Abstract
Background
Global pulmonary function tests lack region specific differentiation that might influence therapy in severe chronic obstructive pulmonary disease (COPD) patients. Therefore, the aim of this work was to assess the degree of expiratory 3rd generation bronchial lumen collapsibility in patients with severe COPD using chest-computed tomography (CT), to evaluate emphysema-phenotype, lobar volumes and correlate results with pulmonary function tests.
Methods
Thin-slice chest-CTs acquired at end-inspiration & end-expiration in 42 COPD GOLD IV patients (19 females, median-age: 65.9 y) from November 2011 to July 2014 were re-evaluated. The cross-sectional area of all segmental bronchi was measured 5 mm below the bronchial origin in both examinations. Lung lobes were semi-automatically segmented, volumes calculated at end-inspiratory and end-expiratory phase and visually defined emphysema-phenotypes defined. Results of CT densitometry were compared with lung functional tests including forced expiratory volume at 1 s (FEV1), total lung capacity (TLC), vital capacity (VC), residual volume (RV), diffusion capacity parameters and the maximal expiratory flow rates (MEFs).
Results
Mean expiratory bronchial collapse was 31%, stronger in lobes with homogenous (38.5%) vs. heterogeneous emphysema-phenotype (27.8%, P=0.014). The mean lobar expiratory volume reduction was comparable in both emphysema-phenotypes (volume reduction 18.6%±8.3% in homogenous vs. 17.6%±16.5% in heterogeneous phenotype). The degree of bronchial lumen collapsibility, did not correlate with expiratory volume reduction. MEF25 correlated weakly with 3rd generation airway collapsibility (r=0.339, P=0.03). All patients showed a concentric expiratory reduction of bronchial cross-sectional area.
Conclusions
Changes in collapsibility of 3rd generation bronchi in COPD grade IV patients is significantly lower than that in the trachea and the main bronchi. Collapsibility did not correlate with the reduction in lung volume but was significantly higher in lobes with homogeneous vs. heterogeneous emphysema phenotype. Changes in the 3rd generation bronchial calibres between inspiration and expiration are not predictive for the degree of small airway collapsibility and related airflow limitation.
Keywords: Chronic obstructive pulmonary disease (COPD), emphysema, lung volume, computed tomography (CT)
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common pulmonary diseases in the western world and one of the leading causes of death (1). It is defined by airflow obstruction through emphysema or airway narrowing or their combination (2). Chronic inflammation and irritants are risk factors for airway mural weakening with increased bronchial collapse (3). Besides smoking, exposure to air pollution, age, alpha-1-antitrypsin deficiency and genetics are also responsible for developing COPD. Pathologic studies confirmed both deficiency and or atrophy of bronchial cartilage in COPD (4). In order to differentiate between COPD patients with a leading parenchymal problem and COPD patients with a primary airway obstruction computed tomography (CT) examination is the leading imaging modality (5,6). Knowledge about it has clinical impact on defining the most suitable treatment strategy. Different attempts have been made to measure the lumen in the large airways as a quantification estimate for airway obstruction and small airway remodelling (7-9). Up to date, only the cross-sectional area of the trachea and the main bronchi were successfully analysed in humans with regard to luminal calibre changes induced by physiological breathing, in the end-inspiratory and end-expiratory phases in both healthy volunteers and patients with bronchial asthma and COPD (10-12). Whereas changes in the luminal area of the large airways are known to vary considerably even in healthy people between forced inspiration and forced expiration, corresponding changes of bronchial cross-sectional area below e.g., in segmental (the 3rd generation) bronchi have not yet been intensively studied. Nevertheless, some reports have suggested that airflow limitation in COPD is closely related to the airway dimensions from the 3rd to the 6th generation bronchi (13,14). Generally, technical limitations have been the reason for delayed investigation of smaller airways. Moreover, the degree of lung parenchymal destruction and its extent e.g., diffusely (homogeneous) vs. patchy (heterogeneous) have been described in the last time as clinical phenotypes pointing on their potential relevance for pre-selection of patients foreseen for interventional lung reduction (15). According to earlier animal experiments, the loss of tethering forces and consecutively of the so-called elastic recoil by homogeneous lung parenchyma destruction should be responsible for increased bronchial collapsibility (16).
Based on a one-center experience in dealing with severe emphysema patients, the aim of this work was to assess retrospectively, to our knowledge for the first time, the degree of expiratory bronchial lumen collapsibility in 3rd generation bronchi using chest-CT taking in consideration the potential impact of the leading emphysema phenotype (homogeneous vs. heterogeneous), lobar volumes and their changes in between end-inspiration and end-expiration. Furthermore, we looked for potential correlations between expiratory 3rd generation bronchial collapsibility and collapsibility of more central maximal expiratory flow rate 75 (MEF75) and more peripheral small airways represented by established lung functional tests (MEF25).
Methods
Patient characteristics
Retrospective archive search identified 42 patients with COPD who underwent chest-CT both at end-inspiration and at end-expiration for COPD-quantification and for planning endoscopic lung volume reduction Between November 2011 and July 2014.
All patients were diagnosed with stage IV COPD. Twenty-eight patients had been smoker (66.6%; mean: 43.4±19.1 pack years), however at the time point of examination only three patients were still smoking (7.1%). Thirty-six of the patients reported shortness of breath at the time of examination (85.7%), 15 had cough (35.7%) and 13 needed permanently oxygen support (30.9%; mean: 2.5±0.7 L O2/min).
The indication to undergo chest-CT was set by the treating physicians in order to evaluate the extension and phenotype of emphysema in all patients as part of a pre-selection process before reference to endoscopic lung volume reduction.
All examinations were analysed for the presence of pulmonary infection, edema or haemorrhage as excluding criteria for final analysis.
Ethics statement
The study was approved by institutional ethics board of Eberhard-Karls University Tübingen (No. 084/2015R).
Chest-CT-technique
Overall 42 CT examinations were performed using a 128-slices multidetector CT scanner (Siemens Definition AS+, Forchheim, Germany) system using a 250–330 mm field of view, a 512×512 reconstruction matrix, 120 kV, 100–150 effective mAs and a tube rotation time of 0.5 ms. All examinations were performed non-enhanced. In all patients, a helical acquisition was obtained from the apex to the base of the lungs at the end-inspiratory and the end-expiratory phase, respectively.
Before examination, all subjects were coached and received standardized breathing instructions. Examinations were always performed with the patients in the supine position. Every CT scan was reconstructed at 3 mm slices with a soft (filter, B31f) and a sharp (filter, B70f) reconstruction algorithm for visual assessment and 0.6 mm slices and a soft tissue kernel (filter, B35f) for CT-densitometry and lung volume calculation.
Imaging analysis
All scans were viewed at standard mediastinal windows [level, 35 Hounsfield unit (HU); width, 450 HU] and lung windows (level, −700 HU; width, 1,500 HU). All examinations were viewed by two independent readers (M Horger, C Kloth) with 20 and 2 years’ experience in reading chest-CT. Both readers were blinded to each other and to the patient’s state as well as to the reading results gained in the clinical routine and the laboratory analysis. In case of discrepancy in classifying the emphysema phenotype, a consensus was reached by joint re-reading and consecutive discussion.
Quantitative CT image analysis
The quantitative bronchial lumen assessment (cross-sectional area) was measured after standardized detection of all 3rd generation bronchus by an automatic 3D algorithm “Pulmo 3D” (Siemens Healthcare). The software registered the bronchial tree as well as the bronchus lumen at both end-inspiration and end-expiration (Figure 1) automatically. The software creates a center line along the long axis of each bronchus. For each segmental bronchus the curved center line was controlled. We manually measured in a fixed distance of 0.5 cm below the origin of a segment bronchus for setting the level of cross-sectional bronchial lumen quantification and if necessary corrected the automatical results by a hand drawn ROI in order to measure the bronchial lumen area in each 3rd generation bronchi.
Figure 1.

Overview of segmentation of bronchial tree. Bronchus segmentation is shown in the left lower corner. Distance from the bronchus offspring is signed in 0.5 cm distance and the bronchus lumen is measured with 0.22 cm2.
Expiratory collapsibility of 3rd generation bronchi was determined by the formula (1-cross-sectional areaexp/cross-sectional areainsp) according to the work by Litmanovich et al. (17).
Additionally, the expiratory reduction of bronchial lumen cross-sectional area was classified concentric vs. non-concentric. Symmetry vs. asymmetry in the reduction of bronchial cross sectional area was evaluated subjectively considering a concentric inward movement as physiological.
The emphysema phenotype was classified using the dedicated Pulmo 3D software. Whole lung color-coded maps displaying the number and distribution of emphysema equivalent (density <−950 HU) lung parenchymal areas (clusters) were used for this purpose. Based on this “cluster” analysis we classified visually the emphysema in each lung lobe into homogenous (almost no parenchymal area exhibiting attenuation values >−950 HU left) vs. heterogeneous (a mixture of parenchymal areas (patchy pattern) showing attenuation values >−950 and <−950 HU (Figure 2). The cluster analysis method calculates and displays connected voxels that are below the HU value of −950 HU. All voxels below the LAV value are categorized in four volume clusters, color guided displayed. The following values are specified, by default: 2 mm3 ≤ C1 <8 mm3 in light blue, 8 mm3 ≤ C2 <65 mm3 in light green, 65 mm3 ≤ C3 <187 mm3 in yellow, and 187 mm3 ≤ C4 and above in red (18-21).
Figure 2.
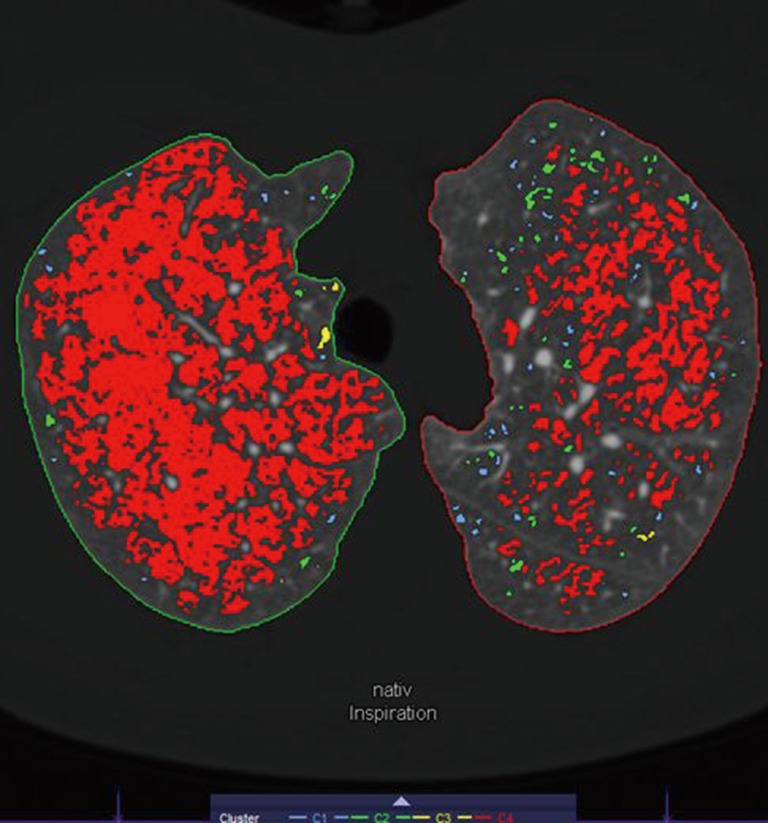
Axial CT image displaying areas of emphysema. Colour guided areas of smaller (blue, green) emphysema or bigger areas (yellow, red) can be identified. In the right lung, a homogenous emphysema phenotype is leading, however in the left lung a heterogeneous phenotype is dominating. CT, computed tomography.
Emphysema phenotypes were visually defined using “cluster” analysis obtained at end-inspiratory phase
Subsequently, we analysed the lung lobar volumes of each patient for each lung lobe using the “Pulmo 3D” software by Syngo.via CT (Siemens Healthcare). The quantification process has been already published (22). We quantified the lung volume separately for each lung and lobe at end-inspiratory and end-expiratory phase and then measured the emphysema-equivalent low-attenuation-values (LAV). The percentage of their volumes below the threshold −950 HU (LAV) was calculated. The latter parameter was determined only at end-inspiration.
Functional lung analysis
Lung function tests were performed using a Masterscreen Body (CareFusion GmbH, Hoechberg, Germany). All data were acquired with a men time difference of 0.17±0.33 y to the CT examination. The following parameters were assessed by the pulmonology department: forced expiratory volume at 1 s (FEV1), total lung capacity (TLC), vital capacity (VC), residual volume (RV), ratio of residual volume over total lung capacity (RV/TLC) and single-breath diffusion capacity for carbon monoxide (DLCOcSB). Additionally, the MEF25, MEF50 and MEF75 was measured in order to estimate the collapse of the larger airways like e.g., 3rd generation bronchi (MEF75), middle-sized bronchi MEF50 and that of the small airways (MEF25).
Statistics
Statistics were calculated with Prism software (GraphPad software, La Jolla, CA, USA). All data are reported as arithmetic mean ± standard deviation (SD). Skewness, kurtosis and D’Agostino-Pearson normality test were used to categorize data. Nonparametric Mann-Whitney test was used for analysis of bronchial diameters and lobular volume. Pearson’s r-correlation was used for MEF25, MEF50 and MEF75. Spearman’s r was determined for all other correlation analysis. For all tests, P values smaller than 0.05 were considered statistically significant.
Influence of emphysema phenotype on expiratory bronchial lumen collapsibility
Overall lobar emphysema was classified homogenous in 30.4% (n=64) or heterogeneous in 66.1% (n=139). In seven lobes (3.5%), CT-densitometry revealed no obvious emphysema. In 11 patients, only one emphysema phenotype (homogenous or heterogeneous) was found (2, homogeneous; 9, heterogeneous), whereas in all other patients a mixed phenotype of emphysema was documented throughout the different lobes. An overview of the emphysema phenotypes and their distributions is shown on Table 1.
Table 1. Overview of emphysema types in the patients. In seven lobes no emphysema were registered.
| Emphysema types | Distribution | ||||
|---|---|---|---|---|---|
| UR | MR | LR | UL | LL | |
| Homogeneous | 15 | 11 | 18 | 8 | 12 |
| Heterogeneous | 26 | 30 | 22 | 32 | 29 |
UR, upper right lobe; MR, middle right lobe; LR, lower right lobe; UL, upper left lobe; LL, lower left lobe.
Considering the emphysema phenotype, expiratory bronchial collapsibility was stronger in patients with homogenous phenotype (38.5%±16.9%) than in those with heterogeneous phenotype (27.8%±4.3%, P=0.014).
Expiratory segment bronchi lumen collapsibility and the effect of corresponding lobar volume changes between inspiration and expiration
The mean cross-sectional area of all segmental bronchi at end-inspiration was 0.15±0.06 cm2 whereas mean cross-sectional area at end-expiration was 0.09±0.05 cm2. The change in percentage from in- to expiration for all individual 3rd generation bronchial lumina yielded an overall mean expiratory bronchial collapse of 31.0%±6.7%.
The mean total lung volume decreased by 19.1% from 6,789.8±1,503.8 mL (inspiration) to 5,492.8±1,217.3 mL (expiration) with a mean lobar reduction of 13.4%±53.3%.
The mean lobar expiratory volume reduction was comparable in both emphysema phenotypes (volume reduction 18.6%±8.3% vs. 17.6%±16.5% in heterogeneous emphysema phenotype, ns).
An overview of lung CT densitometry of all patients is given on Table 2.
Table 2. Overview of lung volumes in mL and computed tomography densitometry with standard deviations of all patients.
| Location | Inspiration | Expiration, volumes (mL) | |||
|---|---|---|---|---|---|
| Volumes (mL) | MLD | LAV | HAV | ||
| Total lung | 6,789.8±1,503.8 | −882.0±17.47 | 27.31±9.49 | 1.02±0.28 | 5,492.76±1,217.31 |
| UL | 1,678.42±647.75 | −887.28±21.47 | 27.04±11.61 | 0.87±0.34 | 1,292.85±378.51 |
| LL | 1,528.82±455.74 | −876.54±24.45 | 25.95±12.25 | 1.16±0.41 | 1,293.33±523.07 |
| UR | 1,427.02±442.26 | −885.80±29.28 | 28.14±14.59 | 0.95±0.40 | 1,281.50±378.51 |
| MR | 623.50±355.36 | −876.71±24.45 | 22.03±10.39 | 1.13±0.43 | 489.66±319.52 |
| LR | 1,664.95±485.89 | −874.53±27.71 | 25.48±11.73 | 1.20±0.43 | 1,292.69±457.67 |
MLD, mean lung density; LAV, low attenuation values; HAV, high attenuation values; UR, upper right lobe; MR, middle right lobe; LR, lower right lobe; UL, upper left lobe; LL, lower left lobe.
Correlation between expiratory bronchial collapsibility and corresponding inspiratory and expiratory lobar volumes
While the measured inspiratory 3rd generation bronchial cross-sectional area showed a moderate correlation with the inspiratory volume in the homogeneous emphysema phenotype, only weak correlations were observed for the heterogeneous emphysema phenotype (r=0.51, P<0.001 and r=0.27, P=0.003). Correlations for expiratory values were not significant. The degree of bronchial lumen collapsibility, did not correlate with the degree of expiratory volume reduction or additional test parameters (P=0.511). Correlations between inspiratory LAV and MLD subdivided in homogenous and heterogeneous lobes is shown on Figure 3. Subdivion of lobular volume changes and corresponding bronchus did not shown significant correlations (UL, r=0.019–0.244; LL, r=−0.098–0.336; UR, 0.087–0.249; MR, r=0.104–0.499; LR, 0.115–0.511; always P=ns).
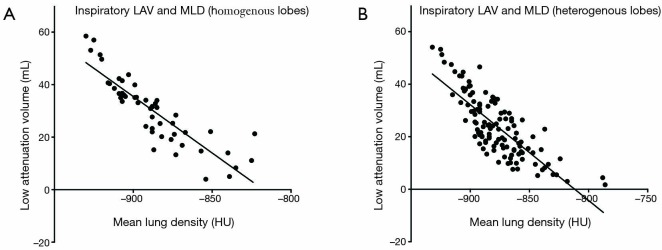
Figure 3.
Statistically significant inverse correlation of low attenuation values (LAV) with mean lung density (MLD) for homogenous lobes (A) vs. heterogeneous lobes (B). A stronger correlation was registered in homogenous (r=−0.92, P=0.0001) vs. heterogeneous emphysema phenotypes (r=−0.78, P=0.0001).
Expiratory changes in the cross-sectional bronchial area (concentric vs. non-concentric)
All patients showed a concentric expiratory reduction of bronchial cross-sectional area.
Functional lung analysis
Lung function tests resulted in a mean TLC of 7.79±1.7 representing 132% of normal lung capacity; the MEF25 was 0.17±0.06 (12.9%±5.7% of normal expiratory volume), and MEF75 was 0.68±0.29 (15.8%±31.1% of normal expiratory volume). The DLCOc was 3.03±1.11 (representing 37.2% of regular capacity). The complete results are displayed on Table 3. According to the measured values, all patients had severe COPD. DLCOcSB did not correlate significant with MEF25/50/75 nor with bronchial collapsibility.
Table 3. Listed clinical parameters.
| Parameter | Mean | SD |
|---|---|---|
| TLC | 7.79 | 1.70 |
| TLC % predicted | 132.01 | 20.52 |
| RV, mL | 5.41 | 1.41 |
| RV % predicted | 242.37 | 64.71 |
| RV % TLC | 68.92 | 8.04 |
| RV % TLC predicted | 172.11 | 22.64 |
| VC, mL | 2.33 | 0.86 |
| VC % predicted | 67.61 | 17.33 |
| FEV1, mL | 0.81 | 0.23 |
| FEV1% predicted | 31.32 | 7.85 |
| PEF | 2.33 | 1.81 |
| PEF predicted | 32.37 | 14.49 |
| MEF 75 | 0.69 | 0.29 |
| MEF 75 predicted | 15.90 | 31.38 |
| MEF 50 | 0.31 | 0.12 |
| MEF 50 predicted | 8.07 | 3.01 |
| MEF 25 | 0.17 | 0.06 |
| MEF 25 predicted | 12.94 | 5.69 |
| DLCOcSB | 3.03 | 1.11 |
| DLCOcSB % predicted | 37.21 | 12.97 |
PFT, pulmonary function testing; 6MWT, 6 minutes working test; TLC, total lung capacity; RV, residual volume; FEV1, forced expiratory volume in 1 s; DLCOc SB, single-breath diffusion capacity for carbon monoxide; Pre, percentage of the measured values to the predicted values; PEF, peak expiratory flow; MEF, maximal expiratory flow rate.
MEF25 correlated with MEF75 (r=0.702, P=0.001). The expiratory lung volume reduction was weakly correlated with MEF25 (r=0.39, P=0.01). There was no statistically significant difference between TLC, FEV1, VC, DLCOcSB and MEF25/75 of the two emphysema types.
Discussion
Our study demonstrates that quantitative end-inspiratory and end-expiratory CT of the 3rd generation airways is feasible in patients with severe COPD and that the underlying predominant emphysema phenotype has a significant impact on the magnitude of expiratory bronchial collapsibility.
Airflow limitation is one major determinant for respiratory failure in patients with COPD. In normal human lungs, the flow-limiting segment is located between the segmental bronchi and the subglottis whereas in severely obstructive lung diseases the flow-limiting segments are supposed to be located in the peripheral airways at all lung volumes (23). Other reports suggested that airflow limitation in COPD was closely related to the airway dimensions from the 3rd to the 6th generation bronchi (13). Again previous reports have asserted that airway dimensions of the large- and intermediate-sized airways reflect airway dimensions in the smaller airways, which are the most important site of airway obstruction in COPD (7,14). The compliance of the extra- and intrapulmonary airways depends finally both on the mechanical structure (bronchial wall characteristics) as well as on the quality of lung parenchyma surrounding the bronchial tree (23). Under physiological conditions, the alveolar attachments confer stability to the airway wall as they pull the airway wall outwards (elastic recoil). Hence, both the surrounding tissues and/or the sub atmospheric pleural pressure exert a tethering force on the intrapulmonary airways. Narrowing of intrapulmonary airways will be, thus physiologically minimized by these tissue forces. However, the expiratory narrowing of intra- and extra-pulmonary airways depends also on their mural characteristics, which vary considerably along the bronchial tree. In healthy volunteers the bronchial collapsibility measured in the main bronchi at forced expiration was reported with >60% (17). The same authors observed even a total collapse of the intermediate bronchi in four volunteers in their series. Similar data have been obtained from measurements of the normal trachea exhibiting a mean expiratory decrease of 35% (range, 11–61%) (24). Cartilage is knowingly limited to the large airways with the proportion of the airway wall occupied by cartilage varying with the airway size. The cartilage in the airway wall can limit the degree of airway narrowing associated with airway smooth muscle contraction. In the trachea and the main bronchi, cartilage occupies the ventral portion of the airway circumference. The c-shaped rings of cartilage provide stability and help to prevent the trachea from collapsing and shutting off the airway. Below the main bronchi, the segmental bronchi (3rd generation) divide into many smaller bronchioles which again ramify into terminal bronchioles. In the tertiary bronchi, the C-shaped cartilage rings are replaced by irregularly arranged plates of hyaline cartilage surrounding the smooth muscle whereas in the smaller intrapulmonary airways the cartilage entirely disappears. Differences in the shape and extent of cartilage throughout the bronchial tree are expected to influence their stability. Thus, in 3rd generation bronchi, the presence of thickened musculature, as well as the reinforcement by cartilage plates is expected to make them more stable against expiratory collapse. Moreover, differences in the consistency between bronchial cartilages of healthy and that of diseased airways is expected to play a major role in the airway motility. Histological studies have demonstrated some degree of atrophy of bronchial cartilage in COPD related to chronic bronchitis (25,26). In contrast, other quantitative reports failed to demonstrate any alteration of cartilage in the bronchi of patients with chronic bronchitis or emphysema (27).
In our study, we set out to evaluate bronchial caliber changes occurring between the end-inspiratory and end-expiratory phase in 3rd generation bronchi in severe COPD. Novel technical equipment makes such measurements possible by first segmenting the lung parenchyma, followed by tracking of the bronchial tree, thus facilitating precise determination of the luminal cross-sectional area in a plane lying perpendicular to the bronchial course. Measurements of cross-sectional area in all segmental bronchi yielded a mean expiratory bronchial collapse of 31%. This is relatively low in comparison with similar measurements performed in the trachea and main bronchi even in healthy volunteers and reflect in our opinion the major structural, local differences along the bronchial tree wall (17). Although we had no corresponding histological validation to our CT-measurements, the low variability in the cross-sectional area between inspiration and expiration in our cohort consisting of exclusively stage IV COPD patients is in support of a less severe damage of the bronchial wall cartilage as previously reported and of the higher stability of 3rd generation bronchial wall (25,26). Expectedly, expiratory bronchial collapsibility was stronger in lobes with homogenous emphysema phenotype (38.5%) than in such exhibiting a heterogeneous emphysema phenotype (27.8%). Notably, the expiratory volume reduction was comparable irrespective of the emphysema phenotype (volume reduction 18.6% for homogeneous emphysema vs. 17.6% for heterogeneous emphysema). Differences in the expansibility and collapsibility of intrapulmonary structures like the bronchi are expected to be influenced by the magnitude of expansion and compression of the surrounding lung parenchyma, the latter being dependent on the regional compliance and airflow resistance (28). Accordingly, in our series, a lesser degree of expiratory bronchial collapsibility was found in patients with the heterogeneous emphysema phenotype, which indirectly suggests more tethering effects due to a higher degree of residual elastic recoil despite comparable values of mean lobar expiratory volume reduction. The weak but significant correlation between the expiratory lung volume reduction and MEF25 is in support of the hypothesis that the airflow resistance in the peripheral smaller airways is playing a major role in this respect in patients with severe COPD. Other variables like e.g., the magnitude of collateral ventilation are also known to affect the balance of intrapulmonary pressure and bronchial collapsibility in this clinical setting. Notably, airway malacia that was described as >50% expiratory reduction of the airway lumen in the trachea of COPD patients seems to spare the 3rd generation bronchi (11). Moreover, the comparably modest expiratory collapse of the 3rd generation bronchi in our cohort consisting mainly of grade IV COPD patients is in support of less significant cartilage destruction in severe COPD patients.
Expectedly and contrary to previous reports dealing with measurements of the lumen of trachea and main bronchi, the expiratory decrease in the cross-sectional area of the bronchi in our series proved always concentric (23). This is thought to be due to the circular support of the bronchial wall by surrounding lung tissue.
Finally, we did not find any statistical correlation between the degree of expiratory collapse in 3rd generation bronchi and the MEF75, which is a lung functional parameter expected to indirectly, represent the expiratory airflow in the larger airways at 75% of the expiratory phase. Nevertheless, MEF75 correlated with MEF25, which in turn correlated with the 3rd generation expiratory bronchial collapse. At this point, our results suggest that airflow limitation could be located behind the third generation bronchi as reported already by Matsuoka et al. (14). However, these authors did not examined all segmental and subsegmental bronchi which limits in our opinion the validity of such statements as the bronchial system is expected to underlie some regional variations in terms of luminal collapsibility dictated by the local anatomy and physiologic influence of thoracic cage mobility. This discrepancy is probably due to the relatively small size of our patient cohort. Hence, changes in the 3rd generation bronchial calibres between inspiration and expiration are not predictive for the degree of small airway collapsibility and related airflow limitation as a general predictor. However, our CT measurements reflect motility of each individual lobe and results therefore are not comparable with those of an overall lung function test.
Our study has some limitations. First, data evaluation was retrospective in character. Second, we did not have any comparable data in healthy volunteers or lower degrees of COPD, which makes understanding of the impact of COPD on inspiratory bronchial distention and expiratory collapsibility impossible.
In conclusion, we could demonstrate that collapsibility of 3rd generation bronchi in COPD grade IV patients is significantly lower than that in the trachea and the main bronchi compared to earlier published data. Collapsibility did not correlate with the reduction in lung volume but was significantly higher in lobes with homogeneous vs. heterogeneous emphysema phenotype. According to our results, changes in the 3rd generation bronchial calibres between inspiration and expiration are not predictive for the degree of small airway collapsibility and related airflow limitation.
Acknowledgements
We acknowledge support by Deutsche Forschungsgemeinschaft and Open Access Publishing Fund of University of Tübingen.
Ethical Statement: The study was approved by institutional ethics board of Eberhard-Karls University Tübingen (No. 084/2015R).
Footnotes
Conflicts of Interest: H Ditt is an employee of Siemens Healthcare. The other authors have no conflicts of interest to declare.
References
- 1.Zhang WJ, Hubbard Cristinacce PL, Bondesson E, et al. MR Quantitative Equilibrium Signal Mapping: A Reliable Alternative to CT in the Assessment of Emphysema in Patients with Chronic Obstructive Pulmonary Disease. Radiology 2015;275:579-88. 10.1148/radiol.14132953 [DOI] [PubMed] [Google Scholar]
- 2.Nakano Y, Muro S, Sakai H, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000;162:1102-8. 10.1164/ajrccm.162.3.9907120 [DOI] [PubMed] [Google Scholar]
- 3.Jokinen K, Palva T, Sutinen S, et al. Acquired tracheobronchomalacia. Ann Clin Res 1977;9:52-7. [PubMed] [Google Scholar]
- 4.Haraguchi M, Shimura S, Shirato K. Morphometric analysis of bronchial cartilage in chronic obstructive pulmonary disease and bronchial asthma. Am J Respir Crit Care Med 1999;159:1005-13. 10.1164/ajrccm.159.3.9712144 [DOI] [PubMed] [Google Scholar]
- 5.Brillet PY, Fetita CI, Saragaglia A, et al. Investigation of airways using MDCT for visual and quantitative assessment in COPD patients. Int J Chron Obstruct Pulmon Dis 2008;3:97-107. 10.2147/COPD.S2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano Y, Müller NL, King GG, et al. Quantitative assessment of airway remodeling using high-resolution CT. Chest 2002;122:271S-275S. 10.1378/chest.122.6_suppl.271S [DOI] [PubMed] [Google Scholar]
- 7.Nakano Y, Wong JC, de Jong PA, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005;171:142-6. 10.1164/rccm.200407-874OC [DOI] [PubMed] [Google Scholar]
- 8.Boulet L, Bélanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med 1995;152:865-71. 10.1164/ajrccm.152.3.7663797 [DOI] [PubMed] [Google Scholar]
- 9.Okazawa M, Müller N, McNamara AE, et al. Human airway narrowing measured using high resolution computed tomography. Am J Respir Crit Care Med 1996;154:1557-62. 10.1164/ajrccm.154.5.8912780 [DOI] [PubMed] [Google Scholar]
- 10.Castagnaro A, Rastelli A, Chetta A, et al. High-resolution computed tomography evaluation of airway distensibility in asthmatic and healthy subjects. Radiol Med 2008;113:43-55. 10.1007/s11547-008-0223-3 [DOI] [PubMed] [Google Scholar]
- 11.Sverzellati N, Rastelli A, Chetta A, et al. Airway malacia in chronic obstructive pulmonary disease: prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickening. Eur Radiol 2009;19:1669-78. 10.1007/s00330-009-1306-9 [DOI] [PubMed] [Google Scholar]
- 12.Koyama H, Ohno Y, Nishio M, et al. Three-dimensional airway lumen volumetry: comparison with bronchial wall area and parenchymal densitometry in assessment of airway obstruction in pulmonary emphysema. Br J Radiol 2012;85:1525-32. 10.1259/bjr/22602417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasegawa M, Nasuhara Y, Onodera Y, et al. Airflow limitation and airway dimensions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:1309-15. 10.1164/rccm.200601-037OC [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka S, Kurihara Y, Yagihashi K, et al. Airway dimensions at inspiratory and expiratory multisection CT in chronic obstructive pulmonary disease: correlation with airflow limitation. Radiology 2008;248:1042-9. 10.1148/radiol.2491071650 [DOI] [PubMed] [Google Scholar]
- 15.Coxson HO, Whittall KP, Nakano Y, et al. Selection of patients for lung volume reduction surgery using a power law analysis of the computed tomographic scan. Thorax 2003;58:510-4. 10.1136/thorax.58.6.510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki H, Nakumura M, Takishma T. Effect of lung parenchyma on bronchial collapsibility during maximum expiratory flow in dogs. Tohoku J Exp Med 1976;118:1-10. 10.1620/tjem.118.1 [DOI] [PubMed] [Google Scholar]
- 17.Litmanovich D, O'Donnell CR, Bankier AA, et al. Bronchial collapsibility at forced expiration in healthy volunteers: assessment with multidetector CT. Radiology 2010;257:560-7. 10.1148/radiol.10100219 [DOI] [PubMed] [Google Scholar]
- 18.Blechschmidt RA, Werthschützky R, Lörcher U. Automated CT image evaluation of the lung: a morphology-based concept. IEEE Trans Med Imaging 2001;20:434-42. 10.1109/42.925296 [DOI] [PubMed] [Google Scholar]
- 19.Bankier AA, De Maertelaer V, Keyzer C, et al. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999;211:851-8. 10.1148/radiology.211.3.r99jn05851 [DOI] [PubMed] [Google Scholar]
- 20.Gietema HA, Schilham AM, van Ginneken B, et al. Monitoring of smoking-induced emphysema with CT in a lung cancer screening setting: detection of real increase in extent of emphysema. Radiology 2007;244:890-7. 10.1148/radiol.2443061330 [DOI] [PubMed] [Google Scholar]
- 21.McGuinness G, Naidich DP, Leitman BS, et al. Bronchiectasis: CT evaluation. AJR Am J Roentgenol 1993;160:253-9. 10.2214/ajr.160.2.8424327 [DOI] [PubMed] [Google Scholar]
- 22.Grosse U, Hetzel J, Gündel L, et al. Impact of endobronchial coiling for lung volume reduction on pulmonary volume and attenuation: preinterventional and postinterventional computed tomography-quantification using separate lobe measurements. J Comput Assist Tomogr 2014;38:779-85. 10.1097/RCT.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 23.Hughes JM, Hoppin FG, Jr, Mead J. Effect of lung inflation on bronchial length and diameter in excised lungs. J Appl Physiol 1972;32:25-35. [DOI] [PubMed] [Google Scholar]
- 24.Murgu SD, Colt HG. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006;11:388-406. 10.1111/j.1440-1843.2006.00862.x [DOI] [PubMed] [Google Scholar]
- 25.Restrepo GL, Heard BE. Air trapping in chronic bronchitis and emphysema: measurements of the bronchial cartilage. Am Rev Respir Dis 1964;90:395-400. [DOI] [PubMed] [Google Scholar]
- 26.Tandon MK, Campbell AH. Bronchial cartilage in chronic bronchitis. Thorax 1969;24:607-12. 10.1136/thx.24.5.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiddens HA, Bogaard JM, de Jongste JC, et al. Physiological and morphological determinants of maximal expiratory flow in chronic obstructive lung disease. Eur Respir J 1996;9:1785-94. 10.1183/09031936.96.09091785 [DOI] [PubMed] [Google Scholar]
- 28.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970;28:596-608. [DOI] [PubMed] [Google Scholar]