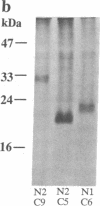
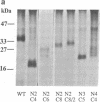
Abstract
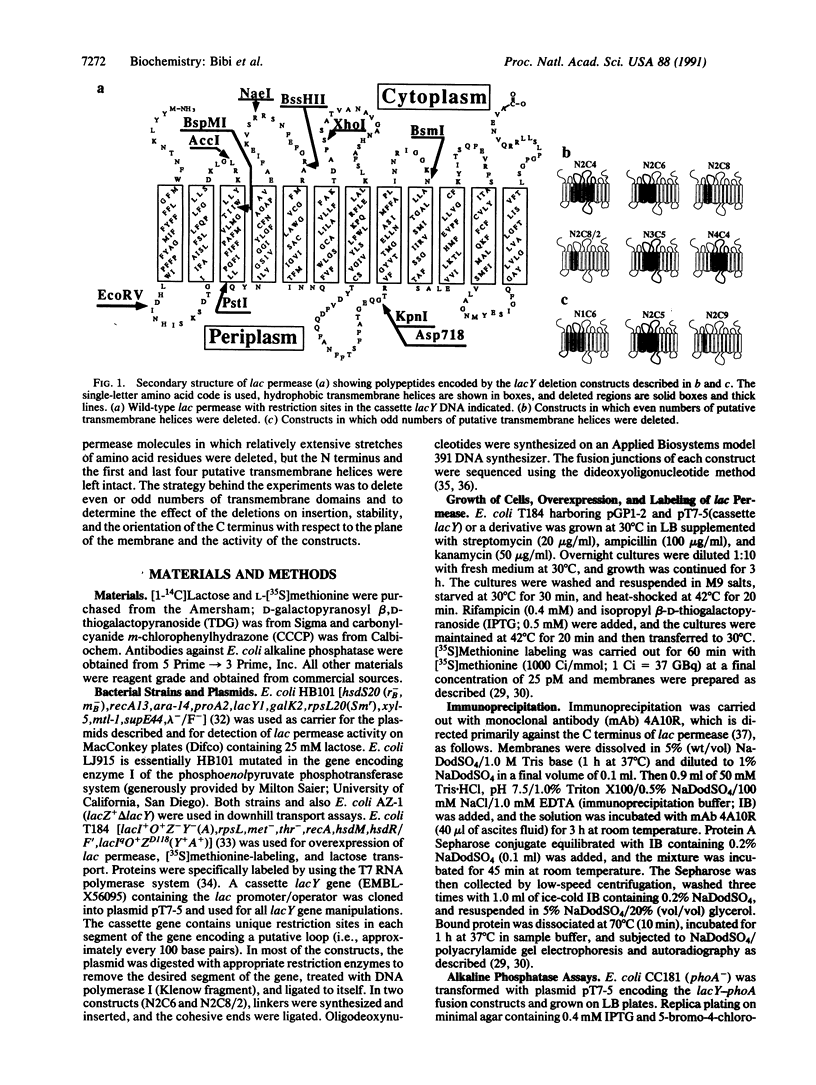
The overall topology of polytopic membrane proteins is thought to result from either the oriented insertion of the N-terminal alpha-helical domain followed by passive insertion of subsequent helices or from the function of independent topogenic determinants dispersed throughout the molecules. By using the lactose permease of Escherichia coli, a well-characterized membrane protein with 12 transmembrane domains and the N and C termini on the cytoplasmic surface of the membrane, we have studied the insertion and stability of in-frame deletion mutants. So long as the first N-terminal and the last four C-terminal putative alpha-helical domains are retained, stable polypeptides are inserted into the membrane, even when an odd number of helical domains is deleted. Moreover, even when an odd number of helices is deleted, the C terminus remains on the cytoplasmic surface of the membrane, as judged by lacY-phoA fusion analysis. In addition, permease molecules devoid of even or odd numbers of putative transmembrane helices retain a specific pathway for downhill lactose translocation. The findings imply that relatively short C-terminal domains of the permease contain topological information sufficient for insertion in the native orientation regardless of the orientation of the N terminus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Calamia J., Manoil C. lac permease of Escherichia coli: topology and sequence elements promoting membrane insertion. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4937–4941. doi: 10.1073/pnas.87.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Herzlinger D., Mitchell R., DeChiara S., Danho W., Gabriel T. F., Kaback H. R. Intramolecular dislocation of the COOH terminus of the lac carrier protein in reconstituted proteoliposomes. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4672–4676. doi: 10.1073/pnas.81.15.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Tahara S. M., Patel L., Goldkorn T., Kaback H. R. Preparation, characterization, and properties of monoclonal antibodies against the lac carrier protein from Escherichia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6894–6898. doi: 10.1073/pnas.79.22.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco N., Viitanen P., Herzlinger D., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 1. Functional studies. Biochemistry. 1984 Jul 31;23(16):3681–3687. doi: 10.1021/bi00311a017. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Schwarz E., Komaromy M., Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984 Oct 15;179(1):125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzlinger D., Carrasco N., Kaback H. R. Functional and immunochemical characterization of a mutant of Escherichia coli energy uncoupled for lactose transport. Biochemistry. 1985 Jan 1;24(1):221–229. doi: 10.1021/bi00322a032. [DOI] [PubMed] [Google Scholar]
- Herzlinger D., Viitanen P., Carrasco N., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984 Jul 31;23(16):3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Bibi E., Roepe P. D. Beta-galactoside transport in E. coli: a functional dissection of lac permease. Trends Biochem Sci. 1990 Aug;15(8):309–314. doi: 10.1016/0968-0004(90)90020-c. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Long S., McCune S., Walker G. C. Symbiotic loci of Rhizobium meliloti identified by random TnphoA mutagenesis. J Bacteriol. 1988 Sep;170(9):4257–4265. doi: 10.1128/jb.170.9.4257-4265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna E., Hardy D., Pastore J. C., Kaback H. R. Sequential truncation of the lactose permease over a three-amino acid sequence near the carboxyl terminus leads to progressive loss of activity and stability. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2969–2973. doi: 10.1073/pnas.88.8.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M. G., Rosenbusch J. P. Topography of lactose permease from Escherichia coli. J Biol Chem. 1988 Nov 5;263(31):15906–15914. [PubMed] [Google Scholar]
- Popot J. L., de Vitry C. On the microassembly of integral membrane proteins. Annu Rev Biophys Biophys Chem. 1990;19:369–403. doi: 10.1146/annurev.bb.19.060190.002101. [DOI] [PubMed] [Google Scholar]
- Rapoport T. A. Protein translocation across and integration into membranes. CRC Crit Rev Biochem. 1986;20(1):73–137. doi: 10.3109/10409238609115901. [DOI] [PubMed] [Google Scholar]
- Roepe P. D., Zbar R. I., Sarkar H. K., Kaback H. R. A five-residue sequence near the carboxyl terminus of the polytopic membrane protein lac permease is required for stability within the membrane. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3992–3996. doi: 10.1073/pnas.86.11.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckler R., Möröy T., Wright J. K., Overath P. Anti-peptide antibodies and proteases as structural probes for the lactose/H+ transporter of Escherichia coli: a loop around amino acid residue 130 faces the cytoplasmic side of the membrane. Biochemistry. 1986 May 6;25(9):2403–2409. doi: 10.1021/bi00357a016. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Seckler R., Wright J. K. Sidedness of native membrane vesicles of Escherichia coli and orientation of the reconstituted lactose: H+ carrier. Eur J Biochem. 1984 Jul 16;142(2):269–279. doi: 10.1111/j.1432-1033.1984.tb08281.x. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Maher P. A., Yaffe M. P. On the transfer of integral proteins into membranes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1960–1964. doi: 10.1073/pnas.84.7.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U., Bieseler B., Ehring R. Limited proteolysis of lactose permease from Escherichia coli. Eur J Biochem. 1986 Jul 15;158(2):423–428. doi: 10.1111/j.1432-1033.1986.tb09770.x. [DOI] [PubMed] [Google Scholar]
- Stochaj U., Ehring R. The N-terminal region of Escherichia coli lactose permease mediates membrane contact of the nascent polypeptide chain. Eur J Biochem. 1987 Mar 16;163(3):653–658. doi: 10.1111/j.1432-1033.1987.tb10914.x. [DOI] [PubMed] [Google Scholar]
- Stochaj U., Fritz H. J., Heibach C., Markgraf M., von Schaewen A., Sonnewald U., Ehring R. Truncated forms of Escherichia coli lactose permease: models for study of biosynthesis and membrane insertion. J Bacteriol. 1988 Jun;170(6):2639–2645. doi: 10.1128/jb.170.6.2639-2645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Bramhall J., Riede I., Wright J. K., Fürst M., Aichele G., Wilhelm U., Overath P. Lactose carrier protein of Escherichia coli. Structure and expression of plasmids carrying the Y gene of the lac operon. Eur J Biochem. 1980;108(1):223–231. doi: 10.1111/j.1432-1033.1980.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Trumble W. R., Viitanen P. V., Sarkar H. K., Poonian M. S., Kaback H. R. Site-directed mutagenesis of cys148 in the lac carrier protein of Escherichia coli. Biochem Biophys Res Commun. 1984 Mar 30;119(3):860–867. doi: 10.1016/0006-291x(84)90853-2. [DOI] [PubMed] [Google Scholar]
- Vogel H., Wright J. K., Jähnig F. The structure of the lactose permease derived from Raman spectroscopy and prediction methods. EMBO J. 1985 Dec 16;4(13A):3625–3631. doi: 10.1002/j.1460-2075.1985.tb04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T., Lodish H. F. Multiple mechanisms of protein insertion into and across membranes. Science. 1985 Oct 25;230(4724):400–407. doi: 10.1126/science.4048938. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. Trans-membrane translocation of proteins. The direct transfer model. Eur J Biochem. 1979 Jun;97(1):175–181. doi: 10.1111/j.1432-1033.1979.tb13100.x. [DOI] [PubMed] [Google Scholar]