Abstract
Background
The prognostic value of the right upper mediastinal lymph node dissection (RUMLND) for patients with middle or lower thoracic esophageal squamous cell carcinoma (ESCC-MLT) is still not well established yet. Our objective is to evaluate the prognostic role of the Sweet procedure plus right upper mediastinal lymph node dissection (MS) by comparing with the Sweet procedure with standard lymph node dissection (SS) in terms of long-term survival.
Methods
Totally 1,477 ESCC-MLT patients underwent radical intent surgery (186 with MS, 1,291 with SS) at our department between January 2007 and September 2013. After propensity score matching (PSM), 186 patients from each group were matched and analyzed. The 5-year survival rates in two groups were compared by detailed stratifications in terms of clinical characteristics.
Results
As for the prognostic role of RUMLND, patients treated with MS tended to obtain higher 5-year survival rate than patients treated with SS in univariate analysis (48.1% vs. 37.4%). Moreover, in multivariate analysis, MS yielded significant higher 5-year survival rate compared with SS (P=0.041). In addition, subgroup analyses of the survival between the MS and SS patients by detailed stratifications demonstrated the survival superiority in the MS group with age <60 years old, TNM stage III, number of lymph node dissection (LND) ≥15, as well as no using of postoperative adjuvant treatment.
Conclusions
The RUMLND in Sweet procedure is an independent prognostic factor for ESCC-MLT patients, especially for those with thoracic middle segment-located tumor, stage III or younger.
Keywords: Esophageal squamous cell carcinoma (ESCC), lymphadenectomy, sweet, prognosis
Introduction
Esophageal carcinoma (EC) is one of the most frequent malignant tumors in the world. In China, more than 95% of the EC are found to be esophageal squamous cell carcinoma (ESCC) (1). Even though esophagectomy with lymph node dissection (LND) is a main surgical approach for locally-advanced EC, the optimal surgical approach has not well established, especially for the extent of lymphadectomy. In other words, it remains unclear whether the addition of extensive LND improves the survival of EC patients (2). A study involving 4,627 surgically resected EC patients from the Worldwide Esophageal Cancer Collaboration database showed that extensive LND was associated with better survival (3). However, some studies indicated that more extensive LND during operation could not improve the survival of EC patients (4,5). Other studies, comparing extended transthoracic resection with limited transhiatal resection for the mid/distal EC, showed that the 5-year overall survival rate only tended to improvement in the former (6,7). A retrospective study involving 391 patients undergoing McKeown procedure (136 with standard LND and 255 with extended LND) showed that the right upper mediastinal (RUM)-LND was not associated with the 5-year survival rate (8). Therefore, further study on the prognostic role of right upper mediastinal lymph node dissection (RUMLND) is necessary.
To optimize the surgical procedure that can decrease early postoperative risk and improve long-term survival in middle or lower thoracic esophageal squamous cell carcinoma (ESCC-MLT) patients, experienced surgeons in our department have started to perform the Sweet procedure plus right upper mediastinal lymph node dissection (MS). The Sweet procedure is generally considered to be able to minimize early postoperative risk, but it is unclear whether it can improve long-term survival by the introduction of RUMLND. We retrospectively compared the MS procedure with the Sweet procedure with standard lymph node dissection (SS) in terms of long-term survival to evaluate the prognostic value of the RUMLND in Sweet procedure for treatment of ESCC-MLT.
Methods
Patients
We collected relevant data of the surgically treated patients who underwent Sweet procedure from the EC database at department of thoracic surgery in our hospital within the study period from January 2007 to September 2013. The patients received neo-adjuvant therapy or video-assisted thoracic surgery (VATS), whose esophagus was not substituted by gastric tube, or who died in postoperative one month were excluded. Finally, a total of 1,477 ESCC-MLT patients undergoing the Sweet procedure were enrolled with 186 patients in MS group and 1291 patients in SS group. Lymph node stations and clinical stages (T, N and TNM) were assessed according to the American Joint Committee on Cancer – Union for International Cancer Control (AJCC-UICC) system (edition 7). All patients were followed up until June 2015 or until death. The data collection and analysis were approved by the Ethics Committee at the West China Hospital of Sichuan University (No. 201649).
Surgical procedures
The SS procedure was carried out in a standard method with esophagogastric anastomosis above the aortic arch without RUMLND. The MS procedure was performed as the Sweet procedure with RUMLND through left posterolateral thoracotomy in the fifth or sixth intercostal. Extend of LND included mainly in the epigastrium around the stomach and the mediastina as well as right upper mediastina (2R, 3p and 4R). RUMLND was performed as follows: pulling up the proximal esophagus to expose the RUM region by an assistant, the surgeon touches 2R, 3p or 4R lymph nodes along the right lateral wall of the trachea by right index finger, then clamps and pulls up the lymph nodes including surrounding tissues using lymph node forceps, finally resects stepwise the lymph nodes by medical electric knife. Though resecting in 2 L lymph node is slightly difficult owing to the left subclavian obstruction and a limited clearance above the aortic arch, resecting 2R lymph node via the left thorax becomes feasible because its deeper position relatively enlarges the small clearance of the anatomy.
The difference between MS and SS procedure was whether or not the RUM lymph nodes including stations 2R, 3p and 4R were resected. RUMLND in Sweet procedure was only performed by sampling rather than systematic LND. The choice of surgical procedure was not randomized and only depended on the habits of the surgeons.
Propensity score matching (PSM)
According to the patient characteristics in the MS group (186 cases); the patients in the SS group (1,291 cases) were matched by PSM method. This method made similar baseline characteristics between two groups in order to create “quasi-random experiment” from retrospective data (9,10). Using a multivariable logistic regression model, we calculated the propensity scores in terms of clinicopathological characteristics (covariates: age, gender, tumor location, tumor grade, T stage, N stage and pathological stage). After 1:1 matching by the ‘Nearest Neighbor’ method (the caliper value: 0.1), 186 out of 1,291 SS patients were matched with the 186 MS patients.
Statistical analysis
Statistical analysis was performed on SPSS 22 (IBM Corporation) and Stata/SE 12.0, with a significance level at two-sided P<0.05. Continuous variables were expressed as mean ± standard deviation. The baseline characteristics of continuous and dichotomous variables between groups were compared by the independent sample t-test and Pearson χ2 test, respectively. The associations of RUM-lymph node metastasis (LNM) with clinicopathological characteristics were evaluated via logistic regression. Survival analyses were compared with the log rank test and Cox’s proportional hazard regression. The independent prognostic factors were identified by the multivariate stepwise Cox’s regression. Survival curves were drawn using Cox’s regression. Subgroup forest plots were analyzed and depicted on Stata/SE 12.0.
Results
Patient characteristics
After excluding the 1,105 unmatched SS patients from the total of 1,477 patients, we finally enrolled and analyzed 186 MS patients and matched 186 SS patients. These matched patients included 80 (21.5%) females and 292 (78.5%) males, whose median age was 60 years (range, 36–80 years). The clinical characteristics were not significantly different between groups (Table 1).
Table 1. Clinical characteristic of patients after matching.
| Factor | MS (n=186) (%) | SS (n=186) (%) | P value |
|---|---|---|---|
| Age | 60.68±7.906 | 59.92±7.707 | 0.349 |
| Gender | >0.999 | ||
| Male | 146 | 146 | |
| Female | 40 | 40 | |
| Tumor location | 0.877 | ||
| Middle | 161 | 163 | |
| Lower | 25 | 23 | |
| Tumor grade | |||
| G1 | 14 | 14 | 0.994 |
| G2 | 79 | 80 | |
| G3 | 93 | 92 | |
| T stage | 0.601 | ||
| Tis + T1a + T1b | 29 | 35 | |
| T2 | 25 | 28 | |
| T3 | 104 | 91 | |
| T4a + T4b | 28 | 32 | |
| N stage | 0.254 | ||
| N0 | 101 | 89 | |
| N+ (N1 + N2 + N3) | 85 | 97 | |
| Pathological stage | 0.327 | ||
| 0 + IA + IB | 21 | 31 | |
| IIA + IIB | 77 | 72 | |
| IIIA + IIIB + IIIC | 88 | 83 | |
| Postoperative adjuvant treatment | 0.329 | ||
| Yes | 71 | 61 | |
| No | 115 | 125 | |
| Total LND | 24.07±9.627 | 20.22±8.159 | <0.001 |
| LND outside RUM | 20.74±8.691 | 20.22±8.159 | 0.555 |
| Total LNM numbers | 1.67±2.852 | 1.27±1.861 | 0.107 |
| Total LNM rates | 85 (45.7) | 97 (52.2) | 0.254 |
| LNM rates outside RUM | 80 (43.0) | 97 (52.2) | 0.097 |
| Middle-lower mediastinal LNM rates | 52 (28.0) | 60 (32.3) | 0.429 |
| Upper-abdominal LNM rates | 58 (31.2) | 54 (29.0) | 0.735 |
MS, the Sweet procedure plus right upper mediastinal lymph node dissection; SS, the Sweet procedure with standard lymph node dissection; RUM, right upper mediastina; LNM, lymph node metastasis; LND, lymph node dissection.
Follow-up
All the enrolled patients were followed up for a median period of 45.8 months (range, 18.7–93.2 months) by telephone from the patient or her/his family members. Also 71 MS patients and 61 SS patients received postoperative adjuvant chemotherapy and/or radiation therapy, but showed no significant difference between groups (P=0.329). By the end of follow-up, 153 of the 372 patients survived, with 35 missed patients (9.4%) and 184 dead patients (49.5%). The median survival time was 42.3 months and the 5-OS rate was 42.8% for the all subjects.
Associations of RUM-LNM with clinicopathological characteristics
Table 2 showed the associations of RUM-LNM with clinicopathological characteristics. The rate of RUM-LNM in the MS group was 19.9% (37/186), of which, 2R, 3p and 4R accounted for 18.8% (35/186), 6.6% (4/61) and 12.5% (1/8), respectively. Of the 614 resected lymph nodes (3.30±2.688, range, 1–19), there were totally 60 RUM-LNMs (0.32±0.864, range, 0–6).
Table 2. The association for RUM-LNM with clinicopathological characteristics.
| Clinical pathologic factor | Cases | RUM-LNM (%) | P value |
|---|---|---|---|
| Age | 0.531 | ||
| ≤60 | 89 | 16 (18.0) | |
| >60 | 97 | 21 (21.6) | |
| Gender | 0.037 | ||
| Male | 146 | 34 (23.3) | |
| Female | 40 | 3 (7.5) | |
| Tumor location | 0.296 | ||
| Middle | 161 | 34 (21.1) | |
| Lower | 25 | 3 (12.0) | |
| Tumor grade | 0.018 | ||
| G1 | 14 | 0 (0.0) | |
| G2 | 79 | 13 (16.5) | |
| G3 | 93 | 24 (25.8) | |
| T stage | 0.004 | ||
| Tis + T1a + T1b | 29 | 1 (3.4) | |
| T2 | 25 | 5 (20.0) | |
| T3 | 104 | 20 (19.2) | |
| T4a + T4b | 28 | 11 (39.3) | |
| Pathological stage | <0.001 | ||
| 0 + IA + IB | 21 | 0 (0.0) | |
| IIA + IIB | 77 | 2 (2.6) | |
| IIIA + IIIB + IIIC | 88 | 35 (39.8) |
RUM, right upper mediastina; LNM, lymph node metastasis.
Survival analysis
For the MS versus SS patients, the median survival periods were 46.7 vs. 34.1 months and the 5-OS rates were 48.1% vs. 37.4%, respectively, with no significant differences tested by univariate analysis (P=0.055) (Table 3). However, multivariate Cox regression showed a significant difference between these two groups (P=0.041) (Figure 1 and Table 3).
Table 3. Survival analysis according to clinical characteristics.
| Factor | Univariate analysis (P value) | Multivariate analysis | ||
|---|---|---|---|---|
| OR | 95.0% CI | P value | ||
| Age (≤60 vs. >60) | 0.901 | 1.085 | 0.802–1.467 | 0.598 |
| Gender (male vs. female) | 0.112 | 0.773 | 0.525–1.139 | 0.193 |
| Location (middle vs. lower) | 0.830 | 0.954 | 0.624–1.459 | 0.828 |
| Grade (G1 + G2 vs. G3) | 0.016 | 1.223 | 0.944–1.584 | 0.128 |
| T stage (Tis/T1/T2/T3) | <0.001 | — | — | — |
| N stage (N0 vs. N+) | <0.001 | — | — | — |
| Pathological stage (I/II/III) | <0.001 | 2.024 | 1.579–2.595 | <0.001 |
| Total numbers of LND (<15 vs. ≥15) | 0.761 | 0.973 | 0.687–1.378 | 0.876 |
| Adjuvant treatment (no vs. yes) | 0.195 | 0.755 | 0.550–1.038 | 0.083 |
| Groups (MS vs. SS) | 0.055 | 1.362 | 1.013–1.831 | 0.041 |
N+, N1+N2+N3; MS, the Sweet procedure plus right upper mediastinal lymph node dissection; SS, the Sweet procedure with standard lymph node dissection; LND, lymph node dissection.
Figure 1.
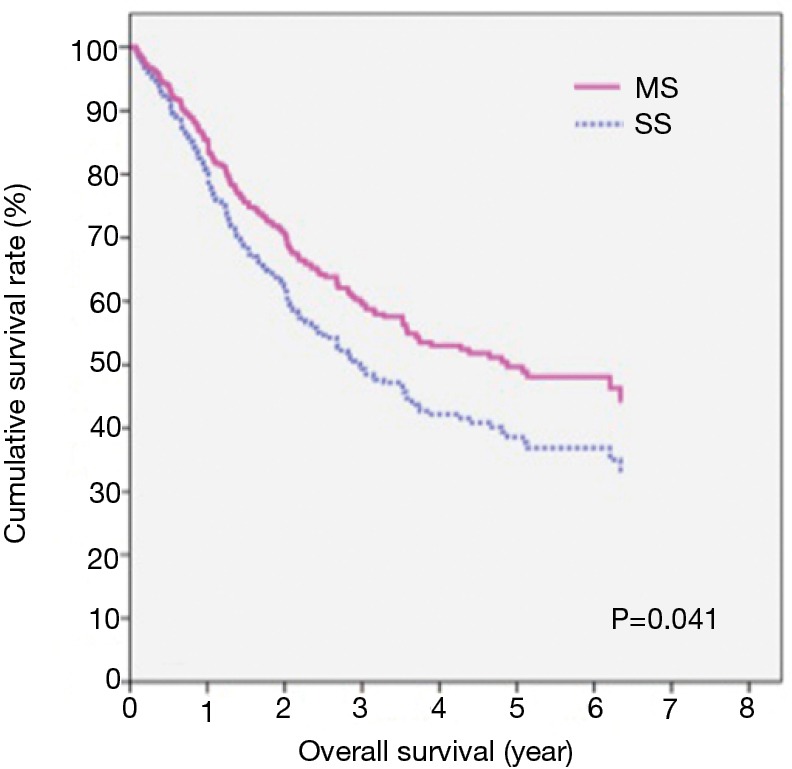
The survival for patients between MS and SS by multivariate cox regression. MS, the Sweet procedure plus right upper mediastinal lymph node dissection; SS, the Sweet procedure with standard lymph node dissection.
Subgroup analyses for the survival between the MS and the SS group
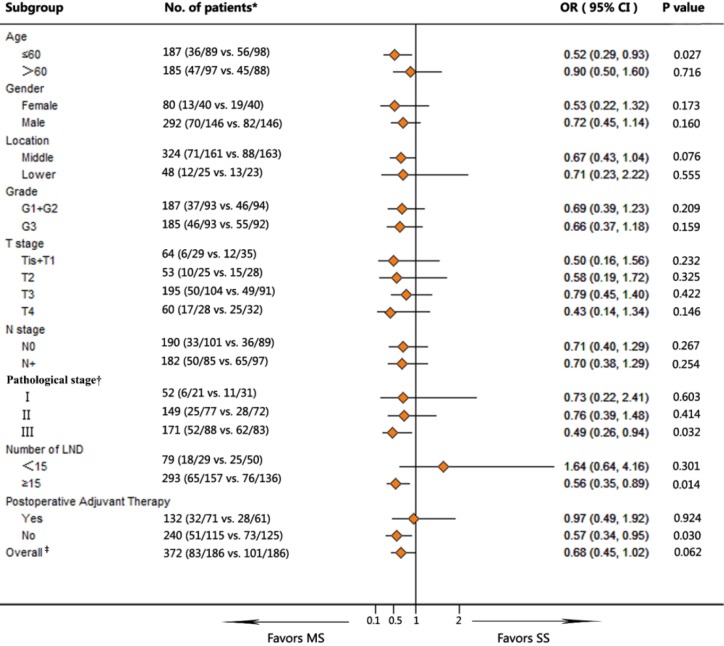
The survival in MS group was significantly higher in subgroup including: age <60, TNM III stage, more than 15 LNDs, and no postoperative adjuvant treatment (P=0.027, 0.032, 0.014 and 0.030, respectively) as compared with SS group. There was a marginal difference in the middle thoracic EC between groups in favor of the MS group (P=0.076; OR =0.67; 95% CI, 0.43–1.04) (Figure 2).
Figure 2.
Subgroup analyses for survival in patients between MS and SS group. †, significant difference among inter-subgroups by multivariate Cox regression analysis (P<0.001); ‡, significant difference between the MS and the SS group by multivariate Cox regression analysis (P=0.041). MS, the Sweet procedure plus right upper mediastinal lymph node dissection; SS, the Sweet procedure with standard lymph node dissection; LND, lymph node dissection.
Discussion
Whether or not RUMLND for ESCC-MLT patients can bring survival benefit remains controversial (2). Furthermore, it is unclear whether the new method by Sweet procedure to perform RUMLND can improve the survival of ESCC-MLT patients. Therefore, we retrospectively explored the factor and significance of RUMLNM in the MS group, and compared the survival of the MS and SS patients in different stratifications for ESCC-MLT. The important role and feasibility to improve survival of ESCC-MLT patients by RUMLND via Sweet procedure were demonstrated. To our knowledge, this was the first study to explore the prognostic value of RUMLND in Sweet procedure for the operation of esophageal cancer.
Previously, a meta-analysis showed that the LNM rate in the upper mediastina was 21.1% for thoracic middle EC and 10.5% for thoracic lower EC (11), which was similar to our result with 19.9% RUM-LNM rate. Therefore, it fitted for the notion of radical resection of esophageal cancer to achieve more survival benefits, despite a sampling lymph nodal resection for RUM. We found the higher RUM-LNM rate was related with poorer tumor differentiation, deeper tumor invasion and more advanced pathological stage. This suggested that RUM lymph nodes should be removed for relatively advanced ESCC-MLT patients. However, as previous report in N1 stage ESCC (12), the LNM of upper mediastinum was significantly correlated with tumor location, which was inconsistent with our results maybe due to our study included N0 to N3 rather than only N1.
Whether extensive LND can improve 5-year survival rate remains debatable. Some studies showed that 5-year survival rates were not significantly different between the extended LND via transthoracic and limited LND via transhiatal for esophageal adenocarcinoma (13-15). Despite no significant difference, other studies showed a trend toward improved 5-year survival with the extended transthoracic approach (6,7,16,17). Similarly, in our series of ESCC-MLT patients, 5-year survival rates between MS and SS groups were not significantly different (48.1% vs. 37.4%) evaluated by univariate analysis (P=0.055), but a better trend in the MS group. Moreover, multivariate analysis showed that the RUMLND and TNM stage were important prognostic factors for the survival of ESCC-MLT patients, and that the MS procedure achieved a significantly higher lymph node resection and better survival than in the SS patients (P=0.041). These were similar with some previous literatures. For instance, the number of LND was an independent prognostic factor for the survival of ESCC patients with N0 (1) and the number of negative lymph nodes was an independent prognostic factor for ESCC patients (18).
In this study, no significant differences were found in the long-term survival rates between patients at age ≤60 and at age >60 (P=0.598). However, with regard to patients aged ≤60 years, the survival in the MS subgroup was significantly superior to that in the SS subgroup (P=0.027). This might be explained by the fact that the patients aged ≤60 years suffered from more advanced EC. For example, a study involving 18,118 EC patients or gastro-esophageal junction carcinoma in the Netherlands showed that despite the comparable survival rates, patients aged ≤50 years presented with more advanced disease stage compared with patients aged >50 years (19).
Tachimori et al. (20) recommended that all patients with thoracic ESCC, regardless of the location, should be performed resection of upper mediastinal lymph nodes that had the highest the index of estimated benefit from lymph node dissection (IEBLD) in patients with middle EC, followed by patients with lower EC. We also found that RUMLND played a more important role in middle versus lower ESCC. The survival of patients in middle ESCC was favorable in the MS group versus the SS group, despite no significant difference.
A study involving 468 patients with cT3NxM0 esophageal cancer with 242 (51.7%) squamous cell carcinomas and 226 (48.3%) adenocarcinomas showed that the transthoracic procedure plus extended LND could prolong the survival of pT3, cT3, and node-positive patients compared with the transhiatal procedure plus limited LND (21). Although no survival differences were found between two groups (MS vs. SS group) in detailed stratifications by tumor location, tumor grade (G1 + G2 and G3), T stage or N stage (N0 and N+), but favorable survival was identified in the MS patients with stage III. Therefore, RUMLND played an important role in locally-advanced ESCC-MLT patients.
The latest National Comprehensive Cancer Network (NCCN) Guideline for EC recommended that the minimum number of LND was 15 (22). Our previous study on ESCC showed that at least six lymph nodal stations should be removed, and even for patients with G3 staging or tumor length >3 cm, the resection of seven lymph nodal stations was recommended (23). In the current study, there was no survival improvement even if the RUM lymph nodes were removed if total resected number was <15, but the survival was superior in patients with RUMLND when the number of LND was ≥15. Therefore, our opinion is the surgeon should remove at least 15 lymph nodes including the RUM lymph nodal stations and even the maximum possible radical LND for locally- advanced ESCC-MLT.
In our series, the survival rates were not significantly different between patients undergoing postoperative adjuvant radiotherapy and/or chemotherapy between two groups. These treatments might improve the survival of patients and thus weakened the comparability between two groups. However, for the patients without receiving these treatments due to many reasons (e.g., the doctor advised for patients with early tumor; the patients and/or the family members disagreed), the survival of MS patients was better than the SS patients. As far as our results are concerned, postoperative adjuvant radiotherapy and/or chemotherapy can improve the long-term survival in the ESCC-MLT patients without RUMLND.
There were several limitations in our study. First, the sample size was small in this retrospective study. Although total cases between the MS and SS groups were matched, comparisons of two groups in the each subgroup stratification were not matched by PSM, and therefore potential bias may exist in our study. In addition, we adjusted tumor grade (G1, G2, G3) into two subgroups (G1 + G2 vs. G3), N stage into node-negative (N0) and node-positive (N+) subgroups, and merged Tis into T1 and stage 0 into stage I due to a limited number of cases. Finally, the status for recurrent laryngeal nerve lymph node could not be identified owing to the lack of this name of lymph node station in retrospective data.
In conclusion, The RUM-LND was the main prognostic factor for ESCC-MLT patients, especially for thoracic middle segment-located tumors, stage III tumor or younger patients. Even if the RUM lymph node was resected during operation, the surgeon should warrant that the number of resected lymph nodes was at least 15 and even the maximum possible radical lymphadenectomy for locally-advanced ESCC-MLT. If a non-ideal LND was performed during operation, postoperative adjuvant radiotherapy and/or chemotherapy may be beneficial.
Acknowledgements
None.
Ethical Statement: The study was approved by the Ethics Committee at the West China Hospital of Sichuan University (No. 201649) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Liu Q, Tan Z, Lin P, et al. Impact of the number of resected lymph nodes on postoperative survival of patients with node-negative oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg 2013;44:631-6. 10.1093/ejcts/ezt097 [DOI] [PubMed] [Google Scholar]
- 2.Herbella FA, Laurino Neto RM, Allaix ME, et al. Extended lymphadenectomy in esophageal cancer is debatable. World J Surg 2013;37:1757-67. 10.1007/s00268-013-2018-5 [DOI] [PubMed] [Google Scholar]
- 3.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. 10.1097/SLA.0b013e3181b2f6ee [DOI] [PubMed] [Google Scholar]
- 4.Wong J, Weber J, Almhanna K, et al. Extent of lymphadenectomy does not predict survival in patients treated with primary esophagectomy. J Gastrointest Surg 2013;17:1562-8; discussion 1569. 10.1007/s11605-013-2259-5 [DOI] [PubMed] [Google Scholar]
- 5.van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015;107. pii: djv043. 10.1093/jnci/djv043 [DOI] [PubMed] [Google Scholar]
- 6.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. 10.1056/NEJMoa022343 [DOI] [PubMed] [Google Scholar]
- 7.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. 10.1097/SLA.0b013e31815c4037 [DOI] [PubMed] [Google Scholar]
- 8.Hsu PK, Huang CS, Hsieh CC, et al. Role of right upper mediastinal lymph node metastasis in patients with esophageal squamous cell carcinoma after tri-incisional esophagectomies. Surgery 2014;156:1269-77. 10.1016/j.surg.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inacio MC, Chen Y, Paxton EW, et al. Statistics in Brief: An Introduction to the Use of Propensity Scores. Clin Orthop Relat Res 2015;473:2722-6. 10.1007/s11999-015-4239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding X, Zhang J, Li B, et al. A meta-analysis of lymph node metastasis rate for patients with thoracic oesophageal cancer and its implication in delineation of clinical target volume for radiation therapy. Br J Radiol 2012;85:e1110-9. 10.1259/bjr/12500248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Chen C, Duan X, et al. Characteristics and risk factors of lymph node metastasis in pN1 stage esophageal squamous cell carcinoma. Zhonghua Wai Ke Za Zhi 2015;53:513-7. [PubMed] [Google Scholar]
- 13.Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg 2014;101:511-7. 10.1002/bjs.9456 [DOI] [PubMed] [Google Scholar]
- 14.Khullar OV, Jiang R, Force SD, et al. Transthoracic versus transhiatal resection for esophageal adenocarcinoma of the lower esophagus: A value-based comparison. J Surg Oncol 2015;112:517-23. 10.1002/jso.24024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ovrebo KK, Lie SA, Laerum OD, et al. Long-term survival from adenocarcinoma of the esophagus after transthoracic and transhiatal esophagectomy. World J Surg Oncol 2012;10:130. 10.1186/1477-7819-10-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganesamoni S, Krishnamurthy A. Three-field transthoracic versus transhiatal esophagectomy in the management of carcinoma esophagus-a single--center experience with a review of literature. J Gastrointest Cancer 2014;45:66-73. 10.1007/s12029-013-9562-y [DOI] [PubMed] [Google Scholar]
- 17.Mariette C, Piessen G. Oesophageal cancer: how radical should surgery be? Eur J Surg Oncol 2012;38:210-3. 10.1016/j.ejso.2011.12.022 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Z, Chen H, Yu W, et al. Number of negative lymph nodes is associated with survival in thoracic esophageal squamous cell carcinoma patients undergoing three-field lymphadenectomy. Ann Surg Oncol 2014;21:2857-63. 10.1245/s10434-014-3665-y [DOI] [PubMed] [Google Scholar]
- 19.van Nistelrooij AM, van Steenbergen LN, Spaander MC, et al. Treatment and outcome of young patients with esophageal cancer in the Netherlands. J Surg Oncol 2014;109:561-6. 10.1002/jso.23533 [DOI] [PubMed] [Google Scholar]
- 20.Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. 10.1007/s10388-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kutup A, Nentwich MF, Bollschweiler E, et al. What should be the gold standard for the surgical component in the treatment of locally advanced esophageal cancer: transthoracic versus transhiatal esophagectomy. Ann Surg 2014;260:1016-22. 10.1097/SLA.0000000000000335 [DOI] [PubMed] [Google Scholar]
- 22.NCCN Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers, Version 2. Available online: http://www.nccn.org, accessed March 6, 2015.
- 23.Peng J, Wang WP, Yuan Y, et al. Adequate lymphadenectomy in patients with oesophageal squamous cell carcinoma: resecting the minimal number of lymph node stations. Eur J Cardiothorac Surg 2016;49:e141-6. 10.1093/ejcts/ezw015 [DOI] [PubMed] [Google Scholar]