Abstract
We compare the efficacy of intratesticular ozone therapy with intraperitoneal ozone therapy in an experimental rat model. For this purpose, 24 rats were divided into four groups including sham-operated, torsion/detorsion, torsion/detorsion plus intraperitoneal ozone (O-IP), and torsion/detorsion plus intratesticular ozone (O-IT). The O-IP ozone group received a 4 mg kg−1 intraperitoneal injection of ozone, and the O-IT group received the same injection epididymally. At 4 h after detorsion, the rats were sacrificed and orchiectomy materials were assessed histopathologically. Spermatogenesis in the seminiferous tubules and damage to the Sertoli cells were histopathologically evaluated in the testes using the Johnsen scoring system. i-NOS and e-NOS activities in the testis tissue were also evaluated. Torsion-detorsion caused a decreased Johnsen score and increased apoptosis of spermatogonial and Sertoli cells. Ozone injection prevented increases in Johnsen score and i-NOS level. e-NOS level of the O-IP group was significantly lower than that of the O-IP group, and i-NOS level of the O-IT group was significantly lower than that of the O-IP group. Local ozone therapy is more effective than systemic ozone therapy at improving IRI-related testicular torsion. Our study is the first to show that the efficacy of intratesticular implementation of ozone therapy is higher than that of intraperitoneal ozone therapy.
Keywords: local therapy, ozone, rat model, testis, torsion
INTRODUCTION
Testicular torsion accounts for 13%–54% of the cases of acute pediatric scrotal disease and is characterized by both testicular tissue hypoxia and necrosis of the germinal cells which leads to infertility.1,2 Although reperfusion is essential for the survival of the ischemic tissue, reperfusion injury can occur due to the generation of reactive oxygen species (ROS) and intratesticular neutrophil intensification.3
The basic pathologic mechanism underlying ischemia-reperfusion injury (IRI) of the testis is not yet completely understood; however, this oxidative stress is characterized by an imbalance between ROS and cellular antioxidative system.4 Several methods can be utilized to prevent IRI, one of which is ozone therapy. Ozone has been investigated as a therapeutic agent for the treatment of different physiopathologic events mediated by ROS.5,6 Judicious controlled ozone administration has been shown to stimulate the endogenous antioxidant defense systems, which prepares the host to face IRI.7,8 This phenomenon is known as ozone oxidative preconditioning and has been reported to be a simple and harmless new method for protecting organs from IRI.8
In this study, we evaluated the efficacy of ozone therapy in preventing IRI after reperfusion in an experimental rat model of unilateral testicular torsion.
METHODS
Animals
Twenty-four healthy 3-month-old male Wistar rats weighing between 150 and 200 g were used. The rats were housed at a temperature of 24 ± 3°C with 12 h light-dark cycle. The animals were fed a standard pellet diet and provided with water ad libitum. A 7-day period of acclimatization was used.
Experimental protocol
The Ethical Committee on Animal Research at Celal Bayar University, Faculty of Medicine, approved the experimental protocol. The rats were randomly allocated into four groups of six rats each as follows:
S group (sham): to determine the effect of the sham operation;
TD group (torsion and detorsion): to determine the effect of testicular torsion and detorsion;
O-IP group (torsion/detorsion plus ozone intraperitoneal treatment): to determine the effect of ozone intraperitoneal treatment;
O-IT group (torsion/detorsion plus ozone intratesticular treatment): to determine the effect of ozone intratesticular treatment.
Treatment schedule
Ozone was generated by ozonator equipment (Medozon Compact-Hab Herrmann apparatebau GmbH, Germany). Ozone was obtained from medical-grade oxygen by means of a silent electric discharge and constituted approximately 3% of the ozone/oxygen gas mixture. The ozone concentration measured using a UV spectrophotometer at 254 nm was 50 μg ml−1. Ozone doses of 4 mg kg−1 were based on the body weight of each rat and administered intraperitoneally and intratesticularly.
Surgical procedures
All surgical procedures were performed using a sterile technique under anesthesia with 50 mg kg−1 ketamine and 45 mg kg−1 xylazine (Ketalar and Citanest, 2%, Eczacύbasύ, Turkey) administered intramuscularly. A left inguinoscrotal incision was performed. Unilateral testicular torsion was created by rotating the left testis 720° in a clockwise direction and fixing it within the hemiscrotum using a 4/0 atraumatic silk suture. After 1 h, the spermatic cord was detorsed, followed by a reperfusion period of 4 h. Ozone was injected intraperitoneally and epididymally 1½ h before reperfusion in the O-IP and O-IT groups, respectively. Four hours after reperfusion, the rats were euthanized and bilateral orchiectomy materials were obtained.
Paraffin tissue follow-up
Samples were fixed in Bouin's solution and dehydrated in 96% alcohol at room temperature for 24 h. Next, the samples were exposed to a solution with a 1:1 ratio of xylene to alcohol for 15 min and purified in a series of 2 xylene solutions for 30 min each. The samples were then exposed to a solution with a 1:1 ratio of xylene to paraffin in a 60°C incubator for 15 min followed by exposure to a series of 2 paraffin solutions for 60 min each. After routine tissue processing (Shandon, Excelsior tissue processor, Thermo Fisher Scientific, Waltham, MA, USA), the tissues were embedded in paraffin.
Histopathologic assessment
After overnight incubation at 60°C and application of a series of 2 xylene solutions for 30 min each, 5 mm paraffin cross-sections were deparaffinized using a Rotary microtome (RM 2135, Leica) assistant. The cross-sections were then treated with an alcohol series with decreasing concentrations from 95% to 60% and then soaked in flowing water for 5 min to complete the rehydration. The cross-sections were stained following standard protocols for hematoxylin and eosin (HE) and periodic acid–Schiff (PAS) staining. In the same manner, after 5 min of soaking under flowing water and treatment with alcohol series of 80% and 95% alcohol solutions, the cross-sections were air-dried. The aim of this procedure was to make each of the samples pellucid within 30 min of being treated with two entellan-xylene mixtures (UN 1866, Merck, Darmstadt, Germany). The cross-sections were then analyzed by light microscope dispersion. A pathologist evaluated the testicular tissues using standard light microscopy. This examination was completed in a random order and a blinded fashion. The histologic sections were graded for testicular injury and spermatogenesis using the Johnsen score (JS). A minimum of 50 tubules were evaluated, and each tubule was given a score from 1 to 10.
Indirect immunohistochemical staining (IHC)
Dissected cross-sections were left in a 60°C incubator overnight and then made pellucid by treatment with 2 xylene solutions of different concentrations for 30 min each. Then, immunohistochemical staining was performed. After removal of the blocking solution from the tissue using primary antibodies for e-NOS and i-NOS, the cross-sections were incubated overnight. The next morning, the cross-sections were soaked 3 times in an anti-mouse biotin-streptavidin hydrogen peroxidase secondary antibody buffer solution (85-9043 Zymed Histostain kit, San Francisco, CA, USA) for 30 min each time. The immunohistochemical stains were assessed by blinded semiquantitative scoring with scores ranging from 1 to 5.
TUNEL IHC
The distribution of apoptotic cell death was measured by the TUNEL method using the DeadEnd Colorimetric TUNEL system (Promega G7130 kit). After overnight incubation at 60°C, 5 μm-thick cross-sections derived from paraffin blocks were deparaffinized with heat. Subsequently, rehydration was completed with xylene treatment for 1 h and treatment with an alcohol series (95%, 80%, 70%, and 60%) with 2 min for each alcohol solution. The tissues were then treated for 5 min in physiologic serum (0.85% NaCl) at room temperature. Then, the cross-sections were treated with phosphate buffer solution (PBS) before being fixed in a 4% paraformaldehyde solution at room temperature for 15 min.
The TUNEL method was performed to detect apoptotic cells. As with the immunohistochemical assessments of the cross-sections, blinded semiquantitative scoring was used to assess the density of the dye with scores ranging from 1 to 5. Averages were calculated. At the end of the study, the rats were euthanized.
Analysis
All statistical analyses were performed using SPSS 16.0 software (SPSS Inc., IBM, Armonk, NY, USA). Differences among the groups were analyzed by the nonparametric Kruskal–Wallis one-way analysis of variance. The Mann–Whitney U-test was also used as a post hoc test for multiple comparisons. Significant differences were accepted at P < 0.05.
RESULTS
We observed significant testicular damage in the TD group. All studied parameters were statistically significantly different in the left testes of the different groups.
In the S group, healthy seminiferous tubules, higher Johnsen scores (4.4 ± 0.5), and spermatogenesis were detected. In the ipsilateral TD group, atrophic seminiferous tubules, lower Johnsen scores (1.2 ± 0.4), testicle cellular edema, hemorrhage, and general pathologic deformations were detected in the contralateral testes. The contralateral testes also showed minimally affected tubule morphology but mostly preserved spermatogenesis. In the O-IP and O-IT groups, tubules with germ cell necrosis were observed and most tubules showed incomplete maturation to the level of primary or secondary spermatocytes; significant rescue of testicular function and mild to moderate interstitial edema were also observed (Figure 1).
Figure 1.
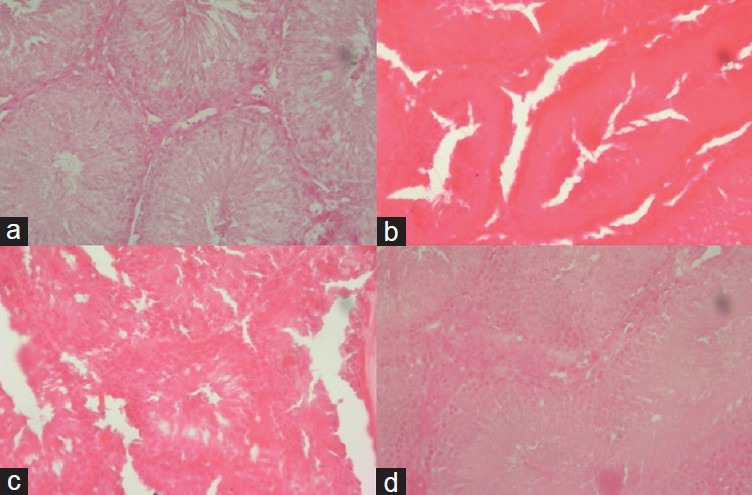
Ipsilateral testis (×200, hematoxylin and eosin stain [HE]). (a) A section from the sham group (S) showing normal histologic findings of preserved spermatogenesis. (b) A section from the TD group showing total infarct and necrosis with infiltration of PMNLs in the interstitial area. (c) A section from the O-IP group showing preserved spermatogenesis up to the level of spermatocytes without apparent interstitial inflammation but with moderate interstitial edema. (d) A section from the O-IT group showing histopathologic findings similar to those of the O-IP group but with fewer spermatocytes.
In the ipsilateral testes, the tissue i-NOS and e-NOS levels were significantly different among all groups, and the differences between the ipsilateral TD and S groups were particularly pronounced. The tissue e-NOS levels were 4.2 ± 0.4, 3.2 ± 0.4, and 2.6 ± 0.5 and the i-NOS levels were 4.2 ± 0.4, 3.4 ± 0.8, and 2.6 ± 0.5 in the TD, O-IP, and O-IT groups, respectively. Tissue e-NOS level was significantly decreased in both the O-IP and O-IT groups compared to the TD group (P < 0.05 and < 0.001, respectively). e-NOS level was not significantly different between the O-IP and O-IT groups (P = 0.14) (Table 1 and Figure 2). Tissue i-NOS level was not significantly different between the O-IP TD groups (P = 0.22), but it was significantly lower in the O-IT group than in the TD group (P ≤ 0.01). e-NOS level was not significantly different between the O-IP and O-IT groups (P = 0.19).
Table 1.
Comparison of Johnsen scores, e-NOS, and i-NOS levels and apoptotic index among the four groups
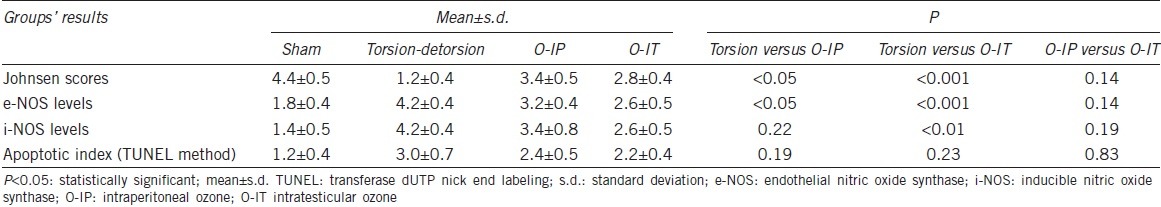
Figure 2.
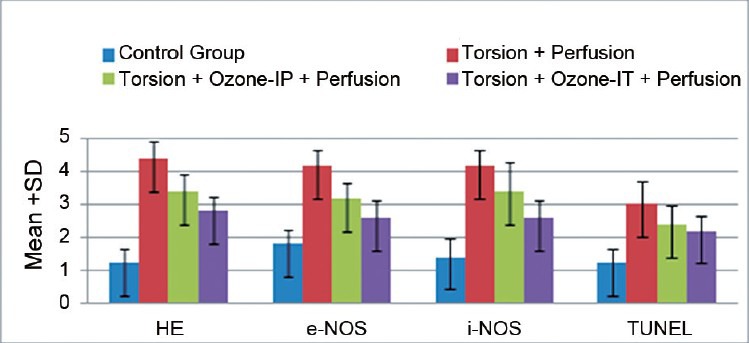
Morphometric analysis of the postozone changes using scores of 1 to 5 for immunohistochemical staining in torsioned rat testis.
DISCUSSION
Testicular torsion is a common urological emergency involving rotation of the testis and twisting of the spermatic cord, which causes restricted blood flow to the affected testis, resulting in testicular atrophy.9,10,11 The main pathophysiological consequence of testicular torsion is ischemia-reperfusion injury of the testis generated by the twisting of the spermatic cord which renders the tissue ischemic, and reperfusion occurs upon release of the twisted cord.9 Ischemia-reperfusion injury involves neutrophil recruitment; generation of reactive oxygen species (ROS), proinflammatory cytokines and adhesion molecules; lipid peroxidation; apoptosis; anoxia; and alteration of the microvascular blood flow, and it can result in infertility.11,12
ROS are produced through normal metabolic reactions and play roles in multiple processes, such as apoptosis and cell signaling.13 ROS also oxidize lipids in the cell and mitochondrial membranes, which alters membrane permeability and disrupts cell integrity.
Ozone therapy is associated with effective regulation of oxidative stress on a cellular level, and studies have identified multiple useful biochemical mechanisms of ozone therapy that elevate antioxidant activity, which is thought to prepare tissue for exposure to oxidative stress.5,7,8,14
The pathophysiology of the anti-inflammatory and antioxidant properties of ozone at therapeutic doses is still unknown as ozone decomposes many different components of the blood. Ozone has been shown to elevate glutathione level, decrease xanthine oxidase production, increase the smooth muscle tone of vessel walls, and affect calcium levels, leading to vasodilation and an increase in erythrocyte glucose level; furthermore, ozone has proven effects in ischemic tissue such as causing an increase in oxygen level.15
Nitric oxide (NO) and NO synthesis (NOS) enzyme subtypes including inducible NOS (i-NOS), endothelial NOS (e-NOS), and neuronal NOS (n-NOS) inside testicular blood vessels have significant impacts on the regulation of oxygen, hormone, and mediator levels and on vessel tone.16
In terms of IRI pathophysiology, the influences of NO and NOS enzymes remain unclear, but NO's antiapoptotic, anti-inflammatory, and vasodilator effects, increase of i-NOS level, and decrease of e-NOS production have been shown to be the underlying causes of increased cell necrosis during reperfusion enhancement.17 Previous research studies showed that one of the results of the increase in melatonin during IRI is i-NOS synthesis inhibition.18
In the experimental study conducted by Ekici et al. using a testicular torsion model, IRI, intraperitoneal ozone therapy, and melatonin application were compared.19 In the melatonin treatment group, a decrease in NO level was observed, and in the ozone therapy group, an increase in NO level was observed. Glutation, inhibition-B, and malondialdehyde levels were stable. The biochemical and histopathological results of melatonin therapy were reported to be comparable to those of intraperitoneal ozone therapy. In studies focused on IRI pathophysiology, NO duct web and NOS subforms were stressed as potentially being responsible for the benefits of ozone therapy. In the present study, we compared the protective outcomes of intraperitoneal (IP) and intratesticular (IT) ozone therapies in testicular tissues using cellular and histochemical analyses. The effects of both ozone therapy types on i-NOS and e-NOS levels were also examined. IP ozone therapy resulted in deregulation of e-NOS and i-NOS levels and a decrease in i-NOS ratio compared to the IT ozone therapy. The i-NOS ratio was also lower in the IP ozone therapy group than in the IP group in the postreperfusion period although this difference was not significant.19
Many studies have examined testicular torsion with IRI and the use of multiple molecules for treatment. Phosphodiesterase type-5 inhibitors are popular for erectile dysfunction, benign prostate hyperplasia, and premature ejaculation and are also investigated in acute testicular injury. Although intraperitoneal vardenafil administration during ischemia in a rat model of testicular torsion resulted in a reduced malondialdehyde level and cellular damage,20 recently Istanbulluoglu et al. demonstrated that vardenafil worsened histopathological changes related to oxidative stress in testicular injury and had no protective effect on testicular ischemia-reperfusion injury in pigs. They showed that the testes of pigs treated with vardenafil had significantly increased numbers of apoptotic cells and levels of i-NOS and e-NOS compared to the sham operation group.21 One recent study investigated the effect of nifedipine on testicular torsion-detorsion injury, and the researchers found that administration of nifedipine before detorsion prevents ischemia/reperfusion cellular damage in testicular tissue.22 Some plant extracts, such as luteolin, apigenin, galangin, catechin, quercetin, rutin, and ternatin, have been reported to have effects on lipid peroxidation. Panax ginseng has been used in Asia as a medical substance for thousands of years and exhibits a wide range of pharmacological and biological activities. Furthermore, ginseng, Korean red ginseng, has been shown to repair testicular dysfunction caused by ischemia-reperfusion in the rat testis by suppressing superoxide production.23 Dietary supplementation with Kastamonu garlic from Turkey resulted in low tissue malondialdehyde levels after testicular torsion in rats, implying attenuation of the generation of free oxygen radicals.24 Ginkgo biloba reduced testicular malondialdehyde and NO levels and the histopathological effects of ischemia-reperfusion injury in rats.25 The net influence of hyperbaric oxygen is unknown, but its inhibitory effects on NO and NOS synthesis have been shown to be more significant than that of ozone therapy in recent studies.26,27 Importantly, molecules used in the treatment in emergent cases of testicular torsion pre- and/or post-operatively must be available for rapid, effective, and easy administration in clinical settings. Our study is the main study in the literature showing that ozone therapy is efficient at treating ischemic damage and intratesticular administration is more practical than intraperitoneal administration. The limitation of our study was the inaccuracy of the measurements of some tissue damage parameters and ROS, such as adenosine, glutathione, superoxide dismutase, and malondialdehyde. Additional studies with well-designed experimental models are needed to clarify the mechanisms through which ozone exerts its effects.
The discovery of antioxidant defense mechanisms and human testicle composition during oxidative stress debilitation is important for determining new antioxidant treatment schedules as to supplement surgery in clinical environments. When evaluated in that respect, local application (IT) of IRI ozone therapy for testicular torsion demands attention in comparison to systemic application (IP) as it is more efficient and is emerging as a possible alternative therapy for testicular torsion.
CONCLUSIONS
Our study showed that ozone therapy (intratesticular and intraperitoneal) was effective for the treatment of IRI due to testicular torsion in a rat model. Intratesticular ozone therapy may be a valuable new approach for the treatment of testicular torsion. Thus, additional studies with different approaches and different molecules will help transition to this therapy from experimental animal studies to clinical trials and then clinical practice for the prevention of ischemia-reperfusion injury after surgical detorsion of testicular torsion.
COMPETING INTERESTS
All authors declared no competing financial interests.
AUTHOR CONTRIBUTIONS
KV and IA performed the immunohistochemical staining and morphologic analyses. OC, RGE, and HT participated in the design of the study, and RGE also participated in the statistical analyses. OC and YI conceived the study, participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.Barada JH, Weingarten JL, Cromie WJ. Testicular salvage and age-related delay in the presentation of testicular torsion. J Urol. 1989;142:746–8. doi: 10.1016/s0022-5347(17)38875-4. [DOI] [PubMed] [Google Scholar]
- 2.Ringdahl E, Teague L. Testicular torsion. Am Fam Physician. 2006;74:1739–43. [PubMed] [Google Scholar]
- 3.Karaguzel E, Kadihasanoglu M, Kutlu O. Mechanisms of testicular torsion and potential protective agents. Nat Rev Urol. 2014;11:391–9. doi: 10.1038/nrurol.2014.135. [DOI] [PubMed] [Google Scholar]
- 4.Akgür FM, Kilinç K, Aktug T. Reperfusion injury after detorsion of unilateral testicular torsion. Urol Res. 1993;21:395–9. doi: 10.1007/BF00300075. [DOI] [PubMed] [Google Scholar]
- 5.Bocci V. Ozone as janus: this controversial gas can be either toxic or medically useful. Mediators Inflamm. 2004;13:3–11. doi: 10.1080/0962935062000197083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Zhan WW, Shen ZJ, Rui WB, Lv C, et al. Blood perfusion of the contralateral testis evaluated with contrast-enhanced ultrasound in rabbits with unilateral testicular torsion. Asian J Androl. 2009;11:253–60. doi: 10.1038/aja.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Sánchez G, Pérez-Davison G, Re L, Giuliani A. Ozone as u-shaped dose responses molecules (hormetins) Dose Response. 2010;9:32–49. doi: 10.2203/dose-response.10-001.Martinez-Sanchez. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Chen H, Xing B, Liu X, Zhan B, Zhou J, et al. Ozone oxidative preconditioning protects the rat kidney from reperfusion injury: the role of nitric oxide. J Surg Res. 2008;149:287–95. doi: 10.1016/j.jss.2007.12.756. [DOI] [PubMed] [Google Scholar]
- 9.Prillaman HM, Turner TT. Rescue of testicular function after acute experimental torsion. J Urol. 1997;157:340–5. [PubMed] [Google Scholar]
- 10.Filho DW, Torres MA, Bordin AL, Crezcynski-Pasa TB, Boveris A. Spermatic cord torsion reactive oxygen and nitrogen species and ischemia-reperfusion injury. Mol Aspects Med. 2004;25:199–210. doi: 10.1016/j.mam.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–59. [PubMed] [Google Scholar]
- 12.Celik O, Kutlu O, Tekcan M, Celik-Ozenci C, Koksal IT. Role of TNF-related apoptosis-inducing ligand (TRAIL) in the pathogenesis of varicocele-induced testicular dysfunction. Asian J Androl. 2013;15:269–74. doi: 10.1038/aja.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altavilla D, Romeo C, Squadrito F, Marini H, Morgia G, et al. Molecular pathways involved in the early and late damage induced by testis ischemia: evidence for a rational pharmacological modulation. Curr Med Chem. 2012;19:1219–24. doi: 10.2174/092986712799320538. [DOI] [PubMed] [Google Scholar]
- 14.Peralta C, Xaus C, Bartrons R, Leon OS, Gelpi E, et al. Effect of ozone therapy oncreactive oxygen species and adenosine production during hepatic ischemia-reperfusion. Free Radic Res. 2000;33:595–605. doi: 10.1080/10715760000301121. [DOI] [PubMed] [Google Scholar]
- 15.Bocci V. The question of balance: The interaction between blood and ozone. In: Valacchi G, Davis P, editors. Oxidants in Biology. Dordrecht, Netherlands: Springer; 2008. pp. 155–65. [Google Scholar]
- 16.Dokucu AI, Oztürk H, Ozdemir E, Ketani A, Büyükbayram H, et al. The protective effects of nitric oxide on the contralateral testis in prepubertal rats with unilateral testicular torsion. BJU Int. 2000;85:767–71. doi: 10.1046/j.1464-410x.2000.00529.x. [DOI] [PubMed] [Google Scholar]
- 17.Shiraishi K, Naito K, Yoshida K. Nitric oxide promotes germ cell necrosis in the delayed phase after experimental testicular torsion of rat. Biol Reprod. 2001;65:514–21. doi: 10.1095/biolreprod65.2.514. [DOI] [PubMed] [Google Scholar]
- 18.Ersoz N, Guven A, Cayci T, Uysal B, Turk E, et al. Comparison of the efficacy of melatonin and 1400W on renal ischemia/reperfusion injury: a role for inhibiting iNOS. Ren Fail. 2009;31:704–10. doi: 10.3109/08860220903085989. [DOI] [PubMed] [Google Scholar]
- 19.Ekici S, Doğan Ekici AI, Öztürk G, Benli Aksungar F, Sinanoğlu O, et al. Comparison of melatonin and ozone in the prevention of reperfusion injury following unilateral testicular torsion in rats. Urology. 2012;80:899–906. doi: 10.1016/j.urology.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 20.Erol B, Tokgoz H, Hanci V, Bektas S, Akduman B, et al. Vardenafil reduces testicular damage following ischemia/reperfusion injury in rats. Kaohsiung J Med Sci. 2009;25:374–80. doi: 10.1016/S1607-551X(09)70530-3. [DOI] [PubMed] [Google Scholar]
- 21.Istanbulluoglu MO, Zor M, Celik A, Cicek T, Basal S, et al. Effects of vardenafil on testicular torsion/detorsion damage: an experimental study in pigs. Urol Int. 2011;86:228–32. doi: 10.1159/000321492. [DOI] [PubMed] [Google Scholar]
- 22.Meštrović J, Drmić-Hofman I, Pogorelić Z, Vilović K, Šupe-Domić D, et al. Beneficial effect of nifedipine on testicular torsion-detorsion injury in rats. Urology. 2014;84:1194–8. doi: 10.1016/j.urology.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Kim GH, Shin JH, Kim KS, Lim JS. Effect of korean red ginseng on testicular tissue injury after torsion and detorsion. Korean J Urol. 2010;51:794–9. doi: 10.4111/kju.2010.51.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ünsal A, Eroglu M, Avci A, Cimentepe E, Guven C, et al. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand J Urol Nephrol. 2006;40:17–22. doi: 10.1080/00365590500407514. [DOI] [PubMed] [Google Scholar]
- 25.Akgül T, Ayyildiz A, Nuhoğlu B, Karagüzel E, Oğüş E, et al. Ginkgo biloba (EGb 761) usage attenuates testicular injury induced by testicular ischemia/reperfusion in rats. Int Urol Nephrol. 2008;40:685–90. doi: 10.1007/s11255-007-9296-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y, Lv Y, Liu YJ, Yang C, Hu HJ, et al. Hyperbaric oxygen therapy in rats attenuates ischemia-reperfusion testicular injury through blockade of oxidative stress, suppression of inflammation, and reduction of nitric oxide formation. Urology. 2013;82:489.e9–e15. doi: 10.1016/j.urology.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Kolski JM, Mazolewski PJ, Stephenson LL, Texter J, Grigoriev VE, et al. Effect of hyperbaric oxygen therapy on testicular ischemia-reperfusion injury. J Urol. 1998;160:601–4. [PubMed] [Google Scholar]


