Abstract
Male circumcision (MC) is reported to reduce human papillomavirus (HPV) prevalence in men. However, the efficacy remains imprecise. The aim of this study was to conduct a systematic review and meta-analysis to assess the relationship between MC and genital HPV infection and genital warts. PUBMED, EMBASE, and Web of Science were searched from inception to March 22, 2015. We identified 30 papers, including a total of 12149 circumcised and 12252 uncircumcised men who were evaluated for the association of circumcision with genital HPV or genital warts. Compared with men who were not circumcised, circumcised men may have had significantly reduced odds of genital HPV prevalence (odds ratio [OR]: 0.68; 95% confidence interval [95% CI]: 0.56–0.82). There was no significant association between MC and genital HPV acquisition of new infections (OR: 0.99; 95% CI: 0.62–1.60), genital HPV clearance (OR: 1.38; 95% CI: 0.96–1.97), and prevalence of genital warts (OR: 1.17; 95% CI: 0.63–2.17). This meta-analysis suggests that circumcision reduces the prevalence of genital HPV infections. However, no clear evidence was found that circumcision was associated with decreased HPV acquisition, increased HPV clearance, or decreased the prevalence of genital warts. More studies are required to evaluate adequately the effect of MC on the acquisition and clearance of HPV infections and prevalence of genital warts.
Keywords: genital warts, human papillomavirus, male circumcision, prevalence
INTRODUCTION
Human papillomavirus (HPV) infection is common and can cause genital warts, invasive cervical cancer in women, and penile and anal cancer in men.1 Cervical cancer is the second most common cancer among women worldwide. Up to 99% of cervical cancers are associated with infection of oncogenic HPV genotypes.2 Therefore, finding interventions that can reduce the risk of HPV infection may have a protective impact on HPV-related diseases, both in men and women.
Male circumcision (MC) is a simple, rapid operation; however, it remains unclear whether it has a protective effect against genital HPV infection. A systematic review of studies conducted by Van Howe et al.3 found no evidence of an association between MC and genital HPV infections. However, two meta-analyses4,5 and several studies6,7,8 found that MC could help reduce HPV infections. Recently, a randomized controlled trial (RCT) with a large patient population demonstrated that MC was not associated with the acquisition and clearance of genital HPV infection.9 Based on the discrepancy between these findings, there is an urgent need to perform an updated meta-analysis on this topic. In the present systematic review and meta-analysis, we added five recent papers1,9,10,11,12 (including 4103 circumcised and 5916 uncircumcised men) to provide a comprehensive survey to address this controversy.
MATERIALS AND METHODS
Data sources and search strategy
PUBMED, EMBASE, and Web of Science were searched from inception until March 22, 2015. The search was performed using the following terms: “circumcision, male,” “HPV,” “papillomaviridae,” “genital diseases, male,” “genital warts,” and “condylomata acuminata.” We also examined the reference lists of all relevant papers. The criteria for eligibility were as follows: (1) evaluate the potential association between MC and HPV infection or MC and genital warts; (2) give a precise description about how MC status was ascertained; and (3) reporting of HPV sampling techniques, sampling sites, and details of the different polymerase chain reaction assays used for HPV DNA detection. We excluded studies if they (1) did not report any of the outcomes of interest, (2) enrolled men who were HIV-positive, (3) had interventions that did not include MC, and (4) contained data that could not be extracted in an appropriate format and any attempts to obtain the relevant data from the authors had failed.
Data extraction and quality assessment
We systematically assessed the quality of all the studies included. Data were extracted independently by two authors (YP-Z and ZW-J) and disagreements were discussed to reach consensus between the two authors or consultation with a third reviewer. We classified as separate studies if more than one outcome (HPV prevalence, HPV acquisition, HPV clearance, and genital warts) was evaluated in one paper. The following data were extracted from the studies: (1) publication details, including first author and year of publication; (2) study design; (3) characteristics of the studied population, including sample size, age range, study population, and country in which the study was conducted; (4) method of ascertaining MC status (self-reported or physical examination); (5) the proportion of circumcised and uncircumcised men; and (6) positive events among circumcised and uncircumcised men.
Statistical analysis
Review Manager version 5.2 software (Cochrane Collaborative, Oxford, UK) was used to integrate all of the individual outcomes. Heterogeneity among the studies was measured by a random-effects model using the χ2 test, P values, and I2 statistics. P < 0.05 was considered statistically significant. Publication bias was estimated by the funnel plot and Begg's rank regression test using STATA version 12.0 software (Stata Corporation, College Station, TX, USA).13 P < 0.1 was considered statistically significant publication bias.
RESULTS
Data retrieval
A total of 5082 citations were identified after the initial database search. After reading the titles and abstracts, 78 papers were retrieved. Fifty-four of these papers were excluded after full-text review. In addition, seven papers were retrieved from the reference lists of all relevant papers.7,8,14,15,16,17 Thus, 30 papers (39 studies) involving a total of 12 149 circumcised and 12 252 uncircumcised men were finally included in this meta-analysis (Figure 1).
Figure 1.
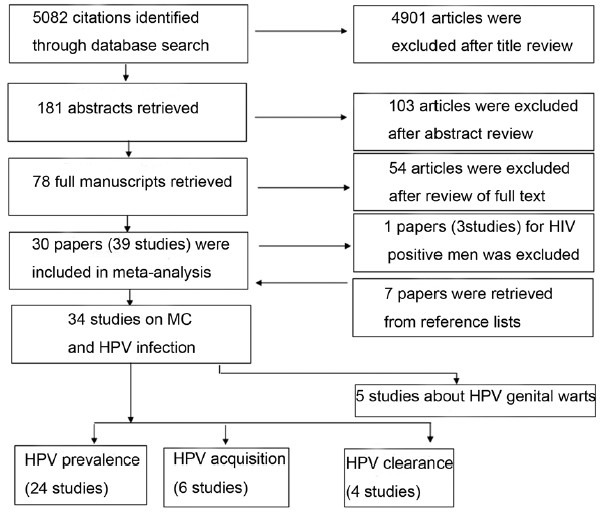
Flowchart of the studies identified in the meta-analysis. MC: male circumcision; HPV: human papillomavirus.
Study characteristics
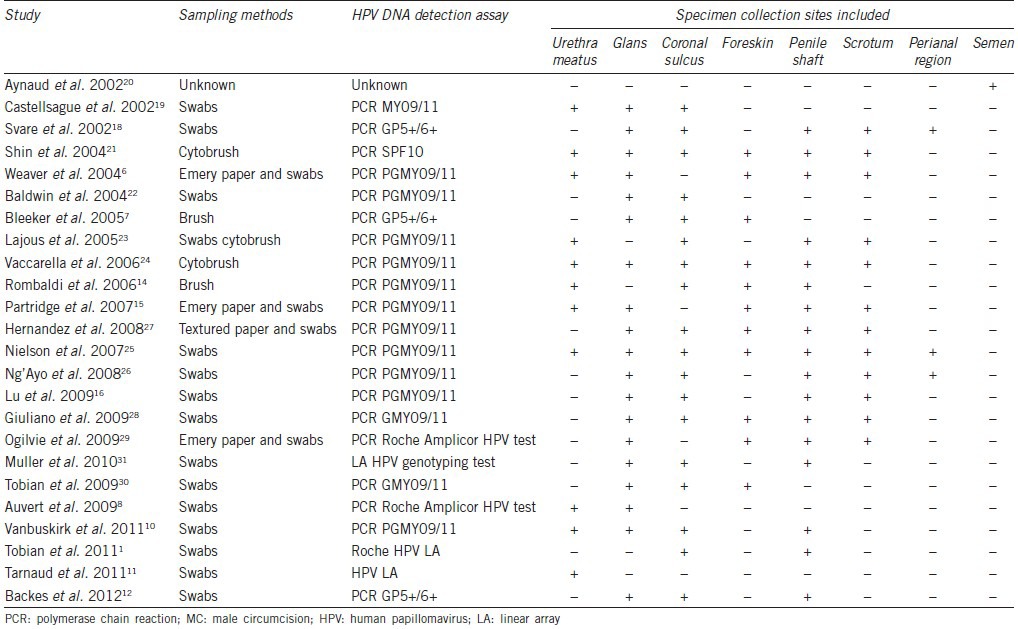
The characteristics of patients enrolled in our meta-analysis are summarized in Tables 1–3. Twenty-four studies evaluated the association between MC and HPV prevalence;1,6,7,8,10,11,12,14,15,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31 six evaluated the association between MC and HPV acquisition;9,10,15,16,23,32 four evaluated the association between MC and HPV clearance;9,16,23,32 and five evaluated the association between MC and genital warts.7,17,33,34,35 HPV specimens were collected from different regions including glans, penile shaft, coronal sulcus, scrotum, foreskin, urethra, and perianal region. All studies measured HPV DNA by polymerase chain reaction. The sampling method and specimen collection sites of studies about MC and HPV prevalence are summarized in Table 4.
Table 1.
Summary of studies reporting on the association between MC and HPV prevalence in men
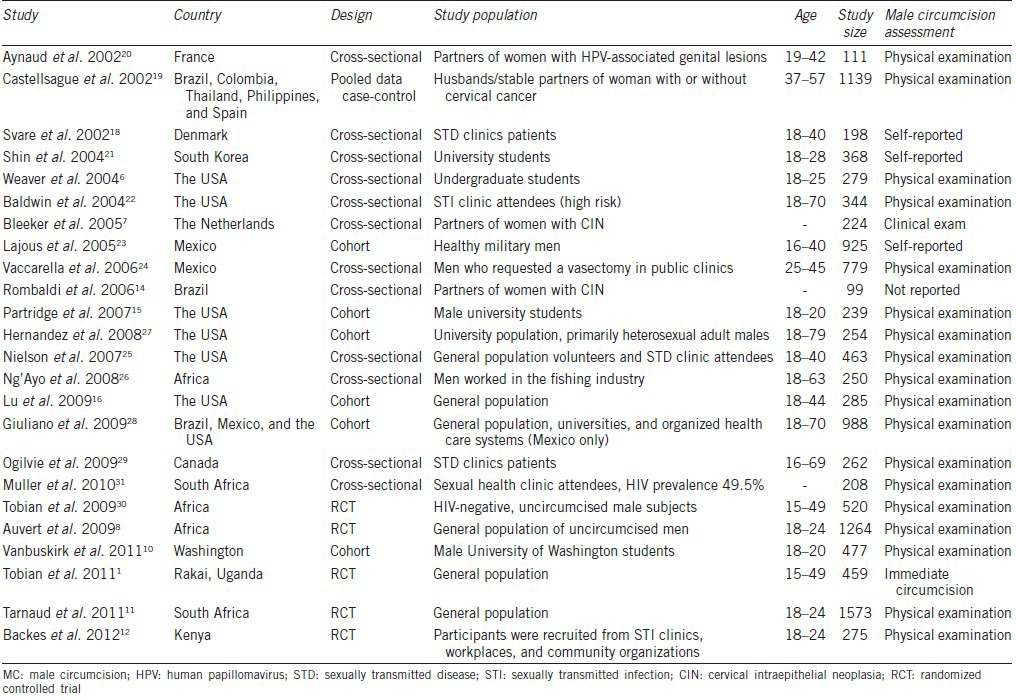
Table 3.
Summary of studies reporting on the association between MC and genital warts in men
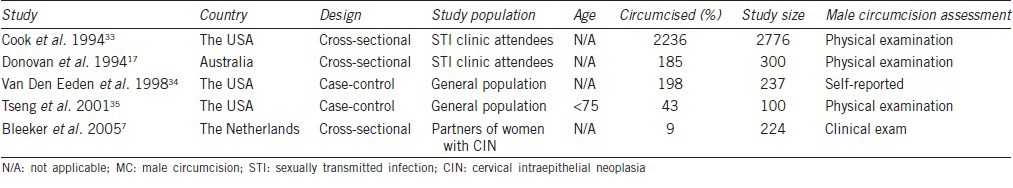
Table 4.
Summary of studies reporting on the association between MC and genital HPV Prevalence in men by sampling method and specimen collection sites
MC and HPV prevalence
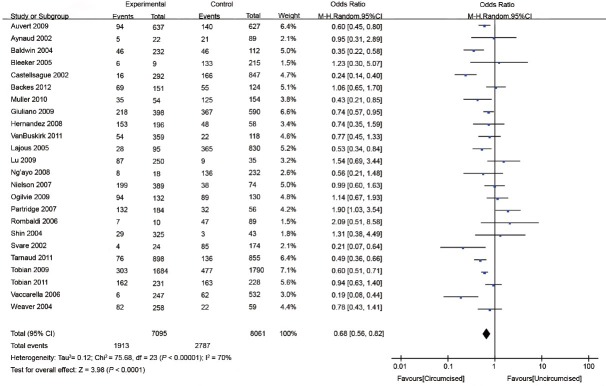
Twenty-four studies evaluated the association between MC and HPV prevalence1,6,7,8,10,11,12,14,15,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31 (Table 1). The random-effects model was applied to calculate the pooled odds ratio (OR) and its 95% CI. HPV-positive rates among circumcised and uncircumcised men ranged from 2.4% to 78.0% and 7.0% to 81.2%, respectively. HPV prevalence was lower in circumcised than in uncircumcised men in 10 of the 24 studies8,11,18,19,22,23,24,27,28,31 but higher in one study.15 In addition, 13 studies showed MC had no effect on HPV prevalence.1,6,7,10,12,14,16,20,21,25,26,29,30 In general, MC significantly reduced the odds of genital HPV prevalence (OR: 0.68; 95% CI: 0.56–0.82), but substantial between-study heterogeneity was observed (I2 = 70%) (Figure 2).
Figure 2.
Forest plot of the studies assessing the association between MC and HPV prevalence. MC: male circumcision; HPV: human papillomavirus.
MC and HPV acquisition
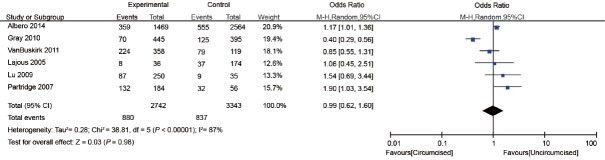
Five cohort studies and one RCT examined the effect of MC on genital HPV acquisition9,10,15,16,23,32 (Table 2). HPV acquisition was defined as follows: a new infection identified in men who were initially negative for any HPV and who acquired one or two or more new HPV infections during the next follow-up or men who were initially positive for a specific HPV genotype but acquired one or more new HPV genotypes during the next follow-up.32 The proportion of men who were circumcised ranged from 17.1% to 87.7%. The interval of follow-up ranged from 12 to 24 months. The proportion of HPV acquisition among circumcised and uncircumcised men ranged from 15.7% to 62.6% and 21.3% to 66.2%, respectively. In general, there was no significant association between MC and genital HPV acquisition (OR: 0.99; 95% CI: 0.62–1.60). Substantial heterogeneity was observed among the studies (I2 = 87%) (Figure 3).
Table 2.
Summary of studies reporting on the association between MC and HPV acquisition and HPV clearance in men
Figure 3.
Forest plot of the studies assessing the association between MC and HPV acquisition. MC: male circumcision; HPV: human papillomavirus.
MC and HPV clearance
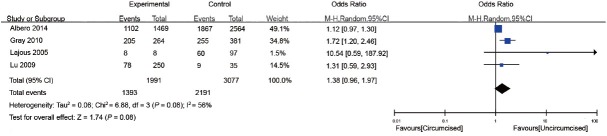
Three cohort studies and one RCT examined the effect of MC on genital HPV clearance9,17,23,32 (Table 2). Clearance was defined as the proportion of men with preexisting HPV, who were negative for that genotype at a subsequent sequential study visit.32 The study population ranged from 105 to 4033. The proportion of men who were circumcised ranged from 7.6% to 87.7%. The interval of follow-up ranged from 12 to 24 months. The proportion of HPV clearance among circumcised and uncircumcised men ranged from 31.2% to 100% and 25.7% to 72.8%, respectively. In general, there was no significant association between MC and genital HPV clearance (OR: 1.38; 95% CI: 0.96–1.97). Substantial heterogeneity was observed among the studies (I2 = 56%) (Figure 4).
Figure 4.
Forest plot of the studies assessing the association between MC and HPV clearance. MC: male circumcision; HPV: human papillomavirus.
MC and genital warts
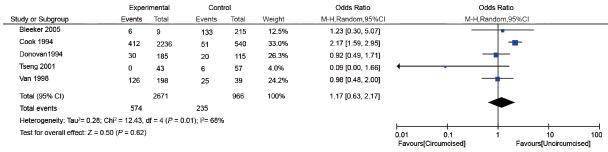
Three cross-sectional two case–control studies examined the effect of MC on genital warts7,17,33,34,35 (Table 3). The study population was men attending sexually transmitted disease (STD) clinics, general population, or partners of women with cervical intraepithelial neoplasia. Three studies assessed current warts7,33,34 and two assessed historic warts.17,35 The proportion of men who were circumcised ranged from 4.0% to 83.5%. In general, there was no significant association between MC and genital warts (OR: 1.17; 95% CI: 0.63-2.17). Substantial heterogeneity was observed among the studies ((I2 = 68%) (Figure 5).
Figure 5.
Forest plot of the studies assessing the association between MC and genital warts. MC: male circumcision.
Publication bias
Begg's funnel plot and Egger's test were performed to assess the publication bias of studies on HPV prevalence. The funnel plots did not reveal any evidence of obvious asymmetry among the 24 studies included (Figure 6). Egger's test was used to provide statistical evidence of funnel plot symmetry. The results still did not suggest any evidence of publication bias (P = 0.271).
Figure 6.
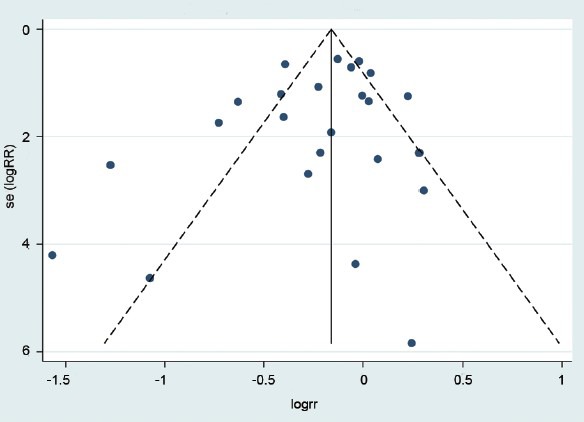
Publication bias in studies on HPV prevalence. HPV: human papillomavirus.
DISCUSSION
The existing evidence, which includes data from case–control, cross-sectional and cohort studies, and RCTs, was analyzed in our meta-analysis to ascertain pooled estimates of the relationship between MC and genital HPV prevalence. Overall, our results revealed that MC reduced the prevalence of genital HPV infection in an average of 32% of men. This means that there is a need to perform three circumcisions to prevent one infection. While a series of studies and our meta-analysis demonstrated an inverse association between MC and HPV prevalence in men, one meta-analysis conducted in September 2006 revealed that there was no significant association between circumcision status and HPV prevalence.3 Because HPV is a topical infection in the skin and mucosa, one possible explanation for the discrepancy may be the varied specimen collection sites in the different studies.
HPV detection varies by anatomical site and evaluating HPV only on the coronal sulcus and urethra might bias the estimated protective efficacy of MC.1 More frequent HPV infection was detected on the coronal sulcus than the shaft in uncircumcised men, suggesting that the moist subpreputial space might provide a more favorable environment for HPV infection.10 When interpreting the effect of different sampling methods on our results, we should note that the effectiveness of sampling methods at different anatomical sites and the sampling method itself may affect the efficacy of the sampling methods. However, it is impossible to make a comment on those effects; thus, the method used to sample HPV may be a source of heterogeneity.
Only a few studies assessed the association between MC and HPV acquisition or clearance. The present meta-analysis suggests no evidence of an effect of decreased HPV acquisition (OR: 0.99; 95% CI: 0.62–1.60)9,10,15,16,23,32 and increased HPV clearance (OR: 1.38; 95% CI: 0.96–1.97).8,16,23,32 However, one RCT conducted in Uganda32 and a cohort study in the USA9 found that MC reduced acquisition of HPV infection. On the contrary, one recently published cohort study9 which enrolled 4033 healthy men and three observational prospective studies15,16,23 suggested that MC was not associated with an overall reduction of genital HPV acquisition, which was consistent with our findings. Although limited data prevented us from performing a subgroup analysis according to sample sites, only a few studies used specimens collected from the scrotum, perianal area, and semen, which might have resulted in selection bias in our meta-analysis. In addition, our results suggested that there was no evidence of MC increasing HPV clearance (OR: 1.38; 95% CI: 0.96–1.97).9,16,23,32 When interpreting the results of our meta-analysis, we must note that HPV has a high rate of spontaneous clearance, and we suggest that the sampling sites also played an important role in the final results. One RCT suggested that MC increased HPV clearance when sampled on the coronal sulcus.32 However, when sampled on the scrotum and penile shaft, Hernandez et al.27 found that HPV clearance was not affected by MC. In addition, when interpreting the differences in findings between reduced prevalence of HPV after MC and no reduction in acquisition or increased clearance after MC, we suggest that this might have been because the study population for HPV acquisition and clearance was smaller than for HPV prevalence. Therefore, the results need to be validated using a larger number of studies.
Our meta-analysis suggested that there was no significant association between MC and genital warts (OR: 1.17; 95% CI: 0.63–2.17).7,17,33,34,35 One study suggested that genital warts were more likely at distal lesions on the penis among uncircumcised men.33 Another study suggested that uncircumcised men were more likely to present with extensive warts.36 In contrast, one prospective cohort study conducted in Kenya37 suggested that the risk of genital warts was not affected by the presence of a foreskin. One plausible explanation for our results may be that genital wart lesions usually appear on the penile shaft; a site that is not often affected by MC.38 As we only found five papers suitable for our meta-analysis,7,17,33,34,35 additional studies are necessary to investigate the relationship between MC and genital warts.
It is plausible that MC might reduce genital HPV infection; however, the mechanism is unclear.19 In uncircumcised men, the inner preputial mucosa is exposed to vaginal and cervical secretions as the foreskin is retracted during intercourse.27 The penile shaft and surface of the foreskin are covered by a keratinized stratified squamous epithelium that could provide a protective effect against HPV infection. However, the foreskin mucosa is not keratinized and might be more susceptible to HPV infections.27 In addition, the moist environment of the foreskin may provide a favorable condition for HPV survival.27 It has been proposed that keratinization of the circumcision scar may also reduce the chance of HPV infection.19 Therefore, MC may reduce the chance of HPV access to epidermal basal cells.
Our meta-analysis included five additional papers1,9,10,11,12 that were not included in the most recent systematic review about MC and genital HPV infection. At the same time, we enrolled an additional 4103 circumcised and 5916 uncircumcised men to provide a comprehensive survey about the relationship between MC and genital HPV infection. As the results of previous meta-analyses3,4,5 and several other studies7,14 showed major differences, it is urgent that an agreement is reached. Even though our results are consistent with the recent two meta-analyses,4,5 our meta-analysis validated the results through using a larger sample size. Compared to the recent two meta-analyses, to reduce the heterogeneity among the enrolled studies, enrollment in our meta-analysis were restricted to HIV-negative men, and one RCT conducted among HIV-positive men was excluded.38
Our meta-analysis had several limitations. First, there was considerable heterogeneity among the studies. This was because of different study types (case–control, cross-sectional, cohort, and RCT), patients coming from different regions, and differences in results between the normal population (lower risk) and those attending sexually transmitted disease (STD) clinics or partners of HPV-infected women (higher risk). It was not possible to run a subset analysis with the existing data; therefore, these factors might have influenced our results. Second, sampling methods and specimen collection sites varied considerably among the included studies. Third, some of the studies were observational, the MC status was ascertained by self-report, and it was hard to give an accurate assessment of the effect of the surgical procedure. At the same time, age at circumcision and different surgical methods may also have affected our results. Finally, our results for HPV acquisition and clearance could have been influenced by a single study providing two-thirds of all the patients and this may have introduced bias to the overall results.
HPV infection has been established as an important cause of invasive cervical cancer in women and penile cancer in men. Our results suggested that MC could reduce the odds of genital HPV prevalence. MC as a useful intervention could reduce the risk of HPV infection in men and may also have a preventive impact on HPV-related diseases both in men and women.
CONCLUSIONS
This meta-analysis suggested that MC was strongly associated with reduced odds of genital HPV prevalence. MC as a useful intervention to prevent HPV infection should be advocated, especially in countries where HPV vaccines are not yet available.
AUTHOR CONTRIBUTIONS
YPZ and ZWJ designed the study, collected the clinical data, and drafted the manuscript. BD and DWY supervised and revised the manuscript. YYK analyzed some of the data. KC and YW performed the literature search and selected the studies. All authors reviewed and approved the manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by a grant from the International Cooperation and Exchange of Science and Technology Commission of Shanghai Municipality (No. 12410709300), a grant from the Guide Project of Science and Technology Commission of Shanghai Municipality (No. 124119a7300) and a grant from the Outstanding Young Talent Training Plan of Shanghai Municipal Commission of Health and Family Planning (No. XYQ2013102).
REFERENCES
- 1.Tobian AA, Kong X, Gravitt PE, Eaton KP, Kigozi G, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int J Cancer. 2011;129:2970–5. doi: 10.1002/ijc.25957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch FX, Shan KV, Manos MM, Munoz N, Sherman M, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.Van Howe RS. Human papillomavirus and circumcision: a meta-analysis. J Infect. 2007;54:490–6. doi: 10.1016/j.jinf.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Albero G, Castellsagué X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus. Sex Transm Dis. 2012;39:104–13. doi: 10.1097/OLQ.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 5.Larke N, Thomas SL, Dos Santos Silva I, Weiss HA. Male circumcision and human papillomavirus infection in men: a systematic review and meta-analysis. J Infect Dis. 2011;204:1375–90. doi: 10.1093/infdis/jir523. [DOI] [PubMed] [Google Scholar]
- 6.Weaver BA, Feng Q, Holmes KK, Kiviat N, Lee SK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189:677–85. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 7.Bleeker MC, Hogewoning CJ, Voorhorst FJ, van den Brule AJ, Berkhof J, et al. HPV-associated flat penile lesions in men of a non-STD hospital population: less frequent and smaller in size than in male sexual partners of women with CIN. Int J Cancer. 2005;113:36–41. doi: 10.1002/ijc.20502. [DOI] [PubMed] [Google Scholar]
- 8.Auvert B, Sobngwi Tambekou J, Cutler E, Nieuwoudt M, Lissouba P, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis. 2009;199:14–9. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albero G, Castellsague X, Lin HY, Fulp W, Villa LL, et al. Male circumcision and the incidence and clearance of genital human papillomavirus (HPV) infection in men: the HPV Infection in men (HIM) cohort study. BMC Infect Dis. 2014;14:75. doi: 10.1186/1471-2334-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanbuskirk K, Winer RL, Hughes JP, Feng Q, Arima Y, et al. Circumcision and acquisition of human papillomavirus infection in young men. Sex Transm Dis. 2011;38:1074–81. doi: 10.1097/OLQ.0b013e31822e60cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarnaud C, Lissouba P, Cutler E, Puren A, Taljaard D. Association of low-risk human papillomavirus infection with male circumcision in young men: results from a longitudinal study conducted in Orange Farm (South Africa) Infect Dis Obstet Gynecol 2011. 2011:1–7. doi: 10.1155/2011/567408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Backes DM, Bleeker MC, Meijer CJ, Hudgens MG, Agot K, et al. Male circumcision is associated with a lower prevalence of human papillomavirus-associated penile lesions among Kenyan men. Int J Cancer. 2012;130:1888–97. doi: 10.1002/ijc.26196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kjaer SK, van den Brule AJ, Bock JE, Poll PA, Engholm G, et al. Determinants for genital human papillomavirus (HPV) infection in 1000 randomly chosen young Danish women with normal Pap smear: are there different risk profiles for oncogenic and nononcogenic HPV types? Cancer Epidem Biomarkers. 1997;6:799–805. [PubMed] [Google Scholar]
- 14.Rombaldi RL, Serafini EP, Villa LL, Vanni AC, Baréa F. Infection with human papillomaviruses of sexual partners of women having cervical intraepithelial neoplasia. Braz J Med Biol Res. 2006;39:177–87. doi: 10.1590/s0100-879x2006000200003. [DOI] [PubMed] [Google Scholar]
- 15.Partridge JM, Hughes JP, Feng Q, Winer RL, Weaver BA, et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–36. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 16.Lu B, Wu Y, Nielson CM, Flores R, Abrahamsen M, et al. Factors associated with acquisition and clearance of human papillomavirus infection in a cohort of US men: a prospective study. J Infect Dis. 2009;199:362–71. doi: 10.1086/596050. [DOI] [PubMed] [Google Scholar]
- 17.Donovan B, Bassett I, Bodsworth NJ. Male circumcision and common sexually transmissible diseases in a developed nation setting. Genitourin Med. 1994;70:317–20. doi: 10.1136/sti.70.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svare EI, Kjaer SK, Worm AM, Osterlind A, Meijer CJ. Risk factors for genital HPV DNA in men resemble those found in women: a study of male attendees at a Danish STD clinic. Sex Transm Infect. 2002;78:215–8. doi: 10.1136/sti.78.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellsague X, Bosch FX, Munoz N, Meijer CJ, Shah KV, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346:1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 20.Aynaud O, Poveda JD, Huynh B, Guillemotonia A, Barrasso R. Frequency of herpes simplex virus, cytomegalovirus and human papillomavirus DNA in semen. Int J STD AIDS. 2002;13:547–50. doi: 10.1258/095646202760159666. [DOI] [PubMed] [Google Scholar]
- 21.Shin HR, Franceschi S, Vaccarella S, Roh JW, Ju YH, et al. Prevalence and determinants of genital infection with papillomavirus, in female and male university students in Busan, South Korea. J Infect Dis. 2004;190:468–76. doi: 10.1086/421279. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin SB, Wallace DR, Papenfuss MR, Abrahamsen M, Vaught LC. Condom use and other factors affecting penile human papillomavirus detection in men attending a sexually transmitted disease clinic. Sex Transm Dis. 2004;31:601–7. doi: 10.1097/01.olq.0000140012.02703.10. [DOI] [PubMed] [Google Scholar]
- 23.Lajous M, Mueller N, Cruz-Valdez A, Aguilar LV, Franceschi S, et al. Determinants of prevalence, acquisition, and persistence of human papillomavirus in healthy Mexican military men. Cancer Epidem Biomarkers. 2005;14:1710–6. doi: 10.1158/1055-9965.EPI-04-0926. [DOI] [PubMed] [Google Scholar]
- 24.Vaccarella S, Lazcano-Ponce E, Castro-Garduño JA, Cruz-Valdez A, Díaz V, et al. Prevalence and determinants of human papillomavirus infection in men attending vasectomy clinics in Mexico. Int J Cancer. 2006;119:1934–9. doi: 10.1002/ijc.21992. [DOI] [PubMed] [Google Scholar]
- 25.Nielson CM, Flores R, Harris RB, Abrahamsen M, Papenfuss MR, et al. Human papillomavirus prevalence and type distribution in male anogenital sites and semen. Cancer Epidem Biomarkers. 2007;16:1107–14. doi: 10.1158/1055-9965.EPI-06-0997. [DOI] [PubMed] [Google Scholar]
- 26.Ng’Ayo MO, Bukusi E, Rowhani-Rahbar A, Koutsky LA, Feng Q, et al. Epidemiology of human papillomavirus infection among fishermen along Lake Victoria Shore in the Kisumu District, Kenya. Sex Transm Infect. 2008;84:62–6. doi: 10.1136/sti.2007.027508. [DOI] [PubMed] [Google Scholar]
- 27.Hernandez BY, Wilkens LR, Zhu X, McDuffie K, Thompson P, et al. Circumcision and human papillomavirus infection in men: a site-specific comparison. J Infect Dis. 2008;197:787–94. doi: 10.1086/528379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giuliano AR, Lazcano E, Villa LL, Flores R, Salmeron J, et al. Circumcision and sexual behavior: factors independently associated with human papillomavirus detection among men in the HIM study. Int J Cancer. 2009;124:1251–7. doi: 10.1002/ijc.24097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogilvie GS, Taylor DL, Achen M, Cook D, Krajden M. Self-collection of genital human papillomavirus specimens in heterosexual men. Sex Transm Infect. 2009;85:221–5. doi: 10.1136/sti.2008.033068. [DOI] [PubMed] [Google Scholar]
- 30.Tobian AA, Serwadda D, Quinn TC, Kigozi G, Gravitt PE, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. New Engl J Med. 2009;360:1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller EE, Chirwa TF, Lewis DA. Human papillomavirus (HPV) infection in heterosexual South African men attending sexual health services: associations between HPV and HIV serostatus. Sex Transm Infect. 2010;86:175–80. doi: 10.1136/sti.2009.037598. [DOI] [PubMed] [Google Scholar]
- 32.Gray RH, Serwadda D, Kong X, Makumbi F, Kigozi G, et al. Male circumcision decreases acquisition and increases clearance of high-risk human papillomavirus in HIV-negative men: a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1455–62. doi: 10.1086/652184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook LS, Koutsky LA, Holmes KK. Circumcision and sexually transmitted diseases. Am J Public Health. 1994;84:197–201. doi: 10.2105/ajph.84.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Den Eeden SK, Habel LA, Sherman KJ, McKnight B, Stergachis A. Risk factors for incident and recurrent condylomata acuminata among men. A population-based study. Sex Transm Dis. 1998;25:278–84. doi: 10.1097/00007435-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Tseng H, Morgenstern H, Mack T, Peters RK. Risk factors for penile cancer: results of a population-based case-control study in Los Angeles County (United States) Cancer Cause Control. 2001;12:267–77. doi: 10.1023/a:1011266405062. [DOI] [PubMed] [Google Scholar]
- 36.Oriel JD. Natural history of genital warts. Br J Vener Dis. 1971;47:1–13. doi: 10.1136/sti.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavreys L, Rakwar JP, Thompson ML, Jackson DJ, Mandaliya K, et al. Effect of circumcision on incidence of human immunodeficiency virus type 1 and other sexually transmitted diseases: a prospective cohort study of trucking company employees in Kenya. J Infect Dis. 1999;180:330–6. doi: 10.1086/314884. [DOI] [PubMed] [Google Scholar]
- 38.Serwadda D, Wawer MJ, Makumbi F, Kong X, Kigozi G, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis. 2010;201:1463–9. doi: 10.1086/652185. [DOI] [PMC free article] [PubMed] [Google Scholar]