Abstract
We investigated the performance characteristics of prostate-specific antigen (PSA) and PSA density (PSAD) in Chinese men. All Chinese men who underwent transrectal ultrasound-guided prostate biopsy (TRUS-PB) from year 2000 to 2013 were included. The receiver operating characteristic (ROC) curves for both PSA and PSAD were analyzed. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at different cut-off levels were calculated. A total of 2606 Chinese men were included. For the ROC, the area under curve was 0.770 for PSA (P < 0.001) and 0.823 for PSAD (P < 0.001). PSA of 4.5 ng ml−1 had sensitivity of 94.4%, specificity of 14.1%, PPV of 29.5%, and NPV of 86.9%; PSAD of 0.12 ng ml−1 cc−1 had sensitivity of 94.5%, specificity of 26.6%, PPV of 32.8%, and NPV of 92.7%. On multivariate logistic regression analyses, PSA cut-off at 4.5 ng ml−1 (OR 1.61, 95% CI 1.05–2.45, P = 0.029) and PSAD cut-off at 0.12 ng ml−1 cc−1 (OR 6.22, 95% CI 4.20–9.22, P < 0.001) were significant predictors for prostate cancer detection on TRUS-PB. In conclusion, the performances of PSA and PSAD at different cut-off levels in Chinese men were very different from those in Caucasians. PSA of 4.5 ng ml−1 and PSAD of 0.12 ng ml−1 cc−1 had near 95% sensitivity and were significant predictors of prostate cancer detection in Chinese men.
Keywords: Chinese, prostate cancer, transrectal ultrasound-guided prostate biopsy, prostate-specific antigen, prostate-specific antigen density
INTRODUCTION
Prostate cancer is the most common type of neoplasm in Europe1 and the second most common cause of cancer death in men.2 The use of prostate-specific antigen (PSA) and PSA density (PSAD) has gained a lot of interest in early prostate cancer diagnosis over the past two decades.3,4,5,6,7 In our locality, a PSA cut-off value of 4.0 ng ml−1 and a PSAD cut-off value of 0.15 ng ml−1 cc−1 are often used to determine which patient to consider transrectal ultrasound-guided prostate biopsy (TRUS-PB). However, the previous reports were largely based on Caucasians, and whether these results are applicable to Chinese men remained largely unknown. Serum PSA levels differ between different races,8,9 and such difference was also observed even among the different ethnicities within Asia.10 There is a need for Chinese-specific data to streamline the utility of PSA and PSAD in Chinese men for considering TRUS-PB. We conducted this study to investigate the performances of PSA and PSAD at different cut-off levels in Chinese men by analyzing Chinese-specific data retrieved from our TRUS-PB database. We further discussed the differences in the performances of PSA and PSAD between Chinese and Caucasians.
METHODS
Data from all Chinese men who underwent TRUS-PB from year 2000 to 2013 was retrieved from our database. Baseline characteristics including age, digital rectal examination (DRE) finding, prostate volume, the number of cores taken during biopsy, PSA level, overall cancer detection rate, and the Gleason score pattern were reviewed. Prostate volume was estimated by transrectal ultrasound using a prolate ellipsoid volume calculation of height times width times length times 0.52. PSAD was calculated by dividing serum PSA concentration by the prostate volume.
We aimed to investigate the performances of PSA and PSAD for prostate cancer detection on TRUS-PB in the Chinese population. Receiver operating characteristic (ROC) curves of PSA and PSAD for prostate cancer detection on TRUS-PB were analyzed. The area under curves (AUCs) for both PSA and PSAD were calculated. Statistical significance was determined by a P value of < 0.05. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) at different PSA cut-off levels (2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5, 5.5, 6.0, 6.5, and 7.0 ng ml−1) and PSAD cut-off levels (0.10, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19, and 0.20 ng ml−1 cc−1) were calculated. The PSA and PSAD cut-off levels with 80%, 90% and 95% sensitivity were determined. Further logistic regression analyses for prostate cancer detection on TRUS-PB were performed to determine the significance of the PSA and PSAD cut-off levels at 95% sensitivity. In model 1, only PSA was included; in model 2, PSA and PSAD were included; in model 3, PSA, PSAD and presence of abnormal DRE were included; and in model 4, PSA, PSAD, presence of abnormal DRE and age were included in the logistic regression analyses. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY).
RESULTS
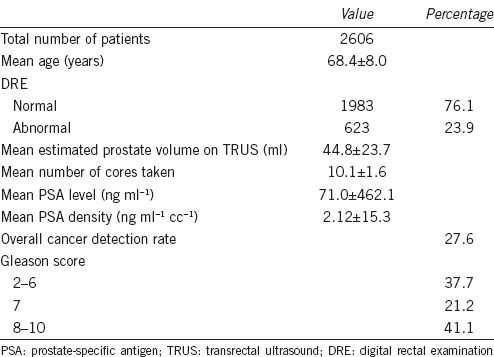
Data from a total of 2606 Chinese men who underwent TRUS-PB from year 2000 to 2013 was retrieved. The mean age of the cohort was 68.4 ± 8.0 years. Among the patients who underwent TRUS-PB, 23.9% of them (623 patients) had abnormal digital rectal examination finding. The mean estimated prostate volume upon transrectal ultrasound was 44.8 ± 23.7 ml. The mean number of cores taken during biopsy was 10.1 ± 1.6. The mean PSA level was 71.0 ± 462.1 ng ml−1. The mean PSAD was 2.12 ± 15.3 ng ml−1 cc−1. The overall cancer detection rate was 27.6%. For patients with confirmed prostate cancer, 37.7% of them had Gleason score of 2–6, 21.2% had Gleason score of 7 and 41.1% had Gleason score of 8–10 (Table 1).
Table 1.
Baseline characteristics of the cohort
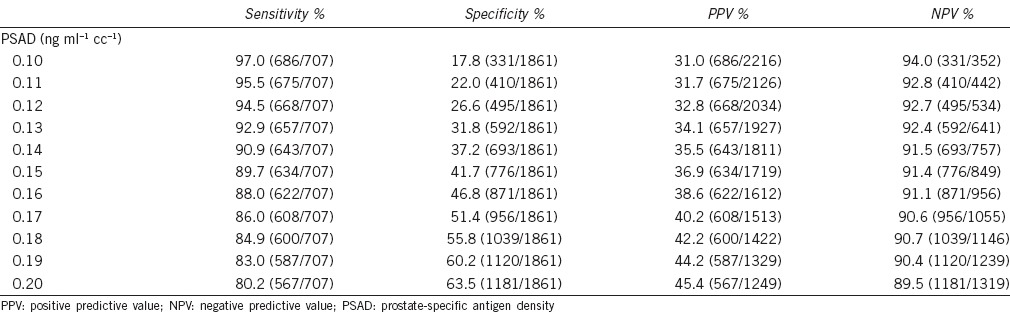
The ROC curves of PSA and PSAD for prostate cancer detection were analyzed (Figure 1). The AUC was 0.770 for PSA (P < 0.001) and 0.823 for PSAD (P < 0.001). For the different PSA cut-off levels (Table 2), a PSA level of 4.5 ng ml−1 had a sensitivity of 94.4%, specificity of 14.1%, PPV of 29.5% and NPV of 86.9%; a PSA level of 5.5 ng ml−1 had a sensitivity of 89.3%, specificity of 31.6%, PPV of 33.2%, and NPV of 88.6%; a PSA level of 7.0 ng ml−1 had a sensitivity of 79.4%, specificity of 50.7%, PPV of 38.0%, and NPV of 86.6%. For the different PSAD cut-off levels (Table 3), a PSAD of 0.12 ng ml−1 cc−1 had a sensitivity of 94.5%, specificity of 26.6%, PPV of 32.8%, and NPV of 92.7%; a PSAD of 0.15 ng ml−1 cc−1 had a sensitivity of 89.7%, specificity of 41.7%, PPV of 36.9%, and NPV of 91.4%; a PSAD of 0.20 ng ml−1 cc−1 had a sensitivity of 80.2%, specificity of 63.5%, PPV of 45.4%, and NPV of 89.5%.
Figure 1.
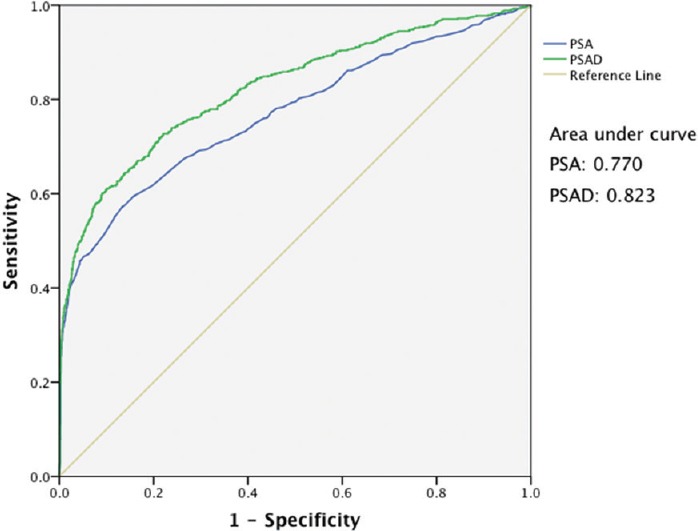
Receiver operating characteristic curves of PSA and PSAD for prostate cancer detection. PSA: prostate-specific antigen; PSAD: prostate-specific antigen density.
Table 2.
Sensitivity, specificity, PPV, and NPV at different PSA cut-off levels
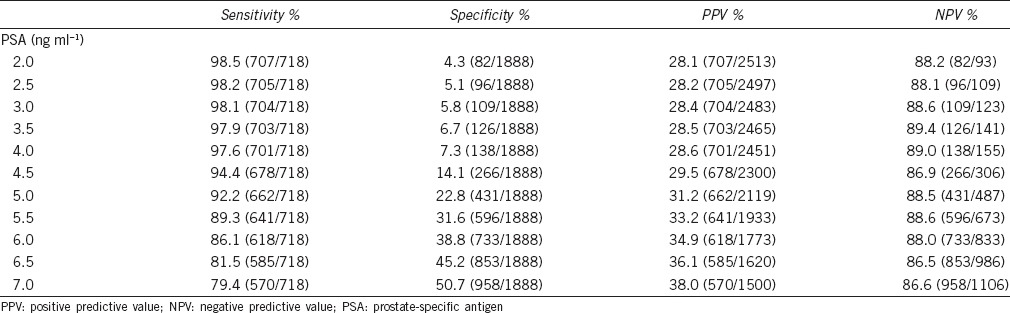
Table 3.
Sensitivity, specificity, PPV, and NPV at different PSAD cut-off levels
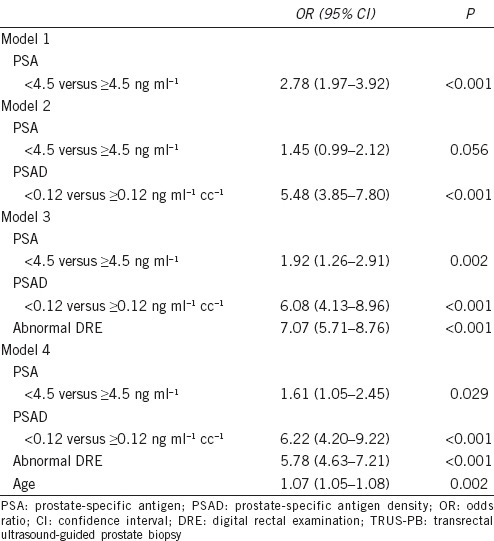
Further logistic regression analyses for prostate cancer detection on TRUS-PB were performed to determine the significance of PSA cut-off at 4.5 ng ml−1 and PSAD cut-off at 0.12 ng ml−1 cc−1 (Table 4). In model 1, PSA was a significant predictor of prostate cancer detection (OR 2.78, 95% CI 1.97–3.92, P < 0.001). In model 2, PSAD was a significant predictor of prostate cancer detection (OR 5.48, 95% CI 3.85–7.80, P < 0.001), while PSA also appeared to increase the risk of prostate detection (OR 1.45, 95% CI 0.99–2.12, P = 0.056). In model 3, PSA (OR 1.92, 95% CI 1.26–2.91, P = 0.002), PSAD (OR 6.08, 95% CI 4.13–8.96, P < 0.001) and presence of abnormal DRE (OR 7.07, 95% CI 5.71–8.76, P < 0.001) were significant predictors of prostate cancer detection. In model 4, PSA (OR 1.61, 95% CI 1.05–2.45, P = 0.029), PSAD (OR 6.22, 95% CI 4.20–9.22, P < 0.001), presence of abnormal DRE (OR 5.78, 95% CI 4.63–7.21, P < 0.001) and age (OR 1.07, 95% CI 1.05–1.08, P = 0.002) were significant predictors of prostate cancer detection.
Table 4.
Logistic regression analyses on prostate cancer detection upon TRUS-PB
DISCUSSION
The investigation on tissue-specific antibodies in the human prostate first started in 1969 by Ablin et al.11 Nadji et al. later characterized PSA as a potential marker for prostatic neoplasms.12 The discovery of PSA has led revolutionary changes in early prostate cancer diagnosis. Catalona et al. conducted a number of important studies on the utility of PSA and PSAD for early prostate cancer detection,3,5,13 and the use of PSA and PSAD has become widely accepted. However, the previous reports were largely based on Caucasians. Whether the results can be applied to Asians remained as an area of controversy. Our TRUS-PB database contained data of all patients who underwent TRUS-PB for suspected prostate cancer in our center from year 2000 onwards. While the decision on TRUS-PB was often made in an individualized approach, the common indications of TRUS-PB in our database were PSA level of >4.0 ng ml−1 and any presence of abnormal DRE finding. We conducted this study to provide Chinese-specific data for investigating the performances of PSA and PSAD in Chinese men.
A total of 2606 Chinese patients were included in our study. PSA and PSAD had good performances in prostate cancer detection with an AUC of 0.770 for PSA (P < 0.001) and 0.823 for PSAD (P < 0.001). Concerning the different PSA cut-off levels, a PSA level of 4.5 ng ml−1 had a sensitivity of 94.4%, specificity of 14.1%, PPV of 29.5% and NPV of 86.9%, and a PSA level of 5.5 ng ml−1 had a sensitivity of 89.3%, specificity of 31.6%, PPV of 33.2% and NPV of 88.6%. In the study by Catalona et al.,5 a PSA cut-off value of 4.0 ng ml−1 had a sensitivity of 78.6% and specificity of 45.8%. With a sensitivity of around 80%, this corresponds to a PSA cut-off value of 7.0 ng ml−1 in our study on Chinese men with a sensitivity of 79.4% and specificity of 50.7%. In the study by Sun et al.14 at a PSA level of 4.0 ng ml−1, the sensitivity was 61.3%–71.3% compared to 97.6% in our study, while the specificity was 85.2%–97.7% compared to 7.3% in our study. The optimal PSA level for considering TRUS-PB in the Chinese population appeared to be very different from that in the Caucasians. The reason why there was such a major difference could not be simply explained by a lower prostate cancer incidence in Chinese men. As the results were based on specified PSA levels, unless there were confounding factors which were more prevalent in Chinese men that might cause falsely elevated PSA, otherwise the number of benign and cancer cases should be proportionate. On multivariate logistic regression analyses, PSA cut-off of 4.5 ng ml−1 was a significant predictor of prostate cancer detection (OR 1.61, 95% CI 1.05–2.45, P = 0.029) after adjusting for PSAD, presence of abnormal DRE and age. While further studies are needed to investigate for potential differences in the natural history and aggressiveness of prostate cancer in the Chinese population, a PSA level of 4.5 ng ml−1 appeared to be an appropriate cut-off level for Chinese men to consider TRUS-PB with a reasonable sensitivity of 94.4%. In our cohort, with reference to a PSA level of 4.5 ng ml−1, TRUS-PB for all men with PSA of more than 4 ng ml−1 will lead to an increase in 151 biopsies (+6.57%), while detecting 23 more prostate cancers (+3.39%).
Being first proposed by Benson et al.7 to distinguish between benign prostatic hypertrophy and prostate cancer, a PSAD cut-off of 0.15 ng ml−1 cc−1 was recommended in earlier studies.15 However, Lujan et al.16 showed that when a PSAD cut-off of 0.15 ng ml−1 cc−1 was used, up to 30.6% of prostate cancer would be missed and suggested that a lower PSAD cut-off should be used. In our cohort, a PSAD of 0.12 ng ml−1 cc−1 had a sensitivity of 94.5%, specificity of 26.6%, PPV of 32.8%, and NPV of 92.7%, and a PSAD of 0.15 ng ml−1 cc−1 had a sensitivity of 89.7%, specificity of 41.7%, PPV of 36.9%, and NPV of 91.4%. In the study by Catalona et al.5 a PSAD of 0.10 ng ml−1 cc−1 had a sensitivity of 78.8% and a specificity of 46.9%. With a sensitivity of around 80%, this corresponds to a PSAD cut-off value of 0.20 ng ml−1 cc−1 in our study on Chinese men with a sensitivity of 80.2% and specificity of 63.5%. One postulation is the physiological difference between Chinese and Caucasians, resulting in a difference in the size of the prostate gland; a smaller prostate volume in Chinese men may result in an increase in PSAD, although further studies are needed to evaluate this postulation. On multivariate logistic regression analyses, PSAD cut-off of 0.12 ng ml−1 cc−1 was a significant predictor of prostate cancer detection (OR 6.22, 95% CI 4.20–9.22, P < 0.001) after adjusting for PSA, presence of abnormal DRE and age. PSAD of 0.12 ng ml−1 cc−1 appeared to be an appropriate cut-off level for Chinese men to consider TRUS-PB with a reasonable sensitivity of 94.5%. With reference to a PSAD of 0.12 ng ml−1 cc−1, TRUS-PB only for men with PSAD of more than 0.15 ng ml−1 cc−1 would miss a total of 34 prostate cancers (5.09%), while sparing 315 biopsies (15.49%) in our cohort.
While PSA is organ-specific but not tumor-specific, its serum level may be altered by various confounding conditions including urinary tract infection, prostatitis, urinary retention, etc. Similarly, PSAD depends on the prostate volume size, which may in turn be proportionate to one's body weight, body height, and body mass index. Unfortunately, we were unable to retrieve such parameters from our database. The major limitation in our study would be the lack of evaluation of potential confounding factors, which may affect PSA and PSAD.
To our knowledge, this is the largest study investigating the performances of PSA and PSAD at different cut-off levels in the Chinese population. Our results provided important information in deciding when to perform TRUS-PB in Chinese men. While there is a lack of data concerning the natural history and aggressiveness of prostate cancer in the Chinese population, PSA of 4.5 ng ml−1 and PSAD of 0.12 ng ml−1 cc−1 appeared to be appropriate levels for Chinese men to consider TRUS-PB. This recommendation was based on a near 95% sensitivity for both PSA (94.4% sensitivity) and PSAD (94.5% sensitivity). While a PSA cut-off value of 4.0 ng ml−1 and a PSAD cut-off value of 0.15 ng ml−1 cc−1 are often used to determine which patient to consider TRUS-PB in our locality, further studies are necessary to investigate the effect of increasing the PSA cut-off value from 4.0 to 4.5 ng ml−1, and decreasing the PSAD cut-off value from 0.15 to 0.12 ng ml−1 cc−1. Balance has to be made to avoid excessive unnecessary biopsies while detecting most if not all of the clinically significant prostate cancers.
AUTHOR CONTRIBUTIONS
JYT and MKY carried out the study. SKY, JHT, WKM, KLH and MKY participated in the study design. JYT, SKY, CKW, BSH and ATN collected the data. JYT, JHT, CKW, BSH, ATN, WKM, and KLH coordinated the study. JYT, SKY and CKW performed statistical analyses. JYT and MKY drafted the manuscript. JHT, WKM, KLH and MKY supervised the study.
COMPETING INTERESTS
All authors declare no competing interest.
REFERENCES
- 1.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–8. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Catalona WJ, Hudson MA, Scardino PT, Richie JP, Ahmann FR, et al. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2037–42. doi: 10.1016/s0022-5347(17)32300-5. [DOI] [PubMed] [Google Scholar]
- 4.Andriole GL, Catalona WJ. Using PSA to screen for prostate cancer. The Washington University experience. Urol Clin North Am. 1993;20:647–51. [PubMed] [Google Scholar]
- 5.Catalona WJ, Richie JP, deKernion JB, Ahmann FR, Ratliff TL, et al. Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves. J Urol. 1994;152:2031–6. doi: 10.1016/s0022-5347(17)32299-1. [DOI] [PubMed] [Google Scholar]
- 6.Benson MC, Whang IS, Olsson CA, McMahon DJ, Cooner WH. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147:817–21. doi: 10.1016/s0022-5347(17)37394-9. [DOI] [PubMed] [Google Scholar]
- 7.Benson MC, Whang IS, Pantuck A, Ring K, Kaplan SA, et al. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–6. doi: 10.1016/s0022-5347(17)37393-7. [DOI] [PubMed] [Google Scholar]
- 8.Oesterling JE, Kumamoto Y, Tsukamoto T, Girman CJ, Guess HA, et al. Serum prostate-specific antigen in a community-based population of healthy Japanese men: lower values than for similarly aged white men. Br J Urol. 1995;75:347–53. doi: 10.1111/j.1464-410x.1995.tb07347.x. [DOI] [PubMed] [Google Scholar]
- 9.DeAntoni EP, Crawford ED, Oesterling JE, Ross CA, Berger ER, et al. Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology. 1996;48:234–9. doi: 10.1016/s0090-4295(96)00091-x. [DOI] [PubMed] [Google Scholar]
- 10.Mochtar CA, Andika RS. The value of prostate-specific antigen in Asia. Ther Adv Urol. 2010;2:77–83. doi: 10.1177/1756287210370329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ablin RJ, Pfeiffer L, Gonder MJ, Soanes WA. Precipitating antibody in the sera of patients treated cryosurgically for carcinoma of the prostate. Exp Med Surg. 1969;27:406–10. [PubMed] [Google Scholar]
- 12.Nadji M, Tabei SZ, Castro A, Chu TM, Murphy GP, et al. Prostatic-specific antigen: an immunohistologic marker for prostatic neoplasms. Cancer. 1981;48:1229–32. doi: 10.1002/1097-0142(19810901)48:5<1229::aid-cncr2820480529>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Moul JW, Hotaling JM, Rampersaud E, Dahm P, et al. Prostate-specific antigen (PSA) and PSA velocity for prostate cancer detection in men aged<50 years. BJU Int. 2007;99:753–7. doi: 10.1111/j.1464-410X.2006.06682.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawachi MH, Bahnson RR, Barry M, Busby JE, Carroll PR, et al. NCCN clinical practice guidelines in oncology: prostate cancer early detection. J Natl Compr Canc Netw. 2010;8:240–62. doi: 10.6004/jnccn.2010.0016. [DOI] [PubMed] [Google Scholar]
- 16.Lujan M, Paez A, Llanes L, Miravalles E, Berenguer A. Prostate specific antigen density. Is there a role for this parameter when screening for prostate cancer? Prostate Cancer Prostatic Dis. 2001;4:146–9. doi: 10.1038/sj.pcan.4500509. [DOI] [PubMed] [Google Scholar]


