Abstract
In couples with infertility, abnormal strict morphology of 0% normal forms (NF) is a criterion to proceed rapidly to in vitro fertilization (IVF). Since no data currently exist, we investigated the outcomes for men with 0% NF to determine reproductive success without the use of assisted reproductive technologies (ART). A cohort of 24 men with 0% NF were identified (2010–2013) with 27 randomly selected men with ≥4% NF as controls. Patient charts were reviewed with men contacted and administered an Institutional Review Board (IRB)-approved telephone questionnaire to ascertain outcomes. After a median follow-up time of 2.5 years, 29.2% of men with 0% NF did not require ART for their first pregnancy (controls = 55.6%, P ≤ 0.05). When all pregnancies were analyzed together, men with 0% NF achieved twenty pregnancies of which 75% did not require IVF (controls = thirty pregnancies; 76.7% did not require IVF). The average age of men and female partners was similar between men with 0% NF and ≥4% NF. All men had normal follicle-stimulating hormone (FSH), testosterone, prolactin, sex hormone-binding globulin (SHBG), and estradiol. Although, global semen parameters were worse in men with 0% NF, when a first pregnancy was a natural conception (NC), 100% of men with 0% NF (n = 7/7) and 37.5% of controls (n = 3/8) went on to have a subsequent pregnancy via NC. Men with 0% NF conceived without IVF in 29.2% of cases compared to 55.6% of controls. Strict morphology should not be used to predict fertilization, pregnancy, or live birth potential. In men with 0% NF, alternative modalities should be considered before immediate IVF.
Keywords: conception, infertility, intrauterine insemination, in vitro fertilization, strict morphology, success
INTRODUCTION
Semen analysis is a critical tool in the assessment of the infertile male; however, the importance of sperm shape, or morphology, is controversial. The ability of sperm morphology to predict in vitro reproductive outcomes was first proposed by Kruger et al.1,2 who noted an inverse relationship between successful oocyte fertilization and sperm morphology. These findings were propagated by a study published in The Lancet3 that noted in men with an increased proportion of sperm with abnormal morphology, and the likelihood of pregnancy was decreased. Indeed, an abnormal strict morphology identifying severe teratozoospermia, or 0% normal forms (NF), has often been used as a criterion to proceed directly to in vitro fertilization (IVF).
While sperm morphology is guided by specific criteria, it still remains subjective to inter- and intra-laboratory differences.4 Furthermore, studies have identified a classification drift between different classification systems (i.e., WHO 2nd, 3rd, etc.)5,6 and in situations where the couples utilize intrauterine insemination (IUI) or IVF, sperm preparation methodologies, techniques, expertise, and sperm selection criteria vary widely. As such, it has been difficult to perform studies to ascertain the predictability of sperm morphology on outcomes.
In the context of these aforementioned limitations, studies have been performed examining sperm morphology and pregnancy outcomes following IUI7,8,9,10,11 and IVF1,12,13,14,15,16,17,18 with variable results.
To date, no studies have been conducted examining teratozoospermia and the likelihood of achieving a natural conception (NC). To further elucidate the importance of strict morphology in NC, we focused only on those with severely abnormal morphology (0% NF).
MATERIALS AND METHODS
After the Institutional Review Board (IRB) approval, a retrospective chart review was conducted on all patients seen at a high volume, tertiary, academic infertility clinic from 2010 to 2013. All men with a strict morphology obtained during work-up for male infertility were examined (n = 156). Those with severe teratozoospermia (0% NF, strict Kruger criteria) were identified, contacted, and administered an IRB-approved telephone questionnaire to ascertain reproductive outcomes. Exclusion criteria included men who refused to participate or who we were unable to contact. Patients who adopted children or used donor sperm for IUI/IVF were also excluded from this analysis. Only live births and current pregnancies were included in the analysis.
When multiple strict morphologies, semen analyses, and hormones were obtained (usually during treatment) only the first, initial values were used. Serum hormone levels were obtained via early-morning venopuncture and analyzed at the same laboratory to minimize fluctuations (Laboratory for Male Reproductive Research and Testing [LMRRT], Baylor College of Medicine [BCM], Houston, Texas, USA). An Access 2, radioimmunoassay system manufactured by Beckman Coulter (Fullerton, California, USA) was utilized. All semen analyses and strict morphologies were also performed at the LMRRT (BCM) to account for differences in technique and equipment.
Strict morphology was evaluated using the Kruger strict criteria. In brief, an aliquot of semen was air-dried and stained using Diff-Quik Stain Set (Baxter Scientific, Deerfield, IL, USA) with two hundred sperm analyzed at ×100 with an oil-immersion lens. NF were judged by the following criteria: (1) oval shaped head that was smooth in configuration; (2) head length, from midpiece to acrosome tip, between 5 μm and 6 μm; (3) head width between 2.5 μm and 3.5 μm; (4) acrosome covering 40%–70% of the head; and (5) no neck, midpiece or tail defects.
Men then were contacted and administered an IRB-approved telephone questionnaire to ascertain reproductive outcomes. The first portion of the survey confirmed that the men were aware of their abnormal morphology (0% NF) and determined whether they achieved pregnancy at any point since their initial semen analysis. In men who reported conception, details regarding the methodologies were ascertained (i.e., NC, IUI, or IVF).
Data were analyzed using Student's t-test for scalar variables. The analysis was performed using Microsoft Excel (Microsoft, Redmond, WA, USA) and GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA) with P ≤ 0.05 considered statistically significant (unless otherwise noted). All values were reported as mean ± standard error of the mean (s.e.m.), unless otherwise noted.
RESULTS
A total of 156 men who had strict morphology obtained during an infertility work-up were obtained. Twenty-four men with a severely abnormal strict morphology (0% NF) were identified and compared to 27 randomly selected control men with ≥4% NF. The average age at time of evaluation of both men and their female partners was not significantly different and neither were the average follow-up times (Table 1). The overall mean follow-up time was 851 ± 49.9 days (n = 51) with a median of 2.5 years. As expected, the semen analysis found that men with 0% NF had, with the exception of volume, significantly worse parameters than control men (Table 1).
Table 1.
Strict morphology, semen analysis, and serum hormone values obtained from patients with 0% NF compared to controls (≥4% NF)
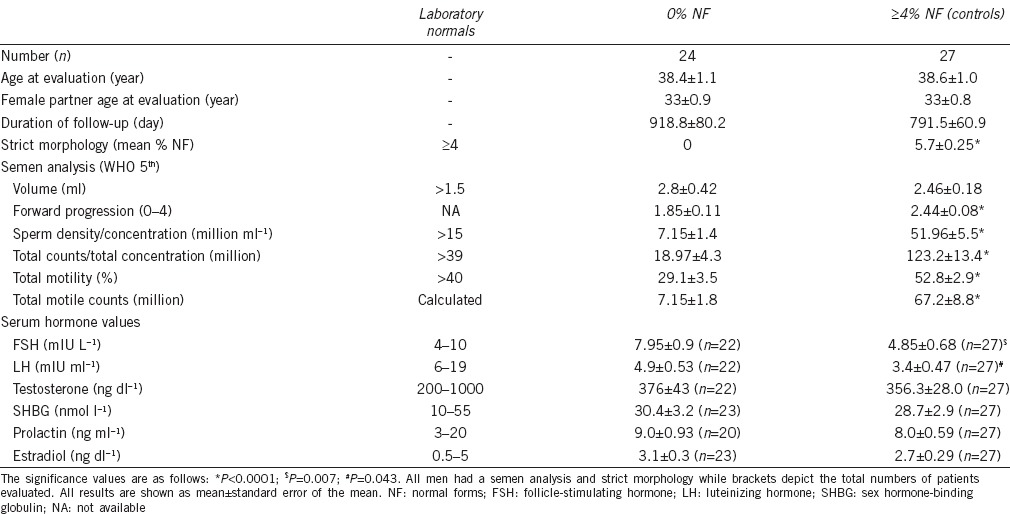
When examining serum hormone levels, testosterone, sex hormone-binding globulin (SHBG), prolactin, and estradiol were similar among the groups (Table 1). The follicle-stimulating hormone (FSH) was with the normal range for both 0% NF and controls; however, it was significantly greater in men with 0% NF. This coincides with the inferior semen analysis findings of these men. Furthermore, the luteinizing hormone (LH) was lower than normal in both groups but significantly higher in men with 0% NF (Table 1).
With regard to the abilities of men to father children (either current pregnancies or live births), 29.2% of men with 0% NF were able to conceive without the use of IVF (n = 7/24) compared to 55.6% of control men (n = 15/27) (P ≤ 0.05) (Figure 1a). In men with 0% NF, a NC occurred 25% of the time (n = 6/24) while controls were able to conceive naturally in 51.8% of cases (P ≤ 0.05) (Figure 1b).
Figure 1.
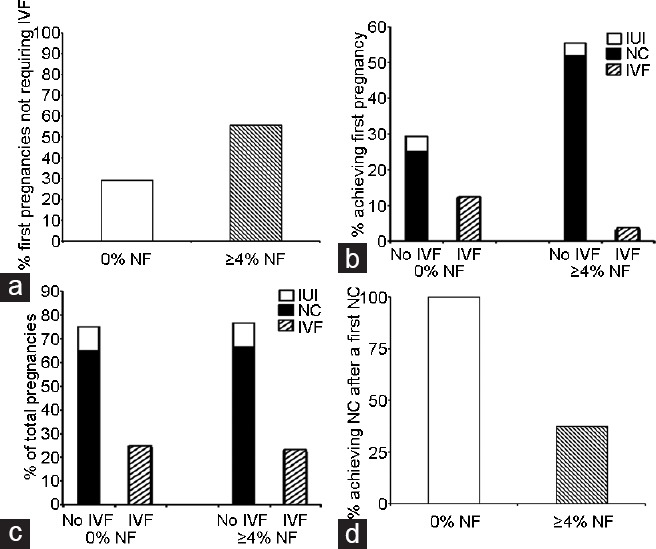
A comparison between pregnancy rates of control men and those with ≥4% NF. (a) Men with 0% NF achieved a first pregnancy and live births without IVF in 29.2% of cases. (b) In men with 0% NF who conceived without use of IVF (Panel A), NC occurred in 25% (n = 6/24). (c) Men with 0% NF achieved twenty pregnancies, with 75% not requiring IVF. (d) In men with 0% NF and in whom the first pregnancy was conceived without IVF (n = 7), 100% had successful NCs on subsequent occasions. NF: normal forms; IVF: in vitro fertilization; NC: natural conception; IUI: intrauterine insemination.
When overall reproductive success was measured, the values between the two groups became even more interesting. Men with 0% NF produced twenty pregnancies and 75% (n = 15/20) did not require IVF while thirty pregnancies were achieved in control men with 76.7% not requiring IVF (Figure 1c). This success was carried forward when the number of subsequent pregnancies was examined (Figure 1d). In cases where men with 0% NF had a child via NC, 100% of them had another child with NC. In contrast, control men were more likely to undergo IVF with repeat pregnancies (Figure 1d).
DISCUSSION
IVF with intracytoplasmic sperm injection (ICSI) is increasingly used to overcome male subfertility. The Center for Disease Control reported in 2010 that children born through IVF now constitute 1.5% of all births. Of the 147 260 cycles performed by the Society for Assisted Reproductive Technique's (SART) member clinics in 2010, 47 090 live-birth deliveries occurred (37% live birth rate).19 The 2014 annual report from SART indicates a growing number of IVF cycles and infants conceived via assisted reproductive techniques. Despite the growing success of IVF, not all assisted reproduction procedures result in pregnancies and even more do not result in the birth of a child.
Among the many factors that influence the outcomes of IVF, semen parameters have traditionally been used as an indicator for oocyte fertilization and attainment of pregnancy in addition to the quality of the oocyte.20 In 1986, Kruger et al.2 published a novel classification system to evaluate sperm morphology and correlate it with the likelihood of IVF outcomes in terms of fertilization and clinical pregnancy.
Variability in IUI and IVF outcomes for men with teratozoospermia has resulted in controversy in how to counsel men with isolated defects in strict morphology. Indeed, some investigators have recommended IVF with ICSI in cases of isolated teratozoospermia.21 The results in the current study suggest that proceeding directly with ART in the face of even 0% NF is unnecessary given that 29.2% of these couples will have a successful NC.
The evaluation of morphology is regarded as subjective due to the fact that it has to be done by the human eye. Furthermore, form and function are two distinct properties.22 As a manner of illustration, consider that spermatozoa in the cervical mucus cannot be proven to be functionally better than those with the same appearance and form in the seminal plasma.23
Previously, Mortimer and Templeton24,25 confirmed the existence of selection for morphologically normal human spermatozoa within the cervical mucus. The authors suggested that “abnormal” spermatozoa may reach the site of fertilization which is in accordance with findings in other species. Later, Mortimer26 by evaluating sperm recovered from the cervical canal, further delineated that the selection of spermatozoa is facilitated by reductions in spermatozoa with midpiece, tail, and other morphological defects which would be expected to impair motility. Despite their efforts and those of others, the presumed importance of strict morphology remains incompletely documented.
Morphology does not indicate anything with regard to the genetic composition of the spermatozoa or the fertilization potential. Given the controversy surrounding morphology, clinicians are left to ponder what to discuss with men who present to their clinics with poor morphology. Given the results of this study, we can comfortably discuss with these patients that NC is indeed possible and, in cases where maternal age allows, should be encouraged. It is likely that IVF is overutilized in the setting of isolated teratozoospermia, particularly when accounting for the known variability between semen analysis results in men.
While this study lacks the rigor of a randomized control trial, attempts were made to limit possible bias and confounders. When considering limitations, recall bias was eliminated by the tendency of parents to remember the birthdays of their offspring. Furthermore, the usage of continuous patients (2010–2014) resolved potential selection bias. Despite this, the population of men seen was limited, mostly, to those experiencing fertility issues and as such, may be different in the general populations. Moreover, IUI and IVF procedures were conducted by different reproductive endocrinologists potentially leading to variable results.
Interestingly, men with 0% NF had worse overall semen analyses compared to control men (Table 1). The finding that these men overcame such an overall “disadvantage” to experience high rates of fertilization and live birth success makes an even stronger argument against the routine use of strict morphology in counseling patients.
The notion that sperm morphology may not be as important as previously thought highlights how a novel systems biology approach to fertility could be considered.27 While the requirement for both partners to have adequately functioning gametes is not disputable, the inter-relationship between the female microenvironment and the male spermatozoa is still unknown. For example, spermatozoa remain in the oviduct for several days by binding to epithelial cells and recently, oviductal epithelial have been shown to interact with spermatozoa and protect against oxidative stress.28 As such, it is tempting to speculate that some oviductal cells may be more nurturing to spermatozoa than others27 – a factor that may be interrelated with morphology.
In summary, men with a complete absence of NF (0%) on strict morphology exhibit high rates of success without IVF. As such, strict morphology should not be used to predict fertilization, pregnancy, or live birth potential. In men with 0% NF, alternative modalities should be considered before immediate IVF.
AUTHOR CONTRIBUTIONS
JRK conceived of the study, participated in its design and coordination, performed statistical analysis, and helped draft the manuscript. RPS conceived of the study, participated in its design and coordination, performed statistical analysis, and helped draft the manuscript. MC carried out data acquisition and statistical analysis and performance of the survey and helped draft the manuscript. DJL conceived of the study, participated in its design and coordination, and helped draft the manuscript. LIL conceived of the study, participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
REFERENCES
- 1.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 2.Kruger TF, Menkveld R, Stander FS, Lombard CJ, Van der Merwe JP, et al. Sperm morphologic features as a prognostic factor in in vitro fertilization. Fertil Steril. 1986;46:1118–23. doi: 10.1016/s0015-0282(16)49891-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 4.Franken DR, Menkveld R, Kruger TF, Sekadde-Kigondu C, Lombard C. Monitoring technologist reading skills in a sperm morphology quality control program. Fertil Steril. 2003;79(Suppl 3):1637–43. doi: 10.1016/s0015-0282(03)00367-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooper TG, Neuwinger J, Bahrs S, Nieschlag E. Internal quality control of semen analysis. Fertil Steril. 1992;58:172–8. [PubMed] [Google Scholar]
- 6.Horte A, Vierula M, Toppari J, Suominen J. Reassessment of sperm morphology of archival semen smears from the period 1980-1994. Int J Androl. 2001;24:120–4. doi: 10.1046/j.1365-2605.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 7.Morbeck DE, Leonard PH, Weaver AL, Shimek KM, Bouwsma EV, et al. Sperm morphology: classification drift over time and clinical implications. Fertil Steril. 2011;96:1350–4. doi: 10.1016/j.fertnstert.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 8.Badawy A, Elnashar A, Eltotongy M. Effect of sperm morphology and number on success of intrauterine insemination. Fertil Steril. 2009;91:777–81. doi: 10.1016/j.fertnstert.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Matorras R, Corcostegui B, Perez C, Mandiola M, Mendoza R, et al. Sperm morphology analysis (strict criteria) in male infertility is not a prognostic factor in intrauterine insemination with husband's sperm. Fertil Steril. 1995;63:608–11. doi: 10.1016/s0015-0282(16)57434-2. [DOI] [PubMed] [Google Scholar]
- 10.Ombelet W, Dhont N, Thijssen A, Bosmans E, Kruger T. Semen quality and prediction of IUI success in male subfertility: a systematic review. Reprod Biomed Online. 2014;28:300–9. doi: 10.1016/j.rbmo.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 11.Grigoriou O, Pantos K, Makrakis E, Hassiakos D, Konidaris S, et al. Impact of isolated teratozoospermia on the outcome of intrauterine insemination. Fertil Steril. 2005;83:773–5. doi: 10.1016/j.fertnstert.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Keegan BR, Barton S, Sanchez X, Berkeley AS, Krey LC, et al. Isolated teratozoospermia does not affect in vitro fertilization outcome and is not an indication for intracytoplasmic sperm injection. Fertil Steril. 2007;88:1583–8. doi: 10.1016/j.fertnstert.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Kihaile PE, Misumi J, Hirotsuru K, Kumasako Y, Kisanga RE, et al. Comparison of sibling oocyte outcomes after intracytoplasmic sperm injection and in vitro fertilization in severe teratozoospermic patients in the first cycle. Int J Androl. 2003;26:57–62. doi: 10.1046/j.1365-2605.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 14.Aboulghar MA, Mansour RT, Serour GI, Amin YM, Kamal A. Prospective controlled randomized study of in vitro fertilization versus intracytoplasmic sperm injection in the treatment of tubal factor infertility with normal semen parameters. Fertil Steril. 1996;66:753–6. doi: 10.1016/s0015-0282(16)58630-0. [DOI] [PubMed] [Google Scholar]
- 15.Goyal HO, Braden TD, Mansour M, Williams CS, Kamaleldin A, et al. Diethylstilbestrol-treated adult rats with altered epididymal sperm numbers and sperm motility parameters, but without alterations in sperm production and sperm morphology. Biol Reprod. 2001;64:927–34. doi: 10.1095/biolreprod64.3.927. [DOI] [PubMed] [Google Scholar]
- 16.Hall J, Fishel S, Green S, Fleming S, Hunter A, et al. Intracytoplasmic sperm injection versus high insemination concentration in-vitro fertilization in cases of very severe teratozoospermia. Hum Reprod. 1995;10:493–6. doi: 10.1093/oxfordjournals.humrep.a135973. [DOI] [PubMed] [Google Scholar]
- 17.Figueiredo H, Tavares A, Ferras L, Couceiro A, Chaves I. Isolated teratozoospermia and in vitro fertilization. J Assist Reprod Genet. 1996;13:64–8. doi: 10.1007/BF02068872. [DOI] [PubMed] [Google Scholar]
- 18.Hotaling JM, Smith JF, Rosen M, Muller CH, Walsh TJ. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2011;95:1141–5. doi: 10.1016/j.fertnstert.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 19.Sunderam S, Kissin DM, Crawford S, Anderson JE, Folger SG, et al. Assisted reproductive technology surveillance – United States, 2010. MMWR Surveill Summ. 2013;62:1–24. [PubMed] [Google Scholar]
- 20.Ghirelli-Filho M, Mizrahi FE, Pompeo AC, Glina S. Influence of strict sperm morphology on the results of classic in vitro fertilization. Int Braz J Urol. 2012;38:519–28. doi: 10.1590/s1677-55382012000400012. [DOI] [PubMed] [Google Scholar]
- 21.McKenzie LJ, Kovanci E, Amato P, Cisneros P, Lamb D, et al. Pregnancy outcome of in vitro fertilization/intracytoplasmic sperm injection with profound teratospermia. Fertil Steril. 2004;82:847–9. doi: 10.1016/j.fertnstert.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Menkveld R, Holleboom CA, Rhemrev JP. Measurement and significance of sperm morphology. Asian J Androl. 2011;13:59–68. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliasson R. Semen analysis with regard to sperm number, sperm morphology and functional aspects. Asian J Androl. 2010;12:26–32. doi: 10.1038/aja.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortimer D, Leslie EE, Kelly RW, Templeton AA. Morphological selection of human spermatozoa in vivo and in vitro. J Reprod Fertil. 1982;64:391–9. doi: 10.1530/jrf.0.0640391. [DOI] [PubMed] [Google Scholar]
- 25.Mortimer D, Templeton AA. Sperm transport in the human female reproductive tract in relation to semen analysis characteristics and time of ovulation. J Reprod Fertil. 1982;64:401–8. doi: 10.1530/jrf.0.0640401. [DOI] [PubMed] [Google Scholar]
- 26.Mortimer D. Sperm recovery techniques to maximize fertilizing capacity. Reprod Dev. 1994;6:25–31. doi: 10.1071/rd9940025. [DOI] [PubMed] [Google Scholar]
- 27.Kovac JR, Lipshultz LI. Interaction between oviductal epithelial cells and spermatozoa underlies a systems biology approach to treating infertility. Fertil Steril. 2013;99:1207–8. doi: 10.1016/j.fertnstert.2012.12.039. [DOI] [PubMed] [Google Scholar]
- 28.Huang VW, Zhao W, Lee CL, Lee CY, Lam KK, et al. Cell membrane proteins from oviductal epithelial cell line protect human spermatozoa from oxidative damage. Fertil Steril. 2013;99:1444–52.e3. doi: 10.1016/j.fertnstert.2012.11.056. [DOI] [PubMed] [Google Scholar]


