPF (8). Therefore, a less risky diagnostic test is crucially important for both therapeutic decisions and prognostication.
As a less invasive alternative, fibred confocal fluorescence microscopy (FCFM) can visualise in vivo lung tissue at the cellular level. A semi-flexible, 1.4-mm diameter probe is passed through the working channel of a standard flexible bronchoscope and advanced to the distal air spaces. It uses point illumination and a pinhole apparatus to block “out of focus” light, enabling microscopic imaging. A field of view of 600 µm × 600 µm with a lateral resolution of 5 µm is generated under the illumination of a 488 nm wavelength laser (9). Endogenous fluorescence allows elastin and tobacco-tar laden macrophages (in smokers) to be identified without the use of intravenous contrast (10). FCFM has already been used to identify malignancy, acute rejection in lung transplantation, amiodarone lung and pulmonary alveolar proteinosis in pilot data (11-13).
As the safety and diagnostic utility of FCFM in patients with ILD is unknown, investigating this became the aim of our study. The patients were classified into two groups: those with chronic fibrosing interstitial pneumonia and those with other ILDs (4). The final diagnoses were based on the multi-disciplinary medical consensus and clinical follow-ups where appropriate. Morphometric analysis of the FCFM images was performed and distinct FCFM patterns were identified. FCFM patterns of the two ILD groups were then compared, and also against computed tomography (CT) patterns of the bronchopulmonary segments from where the FCFM visualisation was done.
Surgical biopsy was not used as the comparator against FCFM because: (I) patients were deemed physiologically too weak to tolerate general anaesthesia for open-lung biopsy; (II) unacceptably high risk of pneumothorax; and (III) current gold-standard for diagnosing IPF is multi-disciplinary medical consensus. While available, bronchoscopic histology/cytology specimens were compared to FCFM images with the appreciation of the limitations of bronchoscopic sampling in ILD. The clinical indication for bronchoscopy was for microbiological sampling and to exclude alternative diagnoses.
Methods
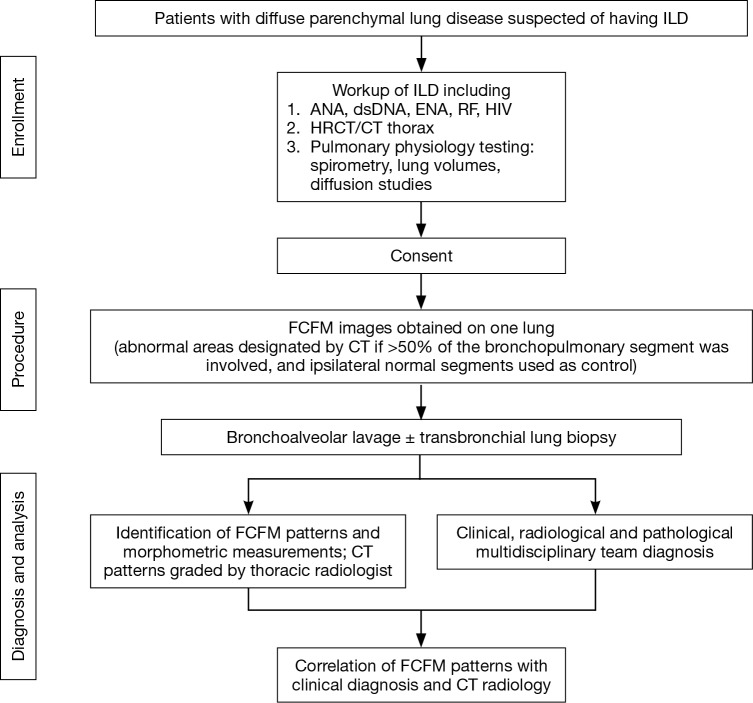
In this single-centred, prospective diagnostic test cross-sectional study, eligible subjects had to have been referred for work-up of ILD (convenience sampling). We included patients 18 to 80 years old who had bilateral diffuse parenchymal lung disease consistent with ILD on the CT thorax. We excluded patients who had contraindication to bronchoscopy such as haemodynamic instability, respiratory failure, or uncorrected coagulopathy. A dedicated thoracic radiologist (Yuen Li Ng) was assigned to identify CT patterns of each bronchopulmonary segment where FCFM was performed. A radiological pattern was attributed to any given segment if more than 50% of that segment was involved on CT (based on the radiologist’s assessment) (14). Nine abnormal CT patterns were identified and were split into three groups. Those in the inflammatory group included ground-glass opacification, nodular opacities, consolidation and bronchial wall thickening. The fibrotic group comprised patterns reflecting reticular changes, traction bronchiectasis, volume loss and honey combing, and thirdly, bronchiolitis group. Patients’ final diagnoses were determined by a clinical-radiological-pathological consensus, and the clinical course of the disease (Figure 1).
Figure 1.
Flow chart of methodology. ILD, interstitial lung diseases; ANA, anti-nuclear antibodies; dsDNA, double stranded DNA; ENA, extractable nuclear antigen antibodies; RF, rheumatoid factor; HRCT, high-resolution computed tomography; FCFM, fibred confocal fluorescence microscopy.
Flexible bronchoscopy was performed under local anaesthesia and moderate sedation using a combination of fentanyl and midazolam. We examined only one side of the bronchial tree that appeared most affected based on pre-procedure CT thorax. This was a safety precaution in case of an inadvertent pneumothorax caused by the miniprobe. The airways were anaesthetized with 1% topical lignocaine in 2 mL aliquots up to a maximum of 200 mg. Secretions were suctioned out before FCFM images (Cellvizio® Mauna Kea Technologies, Paris, France) were obtained. Video clips up to 30 seconds long were recorded and up to ten locations within the lung were visualized. After FCFM imaging, standard bronchoscopic sampling such as bronchoalveolar lavage was performed as clinically indicated. All bronchoscopic procedures were performed by the study investigators: Devanand Anantham, Su Ying Low and Gan Liang Tan.
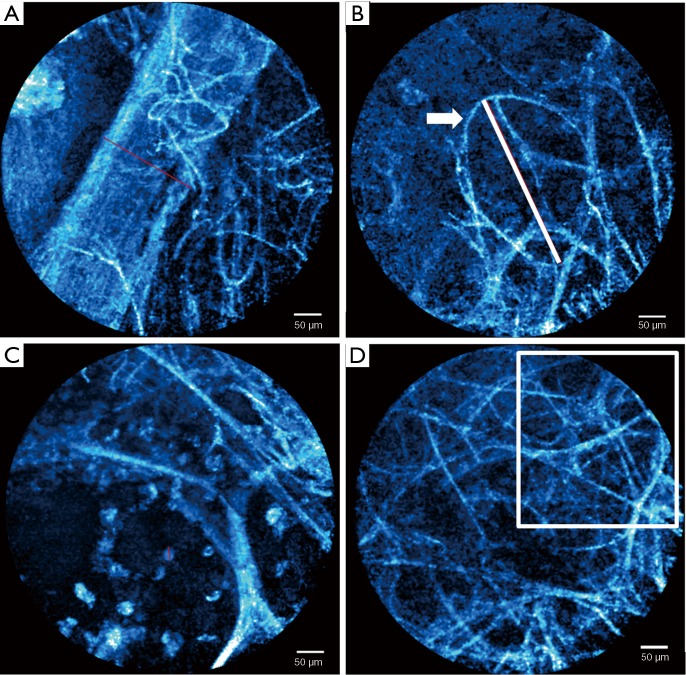
We analysed the recorded videos and still images using a software package (Cellvizio® Viewer 1.6, Mauna Kea Technologies, Paris, France). We excluded out of focus images, cough artefact and mucous obstruction of the lens. Morphometric measurements of alveolar structures (alveolar mouth diameter, alveolar mouth fibre thickness and microvessel diameter) and cellular analysis (number of cellular structures per field of view) were carried out (Figure 2). Distinct patterns such as fibre density on still images from video clips were identified. Representative images/clips of each studied segment were reviewed by the investigators (Devanand Anantham, Su Ying Low, Angela Takano, Yuen Li Ng, Gan Liang Tan and Peng Meng) to allocate a FCFM pattern to each segment by consensus. Investigators were blinded to the final diagnosis during the FCFM pattern allocation. Six FCFM patterns were identified and they were normal, increased fibres, densely-packed fibres, hypercellular, thickened fibres, and others/non-specific. Multidisciplinary meetings between respiratory physicians, pathologists and radiologists at Singapore General Hospital (SGH) were held at the weekly Chest Radiology Conference to determine the final diagnosis for each patient.
Figure 2.
Morphometric measurements of alveolar structures and cellular analyses. (A) Measurement of microvessel diameter, d =182 µm; (B) measurement of alveolar mouth diameter, d =326.8 µm, mouth fibre thickness (arrow) =5.5 µm; (C) counting of cells in one field, n=25; (D) counting of non-alveolar-mouth fibres in one quadrant of the field, n=11.
The patients were divided into two groups: those with chronic fibrosing interstitial pneumonia and those with other ILDs. The FCFM patterns were compared between the patients’ diagnostic groups and CT-bronchopulmonary segment radiology patterns, using binominal comparison with nominal approximation. The sensitivity and specificity to diagnose chronic fibrosing interstitial pneumonia were determined. The relative frequencies of CT radiology patterns in each FCFM group and 95% confidence interval (CI) were calculated by Jeffreys interval method. We used McNemar’s test to compare the CT patterns with the corresponding FCFM pattern. Statistical analyses were performed using SPSS (IBM Corporation, Somers, NY, USA).
This study has been approved by the SingHealth Centralised Institutional Review Board, CIRB Ref: 2012/245/C. (ClinicalTrials.gov Identifier: NCT01624753). Informed consent was obtained from all the participants before FCFM.
Results
Twenty seven patients were enrolled from 1 Mar. 2012 to 1 Jul. 2015 (Table 1). Of these, 18 were male. The median age was 67 years (range, 24–79 years). Fifteen out of 27 patients (56%) had never smoked. Majority were of Chinese ethnicity (24, 89%). Cough was the presenting symptom in 18 patients (67%), while 17 patients (63%) had dyspnoea. There were 16 patients with chronic fibrosing interstitial pneumonia, and 11 patients with other ILDs. Of those with chronic fibrosing interstitial pneumonia, two had IPF, two had connective tissue disease-associated ILD and 12 had NSIP. Of those with other ILDs, three had cryptogenic organizing pneumonia, three diffuse panbronchiolitis, one sarcoidosis, one adenocarcinoma, and three pulmonary infections that presented like ILD.
Table 1. Demographics and clinical characteristics of patients.
| Characteristics | Chronic fibrosing (n=16*) | Other ILD (n=11†) | Overall (n=27) |
|---|---|---|---|
| Age (SD) years | 66 (9.5) | 59 (16.8) | 63.3 (13.7) |
| <50 | 1 | 2 | 3 |
| 50–60 | 4 | 1 | 5 |
| 61–70 | 3 | 5 | 8 |
| >70 | 8 | 3 | 11 |
| Gender | |||
| Male | 11 | 7 | 18 |
| Female | 5 | 4 | 9 |
| Ethnicity | |||
| Chinese | 14 | 10 | 24 |
| Indian | 1 | 0 | 1 |
| Malay | 1 | 1 | 2 |
| Smoking | |||
| Never smoker | 8 | 7 | 15 |
| Ex-smoker‡ | 6 | 1 | 7 |
| Current smoker | 2 | 3 | 5 |
| Presenting symptoms | |||
| Cough | 10 | 8 | 18 |
| Breathless | 12 | 5 | 17 |
| Incidental findings on chest imaging | 3 | 2 | 5 |
| Clinical progress | |||
| Death | 5 | 2 | 7 |
| Following up with pulmonologist | 11 | 7 | 18 |
| Discharged | 0 | 2 | 2 |
| FCFM side | |||
| Left lung | 7 | 2 | 9 |
| Right lung | 9 | 9 | 18 |
*, chronic fibrosing group included six usual interstitial pneumonia patterns (two IPF, two connective tissue disease-associated ILD and two fibrotic NSIP) and ten non-specific interstitial pneumonia; †, other ILD group included three organizing pneumonia, three infective pneumonia (two Pneumocystis jirovecii and one Streptococcus pneumonia), three diffuse bronchiolitis, one sarcoidosis, one lung adenocarcinoma); ‡, ex-smoker: stopped smoking for at least 6 months; ILD, interstitial lung diseases; FCFM, fibred confocal fluorescence microscopy.
Procedure details
The median procedure duration from bronchoscopic intubation to extubation was 15 min (range, 10–35 min). This included time for regular bronchoscopic sampling such as bronchoalveolar lavage (n=27) and transbronchial biopsy (n=10). It is estimated that acquiring the FCFM video sequences took 5–7 minutes. The median number of segments entered for FCFM was seven per patient (range, 4–9 per patient). Moderate sedation was used for all procedures with median doses of 2.5 mg for midazolam (range, 1.5–7.5 mg) and 50 mcg for fentanyl (range, 0–100 mcg). We made 218 attempts to enter the distal airspace of 184 bronchopulmonary segments with the miniprobe. To get clear images, we attempted 22 segments twice, thrice on six segments. We skipped the apico-posterior segment of the left upper lobe (LB1 and LB2) because of the inherent stiffness of the miniprobe and anatomical considerations.
Complications: one patient had a small (<10%) pneumothorax that was managed conservatively without chest drain insertion. This patient had ipsilateral bronchoscopic sampling with transbronchial biopsy, so the complication cannot be solely attributed to the FCFM.
CT characteristics
A total of 184 bronchopulmonary segments were studied (Table 2). Some segments had more than one CT pattern attributed to them because of co-existing patterns. Ground-glass opacification, reticular changes, traction bronchiectasis, volume loss and honey combing patterns were more frequently seen in the chronic fibrosing interstitial pneumonia group (P<0.05), while nodular opacities, consolidation and bronchiolitis were seen more in patients with other ILDs (P<0.05).
Table 2. Computed tomography (CT) radiology patterns in chronic fibrosing interstitial pneumonia and the other interstitial lung diseases (ILDs).
| Patterns | Chronic fibrosing (n=107, %) | Other ILD (n=77, %) | Overall (n=184, %) |
|---|---|---|---|
| Normal | 13 (12.1) | 5 (6.5) | 18 (9.8) |
| Inflammatory | 66 (61.7) | 69 (89.6) | 135 (73.4)* |
| Ground-glass opacification | 61 (57.0) | 32 (41.6) | 93 (50.5)† |
| Nodular | 1 (0.9) | 35 (45.5) | 36 (19.6)* |
| Consolidation | 3 (2.8) | 13 (16.9) | 16 (8.7)* |
| Bronchial wall thickening | 6 (5.6) | 9 (11.7) | 15 (8.2) |
| Fibrotic | 76 (71.0) | 22 (28.6) | 98 (53.3)† |
| Reticular | 69 (64.5) | 16 (20.8) | 85 (46.2)† |
| Traction bronchiectasis | 31 (29.0) | 7 (9.1) | 38 (20.7)† |
| Volume loss | 17 (15.9) | 4 (5.2) | 21 (11.4)† |
| Honey combing | 11 (10.3) | 0 (0) | 11 (6.0)† |
| Bronchiolitis | 1 (0.9) | 11 (14.3) | 12 (6.5)* |
Binominal proportion test was used with normal approximation to calculate P value. *, P<0.05, radiology patterns are more common in the other ILD group; †, P<0.05, radiology patterns are more common in the chronic fibrosing interstitial pneumonia group.
Morphometric measurements of alveolar and cellular structures
From Figure 2, the mean microvessel diameter was 106.0±45.13 µm, n=63. The mean alveolar mouth fibre thickness was 15.7±6.34 µm, n=123. The mean alveolar mouth diameter was 299.3±61.24 µm, n=33. There were no significant differences between chronic fibrosing interstitial pneumonia and other ILD. The calculated alveolar mouth diameter based on Verbeken’s formula was 304.9 µm [92% × diameter (µm) = 1.2 × age (year) + 203.1, using mean age 64.5 in our study] (15). This is similar with our measured mean value of 299.3±62.2 µm. There was no significant difference (P=0.29) in alveolar mouth diameter between patients with chronic fibrosing interstitial pneumonia (291.4±68.5 µm) and those with other ILDs (304.4±59.0 µm).
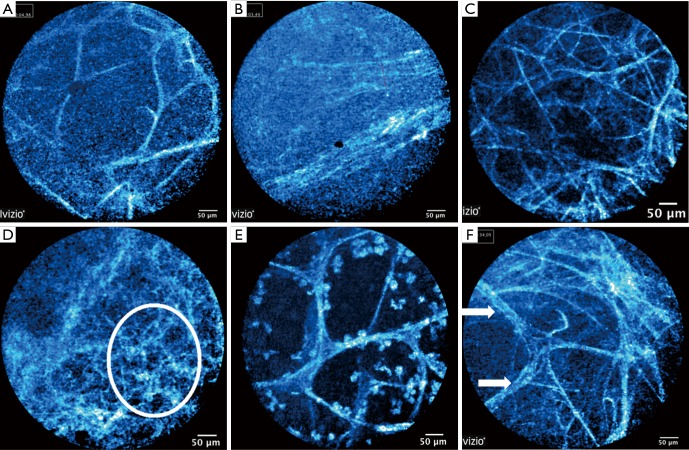
FCFM patterns
The Figure 3 illustrates the six FCFM patterns. Besides the normal or non-specific pattern, any given segment could have more than one FCFM pattern because of co-existing patterns. See Table 3 for detailed description of each pattern. All 31 segments (100%) with the densely-packed fibre pattern had at least five distinct non-alveolar-mouth fibres in at least one quadrant of the image field. 27/31 (87.1%) segments had more than five fibres; and 21/31 (67.7%) segments had > seven fibres. In the segments labelled hypercellular, all 38/38 (100%) had 11 cellular structures in one field; 32/38 (84.2%) segments had 17 cellular structures; and 28/38 (73.7%) segments had 19 cellular structures. Based on these observations, five non-alveolar-mouth fibres and 11 cellular structures were used as thresholds in our FCFM descriptions (Table 3), in conjunction with established descriptions of alveolar ducts and sacs from previous studies (10).
Figure 3.
Fibred confocal fluorescence microscopy (FCFM) patterns. (A) Normal: regular elastin framework of the alveolar rings; (B) others/non-specific: no regular elastin framework of the alveolar rings and loose fibres not recognized as part of alveoli or bronchial wall; (C) increased fibres: normal alveolar structures still visible; (D) densely packed fibres: alveolar structures are absent; (E) hypercellular: cellular structures are visualized within the alveolar space; (F) thickened fibres: normal alveolar structures still visible.
Table 3. Fibred confocal fluorescence microscopy (FCFM) image description.
| FCFM patterns | Description |
|---|---|
| Normal | Regular elastin framework of the alveolar rings and less than four non-alveolar-mouth fibres in any single quadrant of the field (Figure 2D) and ≤11 cellular structures per field and/or microvessels |
| Non-specific | No regular elastin framework of the alveolar rings and loose fibres i.e., not recognized as part of alveoli or bronchial wall |
| Increased fibres | >4 non-alveolar-mouth fibres in at least one quadrant of the field and normal alveolar structures |
| Densely packed fibres | Incomplete or absent alveolar structures and at least five non-alveolar-mouth fibres in any single quadrant of the field and irregular or indistinct fibre pattern i.e., not recognized as part of alveoli or bronchial wall |
| Hypercellular | More than 11 cellular structures in one field (Figure 2C) |
| These cellular structures are visualized within the alveolar space | |
| Thickened fibres | Visual consensus of all investigators |
| Normal alveolar structures | |
| Measurements show that these mean alveolar mouth fibres’ thickness is 21.9±6.17 µm, n=27. The non-thickened fibre thickness is 15.0±5.54 µm, n=111 (P<0.05) |
Relative frequencies of FCFM patterns by diagnosis
From Table 4, the densely packed fibre pattern was observed more frequently in patients with chronic fibrosing interstitial pneumonia (11/16, 68.8%) than in patients with other ILDs (4/11, 36.4%). However, the difference did not reach statistical significance (P=0.097). For the diagnosis of chronic fibrosing interstitial pneumonia, the sensitivity was 68.8% (95% CI, 41.3–89.0%), specificity was 63.6% (95% CI, 30.8–89.1%). In addition, the hypercellular FCFM pattern was more frequently observed in patients with other ILDs (P=0.005). Bronchoscopy was diagnostic in 7/27, 25.9% patients (Table S1), but no relationship was observed between bronchoscopic sampling and FCFM images.
Table 4. Relative frequencies of fibred confocal fluorescence microscopy (FCFM) patterns by diagnostic group.
| FCFM patterns | Patients | ||
|---|---|---|---|
| Chronic fibrosing (n=16, %) | Other ILD (n=11, %) | Overall (n=27, %) | |
| Normal | 9 (56.2) | 9 (81.8) | 18 (66.7) |
| Non-specific | 13 (81.2) | 5 (45.5) | 18 (66.7) |
| Increased fibres | 10 (62.5) | 7 (63.6) | 17 (63.0) |
| Densely packed fibres | 11 (68.8) | 4 (36.4) | 15 (55.6) |
| Hypercellular | 3 (18.8) | 8 (72.7) | 11 (40.7)* |
| Thickened fibres | 5 (31.2) | 2 (18.2) | 7 (25.9) |
Binominal proportion test was used with normal approximation to calculate P value. *, P<0.05. ILD, interstitial lung disease.
Relative frequencies of FCFM patterns by CT radiology
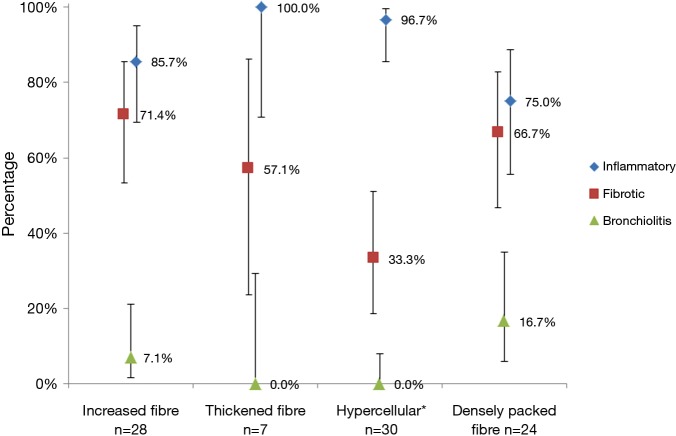
After excluding the normal (n=42) and non-specific (n=30) FCFM images, and those with normal CT bronchopulmonary segments, 77 abnormal FCFM images were analyzed to compare the relative frequency of CT images between FCFM patterns (Figure 4). The co-relation between inflammatory patterns on CT with hypercellular patterns in FCFM was statistically significant (P<0.001). Fibrotic patterns were indistinguishable from inflammatory radiological patterns in the images of thickened fibres, increased fibres, and densely packed fibres (Table S2). We were unable to diagnose bronchiolitis using these four identified abnormal FCFM patterns.
Figure 4.
Relative frequency of computed tomography (CT) radiological image patterns for each category of fibred confocal fluorescence microscopy (FCFM) findings. The markers indicate the frequencies of CT image patterns for each FCFM pattern, the percentages are shown next to the markers. The error bars represent the 95% confidence intervals (CI), approximated by Jeffreys method. For example, in 28 segments with increased fibre FCFM pattern, there were 24 bronchopulmonary segments, 24/28=85.7% (95% CI, 69.5–95.0%) that showed an inflammatory pattern on CT. Note that percentages do not total 100% because coexisting radiological patterns could be found in the same segment. *, P<0.001 by McNemar’s test.
Discussion
FCFM is a potential method for identifying chronic fibrosing interstitial pneumonia in patients with ILD. In the FCFM image, the densely packed fibres pattern is indicative of late-stage scarring and disruption of alveolar structures. We thought increased and thickened fibres indicated an earlier stage of disease, but this proved to be non-discriminatory in the diagnosis based on our data. These hypotheses need to be tested in prospective studies, and where possible, with biopsy co-relation. Because of the poor co-relation with radiology, caution needs to be exercised in using FCFM to identify the optimal bronchopulmonary segment for histological sampling in fibrotic disease. Conversely, the positive association between inflammatory patterns on CT and the hypercellular patterns in FCFM suggest that FCFM can be used to direct bronchoscopic sampling in other ILDs. Differentiation of the observed cellular structures into eosinophils, lymphocytes and neutrophils still proves elusive based on current FCFM technology. We also confirmed that morphological measurements of alveolar mouth dimensions and fibre size are of poor diagnostic utility. This is because of the wide range of values due to respiratory movement and stretching/compression of the fibres by miniprobe manipulation (16).
The small field of view of FCFM and the lack of effective contrast agents to visualize non-elastin structures are also known limitations (17). Additionally, chronic fibrosing interstitial pneumonia and other ILDs are broad categories, each possessing different multiple disease entities. Therefore, based on the data presented, we cannot definitively assert that optical biopsies via FCFM are of any diagnostic value, in ILD diagnosis or management. There are, however, distinct radiological differences between these two categories (Table 2). The architectural disruption seen in densely packed fibres helps us postulate the limited reversibility of the disease process.
The reported complication rate of FCFM is low, with only one reported case of pneumothorax that recovered spontaneously without chest tube insertion or hospitalization (18). Plus, the visceral pleural surface has a characteristically FCFM image of cross-hatched fluorescent linear structures that can be used to identify the limit of advancing the miniprobe (19). There is no current recommendation for the use of fluoroscopy to prevent pneumothorax, but fluoroscopy can be used to identify the edge of the lung. Although FCFM is performed on one side in most studies, preliminary data suggests that it can be safely performed bilaterally (16). Other complications of minor bleeding and transient pain reported elsewhere were not found in our study (10,20).
FCFM can aid in the clinical classification of ILD. However, the promise of optical biopsies remains uncertain, because of the heterogeneity of disease, of radiology, and the difficulty in obtaining lung tissue for histological co-relation. Much work is also needed in the field of bronchoscopy to standardize the FCFM procedure, the acquisition of video sequences and interpretation of images. Current confocal microscopy technology will also need further development before it can be used in place of more invasive tissue biopsies.
Acknowledgements
The authors thank Dr. Palash Ghosh, PhD, Centre for Quantitative Medicine, Duke-NUS Graduate Medical School for his advice and support. The authors appreciate the support of Duke-NUS/SingHealth Academic Medicine Research Institute in editing this paper.
Table S1. Bronchoalveolar lavage, forceps transbronchial lung biopsy and lung function test results with respective fibred confocal fluorescence microscopy (FCFM) pattern from targeted segment.
| No. | Location | Bronchoalveolar lavage | Transbronchial lung biopsy | FVC, L (predicted, %) | DLCO, mM/min/kPa (predicted, %) | FCFM pattern |
|---|---|---|---|---|---|---|
| 1 | LB4, LB5 | >1% eosinophils: eosinophilic cell pattern | – | 1.91 (62.0) | 2.69 (36.0) | Densely packed fibre |
| 2 | RB8 | >3% neutrophils: neutrophilic and eosinophilic cell pattern | – | 1.27 (47.0) | 1.35 (21.0) | Could not penetrate airway wall |
| 3 | RB4, RB5 | Predominance of macrophages. No malignant cells seen | – | 1.56 (65.0) | 2.00 (32.0) | FCFM done on contralateral side |
| 4 | LB3 | Rare markedly atypical cells seen, suspicious for malignancy | Adenocarcinoma of lung origin | NA | NA | Increased fibres |
| 5 | RB4 | Lymphocytic and eosinophilic cell pattern | Pneumonitis and organizing pneumonia | 1.58 (61.0) | 2.27 (36.0) | Fibrotic fibre pattern |
| 6 | RB2, RB6 | Acute inflammatory smear. Negative for malignant cells | – | 2.02 (92.0) | NA | Increased fibres |
| 7 | RB7 | Eosinophilic and neutrophilic pattern | – | 1.45 (67.0) | 3.08 (53.0) | FCFM done on contralateral side |
| 8 | RB5 | Negative for malignant cells | No granulomatous inflammation or malignancy | NA | NA | Fibrotic fibre pattern |
| 9 | LB4, LB5 | Predominance of macrophages, low chronic inflammatory yield | No granulomatous inflammation or malignancy | 2.56 (83.0) | 6.67 (96.0) | Increased fibres and densely packed fibres |
| 10 | LB4, LB5 | >3% neutrophils: neutrophilic and eosinophilic cell pattern | No lung parenchyma present | 1.06 (63.0) | NA | Could not penetrate airway wall |
| 11 | LB7–10 | >3% neutrophils: neutrophilic cell pattern | – | 1.72 (55.0) | 4.31 (59.0) | Increased fibres, hypercellular and densely packed fibres |
| 12 | RB10 | Inflammatory yield identified | No lung parenchyma present | 2.16 (62.0) | 3.47 (47.0) | FCFM done on alternative segments |
| 13 | LB4 | Reactive cells consistent with alveolar pneumocytes are seen | Pneumonitis and organizing penumonia | 2.61 (75.0) | NA | Hypercellular |
| 14 | RB4, RB5 | Acute inflammatory smear | – | 2.16 (63.0) | 5.45 (72.0) | Could not penetrate airway wall |
| 15 | RB4 | No malignant cells seen | – | 2.23 (65.0) | 2.82 (38.0) | Non-specific pattern |
| 16 | RB4, RB5 | Numerous lymphoplasmacytic cells seen | Pneumonitis and organizing penumonia | 2.78 (87.0) | 3.25 (46.0) | Increased fibres, densely packed fibres |
| 17 | RB4, RB5 | >3% neutrophils: neutrophilic and eosinophilic cell pattern | – | 2.85 (66.0) | 5.69 (64.0) | Increased fibres |
| 18 | RB1 | >3% neutrophils: neutrophilic cell pattern | – | 2.85 (69.0) | 6.45 (72.0) | Thin, irregular fibres |
| 19 | RB4, RB5 | Predominance of macrophages | – | 3.20 (83.0) | 5.97 (69.0) | Thickened fibres, densely packed fibres |
| 20 | RB4, RB5 | Predominance of macrophages | Focal, intra-alveolar, organising hemorrhage | NA | NA | Thin, broken fibres |
| 21 | RB5 | >3% neutrophils: neutrophilic cell pattern | – | 2.59 (60.0) | 3.68 (39.0) | Only blood vessels seen |
| 22 | RB6 | >3% neutrophils: neutrophilic and eosinophilic cell pattern | – | NA | NA | Thin fibres |
| 23 | RB6 | Lymphocytic cell pattern | – | 1.57 (83.0) | 2.32 (43.0) | Thick fibres |
| 24 | LB4, LB5 | >3% neutrophils: neutrophilic cell pattern | – | 1.46 (52.0) | NA | Could not penetrate airway wall |
| 25 | RB1–3, RB5 | Positive for Pneumocystis Jirovecii | Interstitial and bronchiolar, lymphohistiocytic infiltrate | NA | NA | Hypercellular |
| 26 | RB4, RB5 | Lymphocytic cell pattern | Non necrotising granulomatous inflammation | 3.09 (64.0) | 7.45 (68.0) | Increased fibres, hypercellular |
| 27 | RB1–3 | Positive for Pneumocystis Jirovecii | – | NA | NA | Hypercellular |
Table S2. Fibred confocal fluorescence microscopy (FCFM) patterns in each group of interstitial lung diseases (ILD).
| Diagnosis | Number of patients | FCFM patterns |
|---|---|---|
| NSIP | 10 | Normal, increased fibres, densely-packed fibres, hypercellular, thickened fibres and others/non-specific |
| IPF and fibrotic NSIP | 4 | Normal, increased fibres, densely-packed fibres and others/non-specific |
| Connective tissue disease-associated ILD | 2 | Normal, increased fibres, densely-packed fibres and others/non-specific |
| Cryptogenic organising pneumonia | 3 | Normal, increased fibres, densely-packed fibres, hypercellular and others/non-specific |
| Diffuse panbronchiolitis | 3 | Normal, increased fibres, densely-packed fibres, hypercellular, thickened fibres and others/non-specific |
| Pulmonary infections | 3 | Normal, hypercellular and thickened fibres |
| Sarcoidosis | 1 | Normal, increased fibres, hypercellular and others/non-specific |
| Adenocarcinoma | 1 | Normal, increased fibres, densely-packed fibres and hypercellular |
NSIP, nonspecific interstitial pneumonia.
Ethical Statement: This study has been approved by the SingHealth Centralised Institutional Review Board, CIRB Ref: 2012/245/C. (ClinicalTrials.gov Identifier: NCT01624753). Informed consent was obtained from all the participants before FCFM.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.American Thoracic Society. European Respiratory Society . American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [DOI] [PubMed] [Google Scholar]
- 2.Gibson GJ, Loddenkemper R, Lundbäck B, et al. Respiratory health and disease in Europe: the new European Lung White Book. Eur Respir J 2013;42:559-63. 10.1183/09031936.00105513 [DOI] [PubMed] [Google Scholar]
- 3.Gomez AD, King TE., Jr Classification of diffuse parenchymal lung disease. Prog Respir Res 2007;36:2-10. [Google Scholar]
- 4.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harari S, Caminati A. Update on diffuse parenchymal lung disease. Eur Respir Rev 2010;19:97-108. 10.1183/09059180.00002510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis 2013;30:3-16. [PubMed] [Google Scholar]
- 8.Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006;100:1753-9. 10.1016/j.rmed.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 9.Anantham D. Confocal Imaging. In: Ernst A, Herth FJ. editors. Principles and Practice of Interventional Pulmonology. New York, NY: Springer-Verlag, 2013:227-35. [Google Scholar]
- 10.Thiberville L, Salaün M, Lachkar S, et al. Human in vivo fluorescence microimaging of the alveolar ducts and sacs during bronchoscopy. Eur Respir J 2009;33:974-85. 10.1183/09031936.00083708 [DOI] [PubMed] [Google Scholar]
- 11.Keller CA, Hurst KE, Petrine KD, et al. Probe-based Confocal Laser Endo-microscopy (pCLE) Can Identity Perivascular Cellular Infiltration in Patients with Acute Cellular Rejection. J Heart Lung Transplant 2014;33:S69-70. 10.1016/j.healun.2014.01.222 [DOI] [Google Scholar]
- 12.Salaün M, Roussel F, Bourg-Heckly G, et al. In vivo probe-based confocal laser endomicroscopy in amiodarone-related pneumonia. Eur Respir J 2013;42:1646-58. 10.1183/09031936.00191911 [DOI] [PubMed] [Google Scholar]
- 13.Danilevskaya O, Averyanov A, Lesnyak V, et al. Confocal laser endomicroscopy for diagnosis and monitoring of pulmonary alveolar proteinosis. J Bronchology Interv Pulmonol 2015;22:33-40. 10.1097/LBR.0000000000000126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 15.Verbeken EK, Cauberghs M, Mertens I, et al. The senile lung. Comparison with normal and emphysematous lungs. 1. Structural aspects. Chest 1992;101:793-9. 10.1378/chest.101.3.793 [DOI] [PubMed] [Google Scholar]
- 16.Keller C, Alvarez F, Erasmus D, et al. Probe-based Confocal Laser Endomicroscopy (pCLE) in Lung Transplantation. 3rd International Conference of Cellvizio Users®. 2001. Available online: http://www.cellvizio.net/sites/default/files/u815/Proceedings-2011-v2-6_6-.pdf
- 17.Yserbyt J, Dooms C, Ninane V, et al. Perspectives using probe-based confocal laser endomicroscopy of the respiratory tract. Swiss Med Wkly 2013;143:w13764. [DOI] [PubMed] [Google Scholar]
- 18.Rafeq S, Ernst A, Majid A, et al. Bronchoscopic imaging using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med 2009;179:A5772. [Google Scholar]
- 19.Bhatt NA, Parrish SC, Malafronte P, et al. Characterization of Pleura by Probe-Based Confocal Laser Endomicrosocopy. Am J Respir Crit Care Med 2015;191:A4971. [Google Scholar]
- 20.Newton RC, Kemp SV, Yang GZ, et al. Imaging parenchymal lung diseases with confocal endomicroscopy. Respir Med 2012;106:127-37. 10.1016/j.rmed.2011.09.009 [DOI] [PubMed] [Google Scholar]