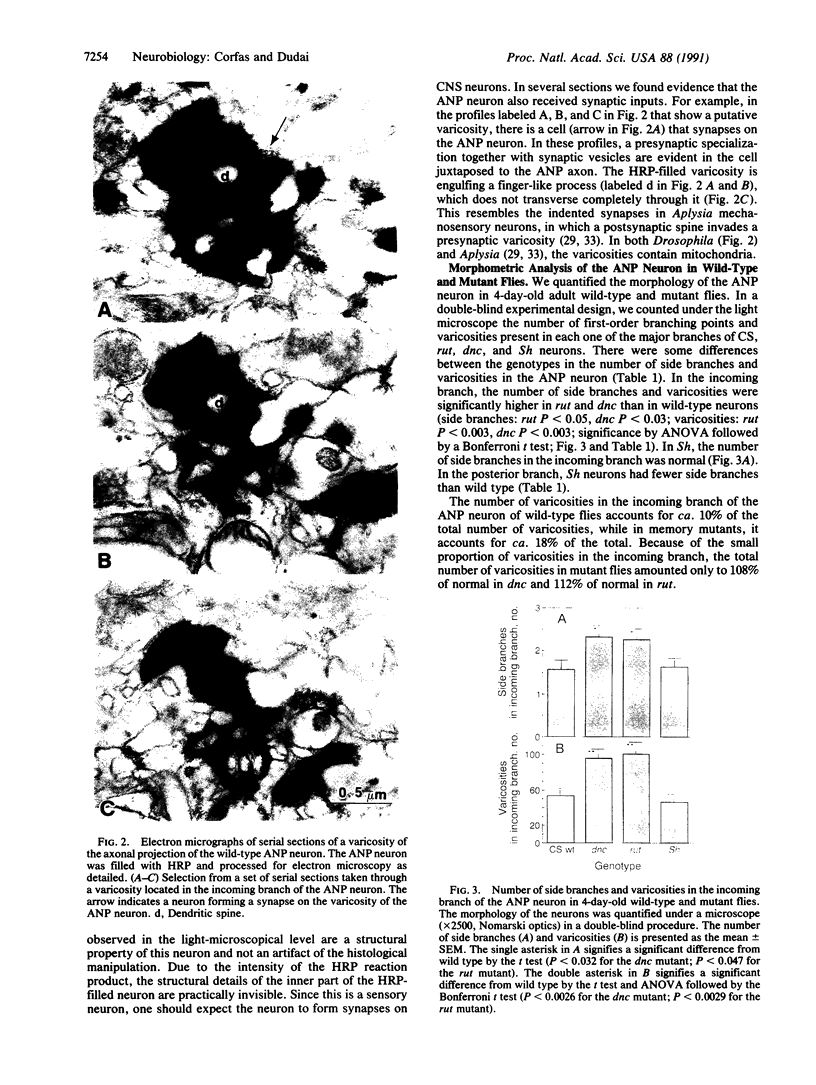
Abstract
Several mutations in Drosophila impair learning and the cAMP cascade. We report here that the fine morphology of an identified mechanosensory neuron is abnormal in two of these mutants, dunce (dnc) and rutabaga (rut). The neuron innervating the antero-notopleural bristle was filled with horseradish peroxidase and studied at the light- and electron-microscopy level. In the mutants dnc and rut, this neuron has an abnormally large number of side branches and varicosities in a defined segment of the axon. In wild-type flies, age tends to decrease the number of side branches and variacosities in the same axonal segment that is affected by the mutations. Ultrastructural studies are compatible with the interpretation that the varicosities are potential synaptic sites. The results suggest that the cAMP cascade plays a role in shaping neuronal connectivity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey C. H., Chen M. Long-term memory in Aplysia modulates the total number of varicosities of single identified sensory neurons. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2373–2377. doi: 10.1073/pnas.85.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Morphological basis of short-term habituation in Aplysia. J Neurosci. 1988 Jul;8(7):2452–2459. doi: 10.1523/JNEUROSCI.08-07-02452.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Thompson E. B., Castellucci V. F., Kandel E. R. Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. J Neurocytol. 1979 Aug;8(4):415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Thompson E. B. Indented synapses in Aplysia. Brain Res. 1979 Sep 7;173(1):13–20. doi: 10.1016/0006-8993(79)91091-6. [DOI] [PubMed] [Google Scholar]
- Balling A., Technau G. M., Heisenberg M. Are the structural changes in adult Drosophila mushroom bodies memory traces? Studies on biochemical learning mutants. J Neurogenet. 1987 Apr;4(2-3):65–73. [PubMed] [Google Scholar]
- Brandon J. G., Coss R. G. Rapid dendritic spine stem shortening during one-trial learning: the honeybee's first orientation flight. Brain Res. 1982 Dec 2;252(1):51–61. doi: 10.1016/0006-8993(82)90977-5. [DOI] [PubMed] [Google Scholar]
- Burg M. G., Wu C. F. Central projections of peripheral mechanosensory cells with increased excitability in Drosophila mosaics. Dev Biol. 1989 Feb;131(2):505–514. doi: 10.1016/s0012-1606(89)80021-1. [DOI] [PubMed] [Google Scholar]
- Byers D., Davis R. L., Kiger J. A., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster. Nature. 1981 Jan 1;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Corfas G., Dudai Y. Adaptation and fatigue of a mechanosensory neuron in wild-type Drosophila and in memory mutants. J Neurosci. 1990 Feb;10(2):491–499. doi: 10.1523/JNEUROSCI.10-02-00491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G., Dudai Y. Habituation and dishabituation of a cleaning reflex in normal and mutant Drosophila. J Neurosci. 1989 Jan;9(1):56–62. doi: 10.1523/JNEUROSCI.09-01-00056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRiemer S. A., Macagno E. R. Light microscopic analysis of contacts between pairs of identified leech neurons with combined use of horseradish peroxidase and lucifer yellow. J Neurosci. 1981 Jun;1(6):650–657. doi: 10.1523/JNEUROSCI.01-06-00650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. Neurogenetic dissection of learning and short-term memory in Drosophila. Annu Rev Neurosci. 1988;11:537–563. doi: 10.1146/annurev.ne.11.030188.002541. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Sher B., Segal D., Yovell Y. Defective responsiveness of adenylate cyclase to forskolin in the Drosophila memory mutant rutabaga. J Neurogenet. 1985 Dec;2(6):365–380. doi: 10.3109/01677068509101423. [DOI] [PubMed] [Google Scholar]
- Dudaí Y., Uzzan A., Zvi S. Abnormal activity of adenylate cyclase in the Drosophila memory mutant rutabaga. Neurosci Lett. 1983 Dec 2;42(2):207–212. doi: 10.1016/0304-3940(83)90408-1. [DOI] [PubMed] [Google Scholar]
- Fifková E. A possible mechanism of morphometric changes in dendritic spines induced by stimulation. Cell Mol Neurobiol. 1985 Jun;5(1-2):47–63. doi: 10.1007/BF00711085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Q., Macagno E. R. Extension and retraction of axonal projections by some developing neurons in the leech depends upon the existence of neighboring homologues. I. The HA cells. J Neurobiol. 1987 Jan;18(1):43–59. doi: 10.1002/neu.480180105. [DOI] [PubMed] [Google Scholar]
- Gao W. Q., Macagno E. R. Extension and retraction of axonal projections by some developing neurons in the leech depends upon the existence of neighboring homologues. II. The AP and AE neurons. J Neurobiol. 1987 May;18(3):295–313. doi: 10.1002/neu.480180305. [DOI] [PubMed] [Google Scholar]
- Ghysen A. The projection of sensory neurons in the central nervous system of Drosophila: choice of the appropriate pathway. Dev Biol. 1980 Aug;78(2):521–541. doi: 10.1016/0012-1606(80)90351-6. [DOI] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Horn G., Bradley P., McCabe B. J. Changes in the structure of synapses associated with learning. J Neurosci. 1985 Dec;5(12):3161–3168. doi: 10.1523/JNEUROSCI.05-12-03161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil R. E., Dubin M. W., Scott G., Stark L. A. Elimination of action potentials blocks the structural development of retinogeniculate synapses. Nature. 1986 Sep 11;323(6084):156–158. doi: 10.1038/323156a0. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Lnenicka G. A., Murphey R. K. The refinement of invertebrate synapses during development. J Neurobiol. 1989 Jul;20(5):339–355. doi: 10.1002/neu.480200507. [DOI] [PubMed] [Google Scholar]
- Muller K. J., McMahan U. J. The shapes of sensory and motor neurones and the distribution of their synapses in ganglia of the leech: a study using intracellular injection of horseradish peroxidase. Proc R Soc Lond B Biol Sci. 1976 Nov 12;194(1117):481–499. doi: 10.1098/rspb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Murphey R. K., Johnson S. E., Sakaguchi D. S. Anatomy and physiology of supernumerary cercal afferents in crickets: implications for pattern formation. J Neurosci. 1983 Feb;3(2):312–325. doi: 10.1523/JNEUROSCI.03-02-00312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphey R. K., Lemere C. A. Competition controls the growth of an identified axonal arborization. Science. 1984 Jun 22;224(4655):1352–1355. doi: 10.1126/science.6729457. [DOI] [PubMed] [Google Scholar]
- Purves D., Hadley R. D., Voyvodic J. T. Dynamic changes in the dendritic geometry of individual neurons visualized over periods of up to three months in the superior cervical ganglion of living mice. J Neurosci. 1986 Apr;6(4):1051–1060. doi: 10.1523/JNEUROSCI.06-04-01051.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Purves D., Voyvodic J. T., Magrassi L., Yawo H. Nerve terminal remodeling visualized in living mice by repeated examination of the same neuron. Science. 1987 Nov 20;238(4830):1122–1126. doi: 10.1126/science.3685967. [DOI] [PubMed] [Google Scholar]
- Rehn B., Breipohl W., Mendoza A. S., Apfelbach R. Changes in granule cells of the ferret olfactory bulb associated with imprinting on prey odours. Brain Res. 1986 May 14;373(1-2):114–125. doi: 10.1016/0006-8993(86)90321-5. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981 Sep 17;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. T. Formation of retinotopic connections: selective stabilization by an activity-dependent mechanism. Cell Mol Neurobiol. 1985 Jun;5(1-2):65–84. doi: 10.1007/BF00711086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully T., Quinn W. G. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985 Sep;157(2):263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Udin S. B. Abnormal visual input leads to development of abnormal axon trajectories in frogs. Nature. 1983 Jan 27;301(5898):336–338. doi: 10.1038/301336a0. [DOI] [PubMed] [Google Scholar]
- Wu C. F., Haugland F. N. Voltage clamp analysis of membrane currents in larval muscle fibers of Drosophila: alteration of potassium currents in Shaker mutants. J Neurosci. 1985 Oct;5(10):2626–2640. doi: 10.1523/JNEUROSCI.05-10-02626.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]







