Abstract
Recent studies have reported that caspase 7 has an apoptotic and nonapoptotic function. However, the relationship between caspase 7 and spermatogenesis remains unknown. This study aimed to investigate the possible function of caspase 7 during normal and abnormal spermatogenesis. The cleaved form of caspase 7 was detected in testis tissues at different postpartum times (5–14 weeks) by qRT-PCR, Western blot and immunohistochemistry (IHC). Then, the mice models of spermatogenic dysfunction were obtained by busulfan (30 mg kg−1 to further evaluate the potential function and mechanism of caspase 7. qRT-PCR and Western blot results showed that caspase 7 expression was gradually elevated from 5 to 14 weeks, which was not connected with apoptosis. IHC results revealed that caspase 7 was mainly located in spermatogenic cells and Leydig cells. In addition, spermatogenic dysfunction induced by busulfan gradually enhanced the apoptosis and elevated the expression of caspase 3, caspase 6, and caspase 9, but decreased the expression of caspase 7 in spermatogenic cells. However, when spermatogenic cells were mostly disappeared at the fourth week after busulfan treatment, caspase 7 expression in Leydig cells was significantly increased and positively correlated with the expression of caspase 3, caspase 6, and caspase 9. Therefore, these results indicate that caspase 7 has a nonapoptic function that participates in normal spermatogenesis, but also displays apoptotic function in spermatogenic dysfunction.
Keywords: apoptosis, caspase 7, caspases, spermatogenesis, spermatogenic dysfunction
INTRODUCTION
Caspase 7, a member of effector caspases (caspase 3, 6, 7), is involved in apoptosis and inflammation.1,2 Caspase 7 is supposed to be functionally redundant with caspase 3, which can be directly activated by caspase 9 to induce the cleave of cellular substrates during apoptosis.3,4,5,6 However, recent studies demonstrate that caspase 7 and caspase 3 have distinct functions.7,8 For example, caspase 7 knockout mice display normal eye lenses, whereas caspase 3 knockout mice result in marked cataracts at the anterior lens pole.9 Meanwhile, nonapoptotic functions of caspases have been reported.10 The nonapoptotic functions of caspase 3 are observed in bone marrow stem cells and odontogenic epithelial cells.11,12 The nonapoptotic functions of caspase 7 are found in tooth development, which play important roles in the differentiation of cells during odontogenesis.13,14 Moreover, the activated form of caspase 7 is presented in a number of bone cells, but it is not in connection with apoptosis.15 Thus, these studies suggest that caspase 7 has an apoptotic and nonapoptotic function. However, the nonapoptotic function of caspase 7 is rarely reported.
Recent studies report that caspase signal pathways are relative to the apoptosis of germ cells.16,17 However, whether the function of caspase 7 is involved in spermatogenesis remains unknown. This study mainly aimed to investigate the potential functions of caspase 7 during normal and abnormal spematogenesis.
MATERIALS AND METHODS
Animal
All C57 male mice were purchased and raised in Southern Medical University Animal Center. This study was supported by the University's Institutional Animal Care and Use Committee. Mice testis tissues were collected at different postpartum times (P5-P14 weeks).
Animal model construction
C57 male mice with 5 weeks old were randomly distributed to experimental group and control group. Twenty cases of mice in an experimental group were treated with the mixture of busulfan (30 mg kg−1 and dimethyl sulfoxide (DMSO) by intraperitoneal injection, and twenty cases of mice in the control group were treated with the same volume of DMSO solution. Five cases of mice in experimental group and control group were respectively anesthetized each week after the single injection of busulfan. Some fresh testis tissues were stored in a refrigerator at −80°C. Others were fixed with Bouin's solution for 12 h, dehydrated with xylene and embedded in paraffin. The sections of testis tissues were deparaffinized, rehydrated and stained with Hematoxylin-eosin (HE) for histological analysis.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from frozen testis tissues by using Trizol reagent (TAKARA, Japanese) and reversely transcribed into cDNA using Reverse Transcription System (TAKARA, Japanese). The PCR analysis of gene expression was quantitatively tested using the SYBR Green RT-PCR Kit (TAKARA, Japanese). The primer sequences of caspase 3 were (F) 5’- AGGAGGGACGAACACGTCT-3’ and (R) 5’-CAAAGAAGGTTGCCCCAATCT-3’. The primer sequences of caspase 6 were (F) 5’- AGGAGGGACGAACACGTCT-3’ and (R) 5’-CAAAGAAGGTTGCCCCAATCT-3’. The primer sequences of caspase 9 were (F) 5’- AGGAGGGACGAACACGTCT-3’ and (R) 5’-CAAAGAAGGTTGCCCCAATCT-3’. The primer sequences of caspase 7 were (F) 5’- GGACCGAGTGCCCACTTATC-3’ and (R) 5’-TCGCTTTGTCGAAGTTCTTGTT-3’. GAPDH was used as internal control, and the primer sequences were (F) 5’- AGGTCGGTGTGAACGGATTTG-3’ and (R) 5’- TGTAGACCATGTAGTTGAGGTCA-3’. All primers were purified and synthesized by the BGI Company (Shenzhen, China). Real-time PCR cycle conditions were: one cycles of 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 34 s.
Western blot analysis
Total protein was extracted from frozen testis tissues using RIPA buffer (1% NP-40, 0.1% sodium dodecyl sulfate, 5 mmol l−1 EDTA, 0.5% sodium deoxycholate and 1 mmol l−1 sodium orthovanadate) and protease inhibitors. Protein concentration was quantified by the bicinchoninic acid method. Protein lysates (50 μg) were separated by using 12% SDS-PAGE and electrotransferred to the polyvinylidene difluoride (PVDF) membranes (Biosharp, USA). Then, the membranes were blocked in PBST solution and incubated with polyclonal anti-caspase 7 (Dilution: 1:500, Bioworld Technology, USA, catalog numbers: BS3532) and anti-Tubulin (Dilution: 1:1000, Cell Signaling Technology, USA, catalog numbers: 15115) overnight at 4°C. Finally, the membrane was incubated with appropriate second antibody and visualized using enhanced chemiluminescence detection kit (Alpha Innotech, USA).
Immunohistochemical staining and evaluation criteria
All testis tissues were fixed with Bouin's solution for 12 h, dehydrated and embedded in paraffin. Two micrometer-thick deparaffinized sections were deparaffinized, rehydrated and quenched with 3% hydrogen peroxide, and antigenicity was repaired by 0.01 mol l−1 sodium citrate buffer (pH 6.0). Caspase 7 polyclonal antibody (Dilution: 1:100, Bioworld Technology, USA, catalog numbers: BS3532), caspase 3 polyclonal antibody (Dilution: 1:100, Bioworld Technology, USA, catalog numbers: BS6428), caspase 6 polyclonal antibody (Dilution: 1:100, Bioworld Technology, USA, catalog numbers: BS6861) and caspase 9 polyclonal antibody (Dilution: 1:100, Bioworld Technology, USA, catalog numbers: BS1388) were incubated at room temperature for 2 h. Then, the sections were treated with the second antibody at room temperature for 30 min. Finally, the sections were visualized by diaminobenzidine (DAB) and stained with hematoxylin. Fetal bovine serum (FBS) was used to replace the primary antibody and served as negative control. The sections with confirmed positive expression of caspase 7, caspase 3, caspase 6, and caspase 9 were treated as positive controls. Positive expression of caspase 7, caspase 3, caspase 6, and caspase 9 was stained as orange or brown in the nuclear and/or cytoplasm of cells. Higher intensity staining represented a higher level of immunoexpression.
TUNEL assay
All testis tissues were fixed with Bouin's solution for 12 h, dehydrated and embedded in paraffin. Two micrometer-thick deparaffinized sections were deparaffinized, rehydrated and quenched with 1% proteinase K (20 mg ml−1 at 37°C for 30 min. Then, sections were washed with PBS and incabuted with TUNEL mix (45 μl Equilibration Buffer, 1.0 μl biotin-11-dUTP, 4.0 μl TdT Enzyme, and 50 μl reaction buffer) at 37°C for 60 min. After washing with PBS, the sections were stained with DAPI. Apoptotic cell were stained as red using fluorescence microscopy at 570 nm.
Statistical analysis
Statistical analysis was performed by SPSS software (version 18.0; SPSS, Chicago, IL, USA). Independent samples t-test was used to evaluate the difference between two groups. One-way analysis of variance (ANOVA) was used for multiple comparisons. P < 0.05 was defined as statistically significant.
RESULTS
Caspase 7 is expressed in normal spermatogenesis
To investigate the relationship between caspase 7 expression and normal spermatogenesis, we detected the expression of caspase 7 in testis tissues at different postpartum times (5–14 weeks). qRT-PCR and Western blot results showed that caspase 7 expression was gradually elevated from 5 to 14 weeks (Figure 1a and 1b). IHC results (Figure 1c) showed that positive caspase 7 expression was observed in the nuclear and cytoplasm of spermatogonia, spermatocyte, round spermatids, elongated spermatozoon, and Leydig cells. Positive expression of caspase 7 was also observed in the cytoplasm of Leydig cells. HE staining of testes demonstrated that the morphology and number of spermatogenic cells and Leydig cells were normal at postpartum times (Figure 1d). To evaluate whether caspase 7 expression is correlated with apoptosis, we measured the mRNA level of caspase 3 and caspase 9. Results demonstrated that caspase 3 and caspase 9 levels were gradually decreased from 5 to 14 weeks (Figure 1e), suggesting that apoptosis was not actived in testis tissues at postpartum times.
Figure 1.
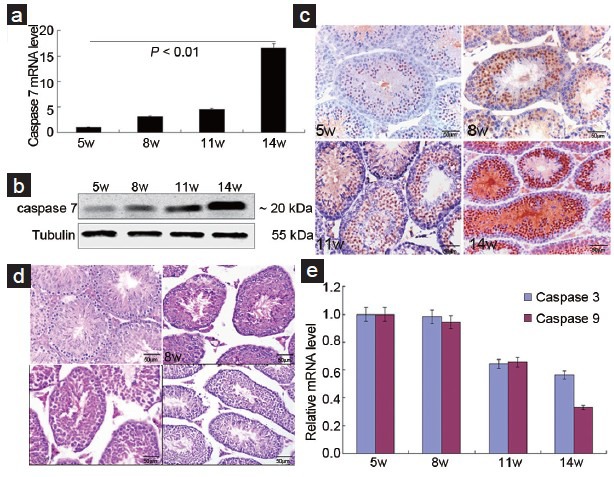
Caspase 7 expression participates in normal spermatogenesis. (a) Caspase 7 mRNA level was detected by qRT-PCR. (b) Caspase 7 protein was detected by Western blot. (c) Caspase 7 protein was detected by IHC (×40). (d) HE staining of testis tissues at different postpartum times (x40). (e) The mRNA levels of caspase 3 and caspase 9 were detected by qRT-PCR. Scale bar = 50 μm.
Caspase 7 expression correlates with spermatogenic dysfunction and germ cells apoptosis
To investigate whether caspase 7 correlated with spermatogenic dysfunction and germ cells apoptosis, we constructed mice models of spermatogenic dysfunction by busulfan. HE staining showed that spermatogenic cells in seminiferous tubules rather than Leydig cells were gradually disappeared at 4 weeks after busulfan treatment (Figure 2a), suggesting that the mice models of spermatogenic dysfunction was successfully obtained. Meanwhile, the morphology and number of spermatogenic cells and Leydig cells in control groups were normal, indicating that DMSO treatment did not impair normal spermatogenesis (data not shown). IHC results revealed that caspase 7 expression in spermatogenic cells was gradually reduced after busulfan treatment (Figure 2b). However, caspase 7 expression in Leydig cells did not significantly changed from 1 to 3 weeks after busulfan treatment (Figure 2b). Unexpectedly, caspase 7 expression in Leydig cells was markedly elevated at the fourth week after busulfan treatment (Figure 2b). Then, we detected the expression of caspase 7 in testis tissues by qRT-PCR (Figure 2c) and Western blot (Figure 2d). The results demonstrated that caspase 7 level was gradually reduced from 1 to 3 weeks. While caspase 7 level at the fourth week was significantly increased compared with those at the third week after busulfan treatment (P < 0.05). To evaluate whether caspase 7 correlated with the apoptosis of germ cells, we detected the levels of caspase 3, caspase 6, and caspase 9 in testis tissues by qRT-PCR and IHC. qRT-PCR results demonstrated that the mRNA levels of caspase 3, caspase 6, and caspase 9 were gradually increased after busulfan treatment (Figure 3a). Meanwhile, IHC results showed that the expression of caspase 3, caspase 6, and caspase 9 was significantly increased in testis tissues from 1 to 3 weeks after busulfan treatment (data not shown). Furthermore, when spermatogenic cells were mostly disappeared at the fourth week after busulfan treatment, the expression of caspase 3, caspase 6, and caspase 9 in testis tissues was still significantly greater compared with those controls (Figure 3b). Thus, these results indicated that the apoptosis in testis tissues was persistently activated after busulfan treatment. To further validate our observations, we evaluated the apoptosis in testis tissues by TUNEL assay. As shown in Figure 4, the apoptosis in testis tissues was hardly detectable in control groups, indicating that the apoptosis was nearly inactivated in normal spermatogenesis. While the apoptosis in testis tissues was significantly enhanced from 1 to 4 weeks after busulfan treatment compared with matched control groups.
Figure 2.
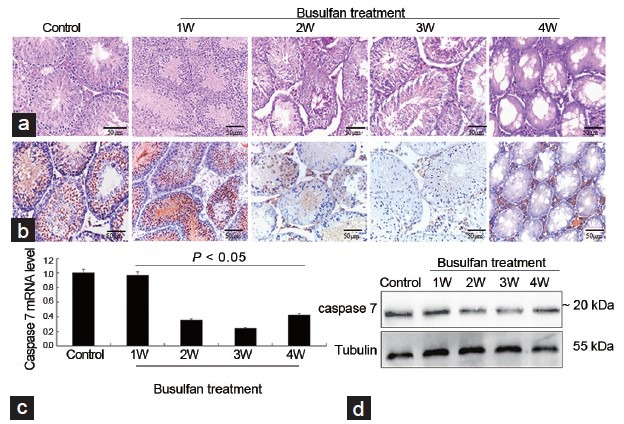
Spermatogenic dysfunction induced by busulfan affects the expression of caspase 7 in testis tissues. (a) HE staining of testis tissues after busulfan treatment (×40). (b) Caspase 7 expression was detected in testis tissues by IHC after busulfan treatment (×40). (c) The mRNA level of caspase 7 in testis tissues was measured by qRT-PCR. (d) The protein level of caspase 7 in testis tissues was measured by Western blot. Scale bar = 50 μm.
Figure 3.
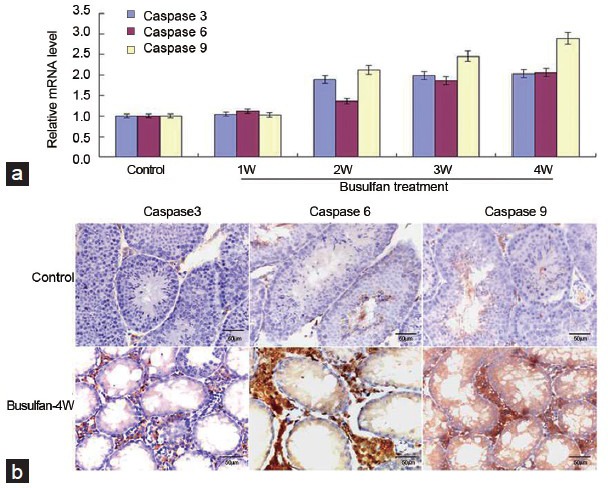
Spermatogenic dysfunction induced by busulfan activates the expression of caspase 3, caspase 6 and caspase 9 in testis tissues. (a) The mRNA levels of caspase 3, caspase 6 and caspase 9 were detected by qRT-PCR. (b) The protein levels of caspase 3, caspase 6 and caspase 9 were detected by IHC at the fourth week after busulfan treatment (×40). Scale bar = 50 μm.
Figure 4.
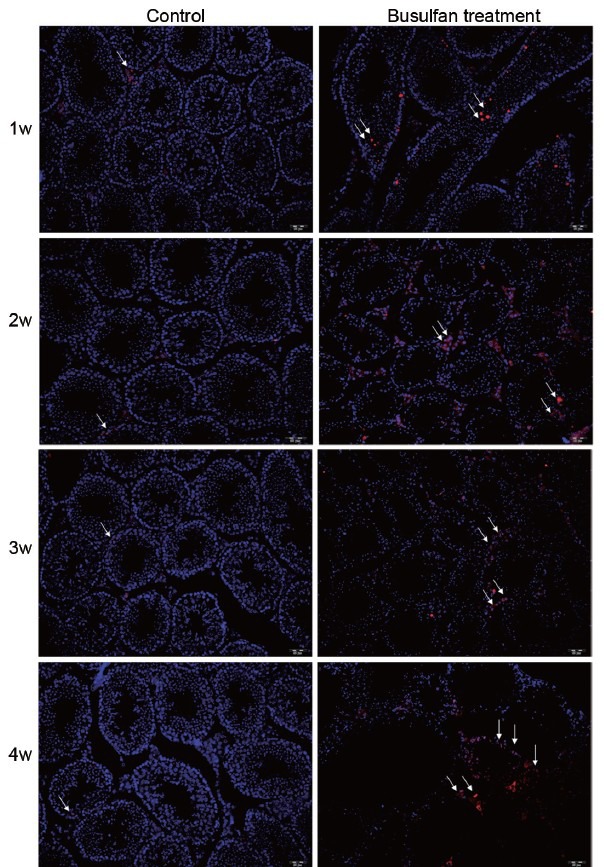
The apoptosis in testis tissues was evaluated by TUNEL assay (×20). White arrow indicated apoptotic cells.
DISCUSSION
In this study, to investigate the potential functions of caspase 7 during spermatogenesis, we first detected the expression of caspase 7 in testis tissues at different postpartum times (5–14 weeks). The qRT-PCR and Western blot results showed that caspase 7 expression was gradually elevated from 5 to 14 weeks. IHC results revealed that caspase 7 was highly expressed in spermatogenic cells and Leydig cells. These observations suggested that caspase 7 might participate with normal spermatogenesis and affect the growth regulation of spermatogenic cells. However, it is well-known that caspase-mediated apoptosis plays important roles in normal spermatogenesis.18,19,20,21 Caspase 7 together with caspase 3 and caspase 6 are effector caspases that can be activated by initiator caspases (caspase 2, caspase 8, caspase 9 and caspase 10) to cause cell apoptosis.22 Thus, these results seemed to be hard to exclude the apoptotic function of caspase 7. Hence, we detected the expression of caspase 3 and caspase 9 that would be activated during apoptosis. Results demonstrated that caspase 3 and caspase 9 levels were gradually decreased in testis tissues from 5 to 14 weeks, suggesting that apoptosis was not activated during testis development. Meanwhile, results also suggested that the cleave of caspase 7 was not resulted from the activation of caspase 9. Furthermore, HE staining of testis tissues demonstrated that the morphology and number of spermatogenic cells were normal. Furthermore, TUNEL assay demonstrated that the apoptosis was nearly inactivated in normal spermatogenesis. Therefore, above observations indicated that caspase 7 exhibited nonapoptotic function that participated in normal spermatogenesis, which might be correlated with the growth regulation of spermatogenic cells.
Although several caspases have been found to be involved in the apoptosis of germ cells,16,17 the relationship between caspase 7 and germ cells apoptosis has never been reported. Then, we constructed the models of spermatogenic dysfunction by busulfan. Current studies have demonstrated that busulfan treatment can markedly cause the apoptosis of germ cells and impair normal spermatogenesis.23,24,25 In this study, our results also showed that normal spermatogenesis was gradually impaired at 4 weeks after busulfan treatment. TUNEL assay also demonstrated that the apoptosis in testis tissues was enhanced after busulfan treatment compared with matched control groups. Moreover, busulfan treatment induced the expression of caspase 3, caspase 6, and caspase 9 during the apoptosis. It is well-known that caspase 7 together with caspase 3 and caspase 6 are effector caspases that can be activated by initiator caspases (caspase 2, caspase 8, caspase 9, and caspase 10) to cause cell apoptosis.22 However, our results indicated that caspase 7 expression in spermatogenic cells was gradually reduced, but its expression in Leydig cells was not significantly changed from 1 to 3 weeks after busulfan treatment. Meanwhile, qRT-PCR and Western blot results showed that caspase 7 expression in testis tissues was gradually decreased at 3 weeks after busulfan treatment. These results indicated that spermatogenic dysfunction induced by busulfan decreased the expression of caspase 7. Meanwhile, these observations also revealed that caspase 7 in spermatogenic cells was not combined with caspase 3, caspase 6, and caspase 9 to cause cell apoptosis, which further validated that caspase 7 had nonapoptotic function that participated in normal spermatogenesis. Furthermore, the nonapoptotic functions of caspase 7 were also found in molar tooth development, dental hard tissues formation, and osteogenesis.12,13,14 Unexpectedly, when spermatogenic cells were mostly disappeared at the fourth week after busulfan treatment, IHC results revealed that caspase 7 expression in Leydig cells was significantly elevated, which was positively correlated with the expression of caspase 3, caspase 6, and caspase 9. Furthermore, qRT-PCR and Western blot results demonstrated that caspase 7 expression in testis tissues was increased at the fourth week. Thus, these results suggested that caspase 7 displayed apoptotic function in Leydig cells, which might be correlated with the stable activation of caspase 3, caspase 6, and caspase 9.
CONCLUSION
These results demonstrate that caspase 7 has a nonapoptotic and apoptotic function during spermatogenesis. Under normal circumstances, caspase 7 predominantly exhibits the nonapoptotic function that participates in normal spermatogenesis. While normal spermatogenesis is persistently impaired and spermatogenic cells are mostly disappeared, caspase 7 expression in Leydig cells may be activated by caspase 3, caspase 6, and caspase 9 to induce apoptosis. Thus, these findings suggest that the location of caspase 7 may impact on its function, which is similar with the recent reports.13,14,15 However, whether the cleaved form of caspase 7 in germ cells is inevitable during normal spermatogenesis remains unclear. Furthermore, whether the depletion of cleaved caspase 7 would lead to spermatogenic dysfunction remains unknown. Therefore, further investigation is needed to validate our findings.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: XMM and BL. Performed the experiments: BL, XMZ and DJL. Analyzed the data: BW and HYW. Contributed reagents/materials/analysis tools: LRZ and FPS. Wrote and revise the manuscript: XMM and BL.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We thank all staffs at the Department of Pathology, Nanfang Hospital and Peking University Shenzhen Hospital. This study was supported by the National Natural Science Foundation of China (No. 81270743), Science and Technology Projects of Guangdong Province (No. 2013B021800147/2014A020212039) and Science and Technology Projects of Shenzhen (No. JCYJ20140415162542992).
REFERENCES
- 1.Erener S, Petrilli V, Kassner I, Minotti R, Castillo R, et al. Inflammasome-activated caspase 7 cleaves PARP1 to enhance the expression of a subset of NF-kappaB target genes. Mol Cell. 2012;46:200–11. doi: 10.1016/j.molcel.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Yang YB, Pandurangan M, Hwang I. Targeted suppression of mu-calpain and caspase 9 expression and its effect on caspase 3 and caspase 7 in satellite cells of Korean Hanwoo cattle. Cell Biol Int. 2012;36:843–9. doi: 10.1042/CBI20120050. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 4.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 5.Brentnall M, Rodriguez-Menocal L, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013;14:32. doi: 10.1186/1471-2121-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamkanfi M, Kanneganti TD. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol. 2010;42:21–4. doi: 10.1016/j.biocel.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–51. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh JG, Cullen SP, Sheridan C, Luthi AU, Gerner C, et al. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci U S A. 2008;105:12815–9. doi: 10.1073/pnas.0707715105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zandy AJ, Lakhani S, Zheng T, Flavell RA, Bassnett S. Role of the executioner caspases during lens development. J Biol Chem. 2005;280:30263–72. doi: 10.1074/jbc.M504007200. [DOI] [PubMed] [Google Scholar]
- 10.Kuranaga E. Beyond apoptosis: caspase regulatory mechanisms and functions in vivo. Genes Cells. 2012;17:83–97. doi: 10.1111/j.1365-2443.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 11.Miura M, Chen XD, Allen MR, Bi Y, Gronthos S, et al. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–13. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumamoto H, Kimi K, Ooya K. Immunohistochemical analysis of apoptosis-related factors (Fas, Fas ligand, caspase-3 and single-stranded DNA) in ameloblastomas. J Oral Pathol Med. 2001;30:596–602. doi: 10.1034/j.1600-0714.2001.301004.x. [DOI] [PubMed] [Google Scholar]
- 13.Matalova E, Vanden Berghe T, Svandova E, Vandenabeele P, Healy C, et al. Caspase-7 in molar tooth development. Arch Oral Biol. 2012;57:1474–81. doi: 10.1016/j.archoralbio.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Matalova E, Lesot H, Svandova E, Vanden Berghe T, Sharpe PT, et al. Caspase-7 participates in differentiation of cells forming dental hard tissues. Dev Growth Differ. 2013;55:615–21. doi: 10.1111/dgd.12066. [DOI] [PubMed] [Google Scholar]
- 15.Svandova E, Lesot H, Vanden Berghe T, Tucker AS, Sharpe PT, et al. Non-apoptotic functions of caspase-7 during osteogenesis. Cell Death Dis. 2014;5:e1366. doi: 10.1038/cddis.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida C, Correia S, Rocha E, Alves A, Ferraz L, et al. Caspase signalling pathways in human spermatogenesis. J Assist Reprod Genet. 2013;30:487–95. doi: 10.1007/s10815-013-9938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClusky LM. The caspase-dependent apoptosis gradient in the testis of the blue shark, Prionace glauca. Reproduction. 2013;145:297–310. doi: 10.1530/rep-12-0216. [DOI] [PubMed] [Google Scholar]
- 18.Dias TR, Rato L, Martins AD, Simoes VL, Jesus TT, et al. Insulin deprivation decreases caspase-dependent apoptotic signaling in cultured rat sertoli cells. ISRN Urol 2013. 2013:970370. doi: 10.1155/2013/970370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cayli S, Sakkas D, Vigue L, Demir R, Huszar G. Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Mol Hum Reprod. 2004;10:365–72. doi: 10.1093/molehr/gah050. [DOI] [PubMed] [Google Scholar]
- 20.Codelia VA, Cisternas P, Moreno RD. Relevance of caspase activity during apoptosis in pubertal rat spermatogenesis. Mol Reprod Dev. 2008;75:881–9. doi: 10.1002/mrd.20822. [DOI] [PubMed] [Google Scholar]
- 21.Lozano GM, Bejarano I, Espino J, Gonzalez D, Ortiz A, et al. Relationship between caspase activity and apoptotic markers in human sperm in response to hydrogen peroxide and progesterone. J Reprod Dev. 2009;55:615–21. doi: 10.1262/jrd.20250. [DOI] [PubMed] [Google Scholar]
- 22.Logue SE, Martin SJ. Caspase activation cascades in apoptosis. Biochem Soc Trans. 2008;36:1–9. doi: 10.1042/BST0360001. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Ok DW, Kwon DN, Chung JI, Kim HC, et al. Murine male germ cell apoptosis induced by busulfan treatment correlates with loss of c-kit-expression in a Fas/FasL- and p53-independent manner. FEBS Lett. 2004;575:41–51. doi: 10.1016/j.febslet.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Mirhoseini M, Saki G, Hemadi M, Khodadadi A, Mohammadi Asl J. Melatonin and testicular damage in busulfan treated mice. Iran Red Crescent Med J. 2014;16:e14463. doi: 10.5812/ircmj.14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Crespo M, Pericuesta E, Perez-Cerezales S, Arenas MI, Lobo MV, et al. Effect of liver growth factor on both testicular regeneration and recovery of spermatogenesis in busulfan-treated mice. Reprod Biol Endocrinol. 2011;9:21. doi: 10.1186/1477-7827-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


