Abstract
The genetic bases and molecular mechanisms involved in the assembly and function of the flagellum components as well as in the regulation of the flagellar movement are not fully understood, especially in humans. There are several causes for sperm immotility, of which some can be avoided and corrected, whereas other are related to genetic defects and deserve full investigation to give a diagnosis to patients. This review was performed after an extensive literature search on the online databases PubMed, ScienceDirect, and Web of Science. Here, we review the involvement of regulatory pathways responsible for sperm motility, indicating possible causes for sperm immotility. These included the calcium pathway, the cAMP-dependent protein kinase pathway, the importance of kinases and phosphatases, the function of reactive oxygen species, and how the regulation of cell volume and osmolarity are also fundamental components. We then discuss main gene defects associated with specific morphological abnormalities. Finally, we slightly discuss some preventive and treatments approaches to avoid development of conditions that are associated with unspecified sperm immotility. We believe that in the near future, with the development of more powerful techniques, the genetic causes of sperm immotility and the regulatory mechanisms of sperm motility will be better understand, thus enabling to perform a full diagnosis and uncover new therapies.
Keywords: antioxidants, calcium, membrane channels, protein kinases, sperm genetic abnormalities, sperm motility
MAIN FACTORS AFFECTING SPERM MOTILITY
Sperm motility is highly dependent on several metabolic pathways and regulatory mechanisms. Besides the involvement of specific gene defects, any abnormalities of these factors could be responsible for cases of poor sperm motility and consequently infertility.
Pathways and regulatory mechanisms involved in sperm motility
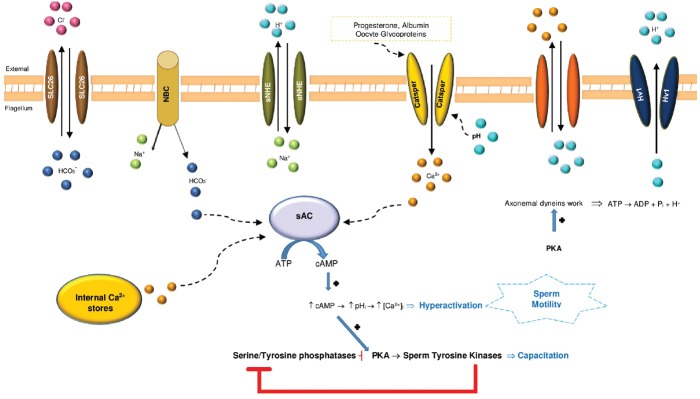
The calcium (Ca2+) pathway and the cyclic adenosine monophosphate (cAMP)-dependent protein kinase or protein kinase A (PKA) pathway are two important metabolic pathways involved in the regulation of sperm motility.1,2 These pathways involve calcium ions, adenylyl cyclases, bicarbonate ions, different membrane channels, and phosphorylation events. All are responsible for the acquisition of competences that will enable sperm to fertilize the oocyte, namely capacitation, hyperactivity, and acrosome reaction (Figure 1).
Figure 1.
Schematic representation of pathways believed of being involved in the regulation of mammalian sperm motility. Activation of a Na+/HCO3− (NBC) co-transporter and the regulation of HCO3−/Cl− by SLC26 transporters increase HCO3− levels.167 The activation of the sperm Na+/H+ exchanger (sNHE) aligned with the activation of the proton channel (Hv1) leads to a raise of the pHi, which activates CatSper, a cation channel of sperm that enables the entry of Ca2+ and thus increases the internal Ca2+ concentration ([Ca2+]).168 Progesterone, a steroid hormone synthesized by the cumulus/granulosa cells, activates CatSper either by binding to the channel itself or to an associated protein.27 Further, albumin, the main protein of human blood plasma and oocyte glycoproteins, together with alkalinization of the sperm cytoplasm also, elevates the internal [Ca2+].169,170 The overall Ca2+ increase may influence glycolysis and the axoneme activity promoting hyperactivation of motility.171 Further, HCO3− and Ca2+ regulate the atypical soluble adenylyl cyclase (sAC), which generates cAMP and that by its turn activates protein kinase A (PKA). PKA induces phosphorylation of axonemal dynein, leading to consumption of ATP and thus increases the pHi. PKA activates sperm tyrosine kinases (with serine and threonine residues) to trigger a cascade of protein phosphorylation involved in sperm motility.24,35,47 Increased cAMP may activate PKA that in turn activates tyrosine kinase and seems to inhibit tyrosine phosphatase.172 The Ca2+ levels are regulated by a plasma membrane Ca2+-ATPase pump (PMCA4), expressed in the principal piece of the axoneme, which extrudes Ca2+ and is essential for hyperactivated motility and male fertility.173
Cellular levels of cAMP are controlled by adenylyl cyclases (ACs) that catalyze an intramolecular cyclization of ATP to cAMP under release of pyrophosphate.3 The mammalian ACs can be separated into two distinct types, transmembrane AC enzymes (tmACs) and soluble AC (sAC, also known as AC10). Soluble AC is directly activated by bicarbonate and Ca2+ and acts as a sensor for ATP, Ca2+, and bicarbonate/CO2 /pH at various intracellular locations. Soluble ACs are the only signaling proteins known to be directly regulated by bicarbonate. Mammalian tmACs, in contrast, are not responsive to bicarbonate. Instead, they are mainly regulated by heterotrimeric G-proteins, as part of the G-protein coupled receptor pathways.3,4 Both ACs are known to play an important role in male fertility. Transmembrane AC is involved in the basic mechanism for motility activation through cAMP-dependent protein phosphorylation and in progressive motility.5,6 Soluble AC is the predominant adenylyl cyclase responsible for the generation of most cAMP in spermatozoa and plays a critical role in cAMP signaling and is involved in the increase in beat frequency in spermatozoa.7,8 Inactivation of sAC gene leads to male sterility given the lack of forward motility.9 Cyclic AMP is thus essential for sperm motility regulation and fertility with reduction of cAMP levels associated with reduced sperm motility (Figure 1).10,11
Calcium is a fundamental regulatory factor for sperm capacitation, hyperactivation, and acrosome reaction. At low intracellular Ca2+ concentrations, flagella beat symmetrically but when Ca2+ levels rise in activated sperm (Ca2+ of 10–40 nM), the waveform becomes more asymmetric, and sperm becomes hyperactivated (Ca2+ of 100–300 nM).12,13 However, high levels of Ca2+ (about 9 μM) suppress motility. This inhibition seems to be due to a decrease of protein phosphorylation (caused by substrate depletion or to conformational changes) induced by Ca2+, which prevents substrate-kinase interactions.14,15 Calcium is also involved in the regulation of dynein-driven microtubule sliding.16 Calmodulin is a key axonemal Ca2+ sensor, and the calmodulin-dependent kinase may mediate this Ca2+ signal. These complexes, localized at the sperm axoneme, are regulated by the central pair complex and radial spokes. Calmodulin regulates motility through direct interaction with protein kinases,17 phosphatases,18 and sAC (Figure 1).7,8
Bicarbonate (HCO3−) ions also play a critical role in the regulation of sperm function (Figure 1). It is an anion of the female reproductive tract transported into sperm during capacitation. An increase in Ca2+ stimulates sAC; it converts ATP to cAMP and increases cAMP levels.19,20 As HCO3− is required for Ca2+ uptake, it causes the same effects. The treatment in vitro with HCO3− evokes Ca2+ entry, which rapidly increases flagellar beat frequency but decreases flagellar beat asymmetry.21 As a result, serine/threonine PKA is activated, which then phosphorylates serine and threonine residues on neighboring proteins to trigger a cascade of protein phosphorylation events.22 The presence of proteins in the fibrous sheath (FS) with PKA anchoring sites strongly suggests that one of the major roles of this structure is to anchor PKA in the principal piece of the flagellum. Cyclic AMP promotes both capacitation and the acrosome reaction and activates PKA (Figure 1).22,23,24 PKA subunits are expressed differentially. The regulatory subunit RIα is expressed throughout male germ cell development, RIIα only appears at the late stages in spermatogenesis, and the catalytic subunit Cα2 is only expressed in sperm. It is believed that the activation of PKA increases flagellar beat frequency and tyrosine phosphorylation to prepare the capacitated sperm for fertilization.22 PKA localizes at the principal piece of the flagellum, and Cα2 null males are completely infertile.25,26
Several Ca2+-permeable-specific channels have been found in sperm based on immunostaining or on the presence of transcripts in spermatogenic cells, such as high voltage-gated Ca2+ channels, cyclic nucleotide-gated channels, cation channels of sperm (CatSper), and transient receptor potential channels (Figure 1). These are a family of alkalinization-activated cation channels (CATSPER-1-4) that are highly conserved in humans. They are the principal Ca2+ channels activated by progesterone in human sperm.27 Mutations in these channels were associated with human infertility and also suggested as a target for development of a male contraceptive.28,29,30,31 Thus, it is likely that Ca2+ plays different roles in distinct stages of the sperm journey.
Phosphorylation is essential in almost every aspect of the cell life, and protein kinases are known to regulate important signaling pathways and cellular processes such as transcription, cell-cycle progression, cell movement, apoptosis, and immunological functions. Protein kinases share a conserved catalytic domain that transfers a phosphate group from ATP and covalently attaches it to specific amino acids with a free hydroxyl group, frequently on both serine and threonine amino acids (serine/threonine kinases). Calcium is important to activate the kinase through limited proteolysis by Ca2+-dependent protease.32 Phosphorylation (Figures 1 and 2) substantially contributes to proper functioning of sperm proteins,33,34 and it seems to be a necessary prerequisite for a sperm to fertilize an oocyte.35,36,37,38 During capacitation, it was detected an increase in the phosphotyrosine content of human FS proteins,39 which makes evident involvement of protein tyrosine phosphorylation in the control of sperm motility.
Figure 2.
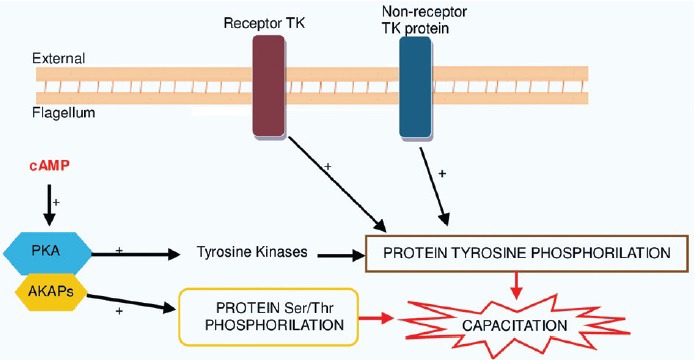
The increase in protein tyrosine phosphorylation during capacitation has been shown to be regulated by a cAMP-dependent pathway involving protein kinase A (PKA), receptor tyrosine kinase pathway, and by the nonreceptor protein tyrosine kinase pathway.35 cAMP has been shown to activate PKA, which in turn regulates protein tyrosine phosphorylation.48 The binding of PKA regulatory subunit to the AKAP family of proteins promotes an increase in tyrosine phosphorylation of sperm proteins by indirect activation of Tyrosine kinases (TKs).174 In human sperm, AKAPs proteins, namely AKAP3 and FSP95, are the most prominent tyrosine phosphorylated proteins during capacitation. Receptors TK is transmembrane proteins having an extracellular ligand binding domain and an intracellular tyrosine kinase domain. Upon extracellular ligand binding, a receptor TK are activated and then phosphorylates it (autophosphorylation) or other proteins. By contrast, nonreceptor protein TK lacks a transmembrane domain, most are soluble intracellular proteins located in the cytoplasm, nucleus, or anchored to the inner leaflet of the plasma membrane. Tyrosine and protein phosphorylation of the sperm flagellar proteins leads to capacitation of human sperm.47,175,176
In human sperm, the A-kinase anchoring proteins (AKAPs) (AKAP3 was formerly called FSP95), Ca2+-binding and tyrosine phosphorylation-regulated protein (CABYR), which is localized in the FS, are the most prominent tyrosine phosphorylated proteins during capacitation.40,41,42,43 Immotile sperm with deficiency in tyrosine phosphorylation do not capacitate properly, and this has been related to altered sperm membrane lipid composition, particularly due to high cholesterol content, which would impair the ability of this sperm to respond to capacitation-inducing stimuli.44,45,46 Sperm protein phosphorylation is highly regulated, and there are several pathways involved.34,35,47
The cAMP-dependent pathway (Figures 1 and 2) can also regulate protein tyrosine phosphorylation by stimulation of PKA activity, because PKA activates some intermediate tyrosine kinases involved in sperm motility.20,48 It was demonstrated that the presence and activity of a kinase (PI 3-kinase) in human sperm and its inhibition results in an increase in intracellular cAMP levels and in tyrosine phosphorylation of the protein AKAP3. This results in the binding of PKA to AKAP3, which is important for motility. These results provide a confirmation that PKA can be targeted to sperm tails by interaction with tyrosine phosphorylated form of AKAP3.49 AKAP scaffolding proteins are thus very important in regulating sperm motility as they sequester enzymes, such as protein kinases and phosphatases with the appropriate substrates to coordinate phosphorylation and dephosphorylation events.50 In humans, AKAP3 and AKAP4 are the most abundant structural FS proteins that anchor cAMP-dependent PKA.43
The role of reactive oxygen species in the acquisition and control of sperm motility
Reactive oxygen species (ROS), such as the superoxide anion (O2−), hydrogen peroxide (H2O2), and nitric oxide (NO−), are chemically reactive molecules resulting from oxygen consumption. At certain concentrations, ROS are of extreme importance to sperm function.51,52 It was shown that O2− triggers hyperactivation and capacitation, since the presence of superoxide dismutase (enzyme that catalyzes the dismutation of O2− into oxygen and H2O2) blocks both events.53,54 Other studies also gave evidence of the involvement of ROS on sperm function by demonstrating that low levels of NO− induce capacitation and that at higher levels, it blocks sperm motility.55,56 Thus, low levels of O2− are required to the capacitation process with H2O2 acting as an inductor of the acrosome reaction with high O2− levels being deleterious for sperm function57 and higher H2O2 levels adversely affect sperm motility parameters.58
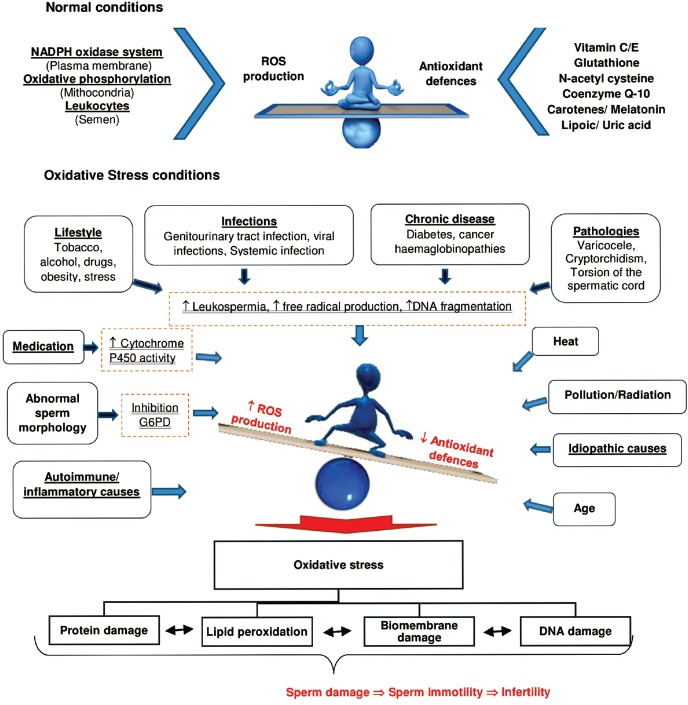
The cAMP/PKA pathway is also dependent of ROS.52,59 ROS are naturally originated in the human ejaculate: (a) sperm by themselves from spontaneous production through the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase system at the level of the sperm plasma membrane,60,61,62,63 or by the natural production of ROS by mitochondria, which is considered the main source of ROS in spermatozoa;64,65 (b) from leukocytes infiltrated into semen.66 When ROS production is heightened or a compelling reduction in the effectiveness of antioxidant defenses arises, an imbalance between ROS production and the biological system's aptitude to withdraw or repair the ROS damage occurs, which is known as oxidative stress (Figure 3). This can be caused by several factors.67 Cytoplasmic droplets (excess of residual cytoplasm) are a result of defective spermiogenesis and a considerable source of ROS.68
Figure 3.
Causes that lead to an oxidative stress condition. Here, these are represented the main mechanisms by which ROS are produced both naturally and in unhealthy conditions. NADPH: nicotinamide adenine dinucleotide phosphate; G6PD: glucose-6-phosphate dehydrogenase.
Leukocytospermia is also positively correlated with an increase in ROS production.69,70 Genitourinary tract infections,71 chronic inflammation,72 and pathologies such as varicocele73 induce ROS production and contribute to sperm immotility. The lifestyle, including smoking,74 dietary deficiencies,75 excessive alcohol consumption,76 psychological stress,77 the contact with environmental pollutants,78 and age,79 are all positively correlated with oxidative stress and linked with sperm immotility. Thus, increased ROS levels have been correlated with decreased sperm motility and with the proportion of various sperm head and tail anomalies,80,81 and some hypotheses have been proposed for this correlation (Figure 3).
One hypothesis is that ROS inhibit the activity of some enzymes such as glucose-6-phosphate dehydrogenase (G6PD), which through the hexose monophosphate pathway controls the intracellular availability of NADPH. Inhibition of G6PD leads to a decrease in the availability of NADPH and a parallel accumulation of oxidized glutathione and reduced glutathione. This leads to a reduction in the antioxidant defenses of the sperm and peroxidation of membrane phospholipids.62,82 Another way of injury may be due to the fact that high ROS levels induce a cascade of events that result in a decrease in axoneme protein phosphorylation and sperm immobilization.83 Advantages of this effect of ROS in sperm function are being used to develop contraceptives. Besides the direct influence on sperm motility, the increase of ROS is also related with an increase of DNA damage.84,85
The control of cell volume and osmolarity is fundamental for sperm motility
The maintenance of a correct cell volume and osmolarity is vital. During maturation and at ejaculation, sperm experiences great changes in its environment, namely rapid changes in the osmotic environment, once the osmolarity of cauda epididymidal fluid (osmol: 342 mmol kg−1) is higher than the contents of uterus (osmol: 284 mmol kg−1).86,87 When sperm encounters hypo or hypertonic environments, they tend to swell or shrink owing to the influx or efflux of water during reestablishment of the osmotic equilibrium. To maintain cell functionality in face of these osmotic changes, sperm possesses volume regulatory abilities, particularly regulatory volume decrease in response to hypotonic challenge (Figure 4).88 Defects in the mechanisms of volume regulation and in the epididymal osmolyte uptake cause an abnormal increase in sperm head volume and angulation of sperm tail that leads to an alteration of movement patterns, compromising forward progression resulting in defects in sperm motility and fertility.86
Figure 4.
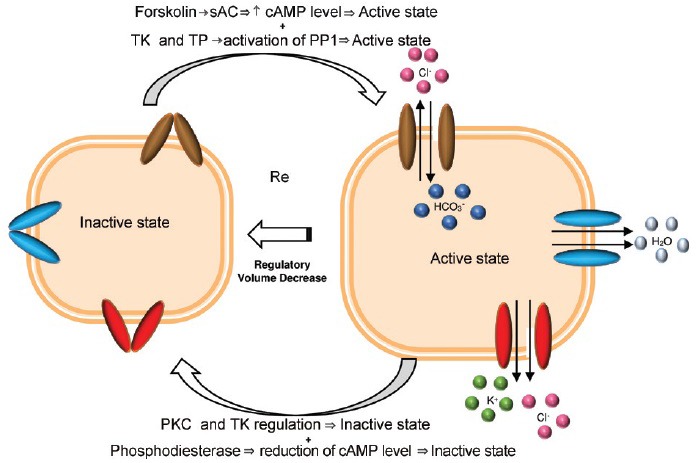
Transmembrane ion transport proteins and mechanisms thought to be involved in the regulatory volume decrease (RVD) of sperm. Reduction of cell volume is characterized by H2O removal via aquaporins (blue tubules) due to K+ and Cl− efflux via K+ and Cl− channels and co-transporters (red tubules) and coupled Cl−/HCO3− exchangers (brown tubules).86,92,94,177 Protein kinase-C (PKC) and tyrosine kinase (TK) are believed to be involved in the signaling sequence that leads to deactivation of the regulatory mechanism by closing and keeping closed the anion channel (inactive state). Inhibition of PKC, probably by dephosphorylation of the residues phosphorylated by PKC, which results in activation of the channel, increases the isotonic cell volume. Protein phosphatase 1 (PP1) may be involved in the signaling sequence that leads to the activation of the regulatory mechanism, possibly by the opening the ion channels (active state) and thus reducing the cell volume. The inhibition of PP1 results in blocked RVD. The TK and tyrosine phosphatase (TP) are believed to be involved in the activation and regulation of PP1 activity. Besides PP1, PKC, TK, and TP regulation is also assumed that cAMP-dependent pathway and Ca2+ channels (not represented) are also involved in regulation of sperm volume. Under hypotonic conditions, forskolin, a potent stimulator of soluble adenylyl cyclase (sAC), activates sAC that in turn leads to an increase in cAMP levels. This increase of cAMP levels may cause an activation of the mechanism of RVD (opening the ion channels). By contrast, activation of phosphodiesterase, an enzyme belonging to a group of enzymes that degrades the phosphodiester bond in the second messenger molecules cAMP, results in decrease of cAMP levels with opposite effects.178
The cytoplasmic droplet found at the midpiece of some sperm is a portion of excessive residual cytoplasm that is normally lost during the final maturation phase of spermiogenesis. The normal connecting piece and upper midpiece contain a small portion of cytoplasm with a few endoplasmic reticulum vesicles. The midpiece is the major site of water influx and cell volume regulation, and these vesicles are important when sperm face hypo-osmotic challenges.89 The cytoplasmic droplet is indeed, really important for sperm function given that spermatozoa without it were immotile due to a defective spermatogenesis.90 In addition to cytoplasmic droplet, it has been reported that sperm osmolytes, namely glutamate and K+91 and K+ and Cl− channels are involved in mechanisms of sperm regulatory volume decrease.92,93 Calcium is also known to be involved in the regulation of cell volume, namely by the activation of Ca2+-dependent K+ channels (Figure 4).94,95
Ejaculated sperm is immersed in the seminal plasma, a medium composed of aliquots of the fluid of the testis, epididymal tail, and the secretions of the accessory sexual glands. It also contains a wide variety of factors that influence the functionality of sperm. Sperm motility is also negatively influenced by seminal osmolarity, as patients with normal motility exhibit a mean value of semen osmolarity significantly lower (Ca2+: 3.36 mmol l−1; osmol: 318 mmol kg−1) than that of patients with low sperm motility (Ca2+: 3.10 mmol l−1; osmol: 345 mmol kg−1).96 Seminal plasma proteins are also considered modulators of sperm function and several important biological roles have been attributed to them.97
The striking reduction of cell volume is one of the most distinct morphological changes during the differentiation of spermatids into sperm and is largely due to osmotically driven fluid efflux. Aquaporins (AQP) may be involved in the rapid reduction of spermatid volume during spermiogenesis, the final step of spermatogenesis.98 In humans, it was detected the presence of the water transport protein AQP3 in the principal piece of ejaculated sperm,99 and of AQP7 in the tail of spermatids and testicular spermatozoa, as well as at the midpiece and the anterior flagellum portion of ejaculated sperm.100
The AQP3 was shown to be essential for sperm volume regulation, which is important for the balance between sperm motility and swelling in response to physiological hypotonicity, since AQP3-deficient sperm exhibited hampered migration in the oviduct, resulting in reduced male fertility.99 Regarding the AQP7 expression, it was observed that its absence in ejaculated sperm of some infertile patients was directly correlated with motility rate.100
Molecular abnormalities and associated flagellar sperm structure
Normal sperm morphology is one of the most informative semen parameters used for infertility diagnosis,101 It is correlated with poor sperm motility,102 as an isolated event was not associated with a decreased probability of pregnancy.103 Abnormalities in the sperm structure can occur as a single defect or attain different sperm components.104 In the large majority of the cases, the ultrastructural analysis of immotile sperm reveals nonspecific flagellar anomalies that include disruption of the normal axoneme pattern in association with other components of the sperm tail.105 Specific defects are however found in dysplasia of FS (DFS) and primary ciliary dyskinesia (PCD).
DFS is characterized by a marked hypertrophy and hyperplasia of the FS. Typically, the annulus is not formed, and the abnormal FS invades the midpiece. It is also frequent to observe absence of the central pair complex and dynein arms (DA) (Figure 5).106,107 It has been estimated that about 20% of DFS cases have a familiar incidence and family tree analysis seems to indicate an autosomic recessive inheritance.108,109 However, there are no consensus if DFS is a genetic disorder. Indeed, no association between DFS and defects in genes that code for AKAP3 and AKAP4 proteins were found.110,111,112
Figure 5.
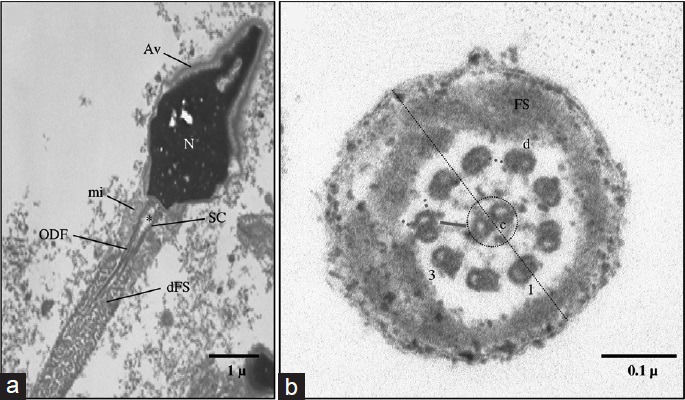
(a) Ultrastructure of sperm from a patient with fibrous sheath dysplasia. Nucleus (n), acrosomal vesicle (AV), segmented columns (SC), proximal centriole (*), mitochondria (mi), and outer dense fibers (ODF). Note the absence of the annulus and the invasion of the midpiece by marked hypertrophy and hyperplasia of the fibrous sheath (FS). (b) Ultrastructure of sperm from a patient with situs inversus. Distal principal piece (PP): axoneme and FS. The 9 peripheral doublets (d) and the radial spokes (green line), the 2 single central microtubules (c), the central bridge (red line) and the fibrilar sheath (dashed circle) are intact. Note the absence of dynein arms (dashed pink lines) and of nexin bridges (dashed blue line), the doublet indicated by a perpendicular line (dotted line) to the central microtubules is named N°.1 with the following numbering in the clockwise direction.
Regarding PCD, it is a genetic, heterogeneous, and autosomal recessive disease that is characterized by cilia immotility due to absence of DA (Figure 5), resulting in recurrent infections of the upper respiratory tract. Absence or dislocation of the central pair complex, defects of radial spokes, and doublet abnormalities are also common. In about 50% of PCD cases, patients present Kartagener syndrome, which is characterized by the combination of situs inversus.106,107 Therefore, investigations into the genetic basis of PCD have started by analysis of DA proteins.113 However, nowadays, several genes associated with flagellar structures are known to be associated with PCD.114
The sperm flagellum is a highly complex structure with several molecular components that are responsible for its assembly, composition, and function (Figure 6). The molecular composition of the human flagellum is still not completely understood and what is known came mainly from the study of model species such as mouse,115 marine invertebrates, and protists.116 As motor of sperm, the axoneme is one of the most studied structures. Due to the high complexity of sperm, any alteration in external and/or internal factors regulating sperm motion as well as in the cellular structure and metabolism involved in generating flagellar beat may result in defects in sperm motility, which consequently results in male infertility. In humans, a strict association between mutations in some genes and alteration in sperm motility is not simple to make due to the high number of variables. Consequently, a list of genes and its relation to sperm motility are still scarce. Nevertheless, due to the degree of conservation of many genes, some, isolated or associated with syndromes, have already been proved to be responsible for some cases of human infertility associated with poor sperm motility.114,117
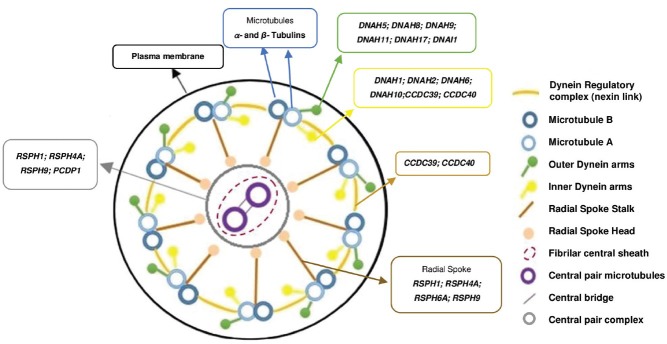
Figure 6.
Scheme of the axoneme components and the main genes known to be associated to each component in Humans. At right is the legend of each component represented in the scheme.
Dyneins are motor proteins that convert the chemical energy contained in ATP into the mechanical energy of movement. The outer and inner DA are composed of heavy chains (HCs), intermediate chains (ICs), and light chains (LCs).116,118 The HC contains the motor machinery that is responsible for transducing chemical energy into directed mechanical force applied to the microtubule surface as it possesses the sites of both ATP hydrolysis and ATP-sensitive microtubule binding; the IC participates in the structural attachment of the DA to flagellar microtubules; and the LC participates in several functions, such as redox-sensitive vicinal dithiols, Ca2+-binding, and intraflagellar transport. The variety of structure and function of these chains indicates that many regulatory mechanisms are present and needed for the proper sperm motility.118,119 Multiple dynein genes are found in the genomes of organisms with motile cilia and flagella.120
At least, five human genes are known to encode for outer DA HC genes such as DNAH5 (dynein, axonemal, and heavy chain 5), DNAH8, DNAH9, DNAH11, and DNAH17. Relative to the inner DA, there are eight human genes such as DNAH1, DNAH2, DNAH3, DNAH6, DNAH7, DNAH10, DNAH12, and DNAH14. The intermediate and light chains are thought to contain at least five genes, including DNAI1 (dynein, axonemal, and intermediate chain 1), DNAI2, DNAL1 (dynein, axonemal, and light chain 1), DNAL4, DNALI1, and NME8 (NME/NM23 family member 8) (National Center for Biotechnology Information-NCBI-database-accessed in October 2014). As these chains regulate DA activity, mutations may result in abnormal ciliary ultrastructure and function and were already associated with syndromes such as PCD.114,117
Another essential structure for sperm function is the dynein regulatory complex (DRC), which functions for dynein regulation and limitation of doublet sliding.121 Some components of the DRC serve primarily to regulate DA activity while others play a role in mediating structural interactions between the DA and the radial spokes.122,123 Four genes were identified as components of the DRC in humans such as DRC1 (dynein regulatory complex subunit 1), DRC7, CCDC39 (coiled-coil domain containing 39), and CCDC40.
The radial spokes and central pair complex are also essential for sperm function since they are important regulators of DA. Mutations that disrupt assembly of the central pair complex generally result in abnormal motility.124,125 Radial spokes and central pair complex are involved in converting simple symmetric bends into the asymmetric waveforms required for forward swimming and in the release of ATP inhibition in a controlled manner.126,127 In addition, the central pair may function as a distributor to provide a local signal to the radial spokes that selectively activates subsets of DA.128 In humans, it has been already described at least seven radial spokes proteins and its encoding genes are RSPH1 (radial spoke-head 1 homolog), RSPH3, RSPH4A, RSPH6A, RSPH9, RSPH10B, and RSPH10B2 (NCBI and UniProt databases, accessed July 2014). One of the most known radial spokes genes is the RSPH1 gene that encodes a radial spoke-head protein that is mainly expressed in respiratory and testis cells. It is important for the proper building of the central pair complex and radial spokes, since mutations in RSPH1 lead to an abnormal axoneme configuration with central pair complex and radial spokes defects,129,130 whereas mutations in RSPH4A and RSPH9 were associated with anomalies in central pair complex.125 Using next generation sequencing, mutations in CCDC39 and CCDC40 genes were also found among individuals with PCD with IDA and central pair complex defects.131
As mitochondria provide part of the energy for motility, dysfunctions of the human mitochondrial sheath as well as of mitochondrial membrane integrity represent the main feature of sperm immotility.65,132 Mitochondrial DNA (mtDNA) mutations/deletions might have several implications to male fertility.133,134,135,36 The integrity and copy number of mtDNA were significantly correlated with sperm count and motility, as they were related to an increase of excessive ROS formation through increased lipid peroxidation in men presenting large-scale mtDNA deletions.135,136,137,138,139
In humans, the lack of the annulus causes a disorganization of the midpiece-principal piece junction with associated sperm immotility, altogether with mitochondrial structural and functional disability.140 Septins (SEPT) are essential structural components of the human annulus.141 It was shown that SEPT4 an SEPT12 are essential for the structural and mechanical integrity of sperm, including proper mitochondrial architecture and establishment of the annulus,141,142 and SEPT7 was shown to be involved in the regulation of sperm morphology and maturation.143 In patients with sperm immotility and annulus defects, a defective labeling for SEPT4 and/or SEPT7 was observed, and these proteins were suggested as biomarkers for monitoring the status of spermiogenesis and sperm quality.144
PREVENTIVE AND THERAPEUTIC APPROACHES TO IMPROVE SPERM MOTILITY
There is no present treatment to sperm immotility due to genetic causes. However, the quality of sperm, which includes sperm motility, can be protected. As discussed above, unhealthy lifestyle habits (recreational toxics, physical inactivity, and excessive use of personal technologies), specific toxic environmental exposures, and several pathologies related to endocrine and cardiovascular diseases are correlated with sperm oxidative stress and can be totally avoided.145,146,147,148,149,150
Other conditions that also increase sperm oxidative stress can be treated by surgery (varicocele)73 by the use of corticosteroids (presence of anti-sperm antibodies following chronic genital tract inflammations)151,152,153 and the correct and timely use of antibiotics for genital tract infections.154,155,156
Based on the knowledge that the human body developed an antioxidant system to keep ROS at an optimum level, several antioxidants have been used to improve sperm motility both in healthy75 and infertile men.157,158
For instance, Vitamin E is a potent peroxyl radical scavenger that functions as a chain-breaking antioxidant. This prevents the propagation of free radicals in membranes and plasma lipoproteins (prevents lipid peroxidation), and decreases the levels of malondialdehyde (an organic compound that is used as a marker for oxidative stress),159 thus improving sperm motility.158,160 Vitamin C (L-ascorbic acid) acts as a reducing agent by donating electrons to various enzymatic reactions. It protects against oxidative stress behaving as a scavenger of ROS (prevents lipid pexoxidation). In addition, by recycling Vitamin E, it protects against DNA damage induced by the H2O2 radical. This molecule is also widely used in preventive treatments.159 Another example is Coenzyme Q10 (ubiquinone), which is a component of the electron transport chain in the mitochondrial respiratory chain. The energy generated is dependent on its availability in the human body. It is also an antioxidant that acts by stabilizing membranes and recycling Vitamin E. It is currently used for treatment of sperm immotility, especially in idiopathic asthenozoospermia.161
Hormonal agents and sperm vitalizers can also be used to improve sperm motility.162 For instance, pentoxifylline, a phosphodiesterase inhibitor, was shown to increase sperm motility163,164 by interfering with the metabolism of cAMP.165
However, although the use of these agents has been positively correlated with sperm motility,166 they can cause adverse effects.155 Consequently, more studies are needed to determine the optimal doses for each compound and establish a solid link with the desired effects.
CONCLUSIONS
This review has explored a little of the complex process underlying sperm motility, which has several pathways and genes involved. In a well-designed process, a minimal alteration may lead to male infertility. Besides preventive measures and some empirical therapies, there is the urgent need for developing a safe and directed therapy based on the genetic causes of sperm immotility and on the pathways that govern sperm motility.
AUTHOR CONTRIBUTIONS
RP Literature search; data analysis and interpretation; text writing. RS Critical discussion; manuscript critical review. AB Patient samples; critical review of the manuscript. MS Study conception and design; data analysis and interpretation; final text writing. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing financial interests.
ACKNOWLEDGMENTS
No particular funding was used for this study. RS, AB, and MS are employees of Public Universities. RP was an MSc student at ICBAS-UP. UMIB is funded by National Funds through Foundation for Science and Technology (FCT) under the Pest-OE/SAU/UI0215/2014.
REFERENCES
- 1.Darszon A, López-Martínez P, Acevedo JJ, Hernández-Cruz A, Treviño CL. T-type Ca2+ channels in sperm function. Cell Calcium. 2006;40:241–52. doi: 10.1016/j.ceca.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Darszon A, Beltrán C, Felix R, Nishigaki T, Treviño CL. Ion transport in sperm signaling. Dev Biol. 2001;240:1–14. doi: 10.1006/dbio.2001.0387. [DOI] [PubMed] [Google Scholar]
- 3.Steegborn C. Structure, mechanism, and regulation of soluble adenylyl cyclases-similarities and differences to transmembrane adenylyl cyclases. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2014;1842:2535–47. doi: 10.1016/j.bbadis.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv. 2002;2:168–84. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 5.Dey S, Roy D, Majumder GC, Bhattacharyya D. Extracellular regulation of sperm transmembrane adenylyl cyclase by a forward motility stimulating protein. PLoS One. 2014;9:e110669. doi: 10.1371/journal.pone.0110669. doi: 10.1371/journal.pone. 0110669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiba K, Inaba K. Distinct roles of soluble and transmembrane adenylyl cyclases in the regulation of flagellar motility in ciona sperm. Int J Mol Sci. 2014;15:13192–208. doi: 10.3390/ijms150813192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–62. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 9.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci U S A. 2004;101:2993–8. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyes A, Martinez R, Luna M, Chavarría ME. Concentrations of calmodulin in sperm in relation to their motility in fertile euspermic and infertile asthenozoospermic men. Int J Androl. 1987;10:507–15. doi: 10.1111/j.1365-2605.1987.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 11.Schlingmann K, Michaut MA, McElwee JL, Wolff CA, Travis AJ, et al. Calmodulin and CaMKII in the sperm principal piece: evidence for a motility-related calcium/calmodulin pathway. J Androl. 2007;28:706–16. doi: 10.2164/jandrol.106.001669. [DOI] [PubMed] [Google Scholar]
- 12.Suarez SS, Varosi SM, Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci U S A. 1993;90:4660–4. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–57. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 14.Gibbons BH, Gibbons IR. Calcium-induced quiescence in reactivated sea urchin sperm. J Cell Biol. 1980;84:13–27. doi: 10.1083/jcb.84.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tash JS, Means AR. Regulation of protein phosphorylation and motility of sperm by cyclic adenosine monophosphate and calcium. Biol Reprod. 1982;26:745–63. doi: 10.1095/biolreprod26.4.745. [DOI] [PubMed] [Google Scholar]
- 16.Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell. 2002;13:3303–13. doi: 10.1091/mbc.E02-04-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marín-Briggiler CI, Jha KN, Chertihin O, Buffone MG, Herr JC, et al. Evidence of the presence of calcium/calmodulin-dependent protein kinase IV in human sperm and its involvement in motility regulation. J Cell Sci. 2005;118:2013–22. doi: 10.1242/jcs.02326. [DOI] [PubMed] [Google Scholar]
- 18.Tash JS, Bracho GE. Regulation of sperm motility: emerging evidence for a major role for protein phosphatases. J Androl. 1994;15:505–9. [PubMed] [Google Scholar]
- 19.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–705. [PubMed] [Google Scholar]
- 20.Luconi M, Porazzi I, Ferruzzi P, Marchiani S, Forti G, et al. Tyrosine phosphorylation of the a kinase anchoring protein 3 (AKAP3) and soluble adenylate cyclase are involved in the increase of human sperm motility by bicarbonate. Biol Reprod. 2005;72:22–32. doi: 10.1095/biolreprod.104.032490. [DOI] [PubMed] [Google Scholar]
- 21.Wennemuth G, Carlson AE, Harper AJ, Babcock DF. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development. 2003;130:1317–26. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]
- 22.Burton KA, McKnight GS. PKA, germ cells, and fertility. Physiology. 2007;22:40–6. doi: 10.1152/physiol.00034.2006. [DOI] [PubMed] [Google Scholar]
- 23.Hyne RV, Garbers DL. Calcium-dependent increase in adenosine 3’,5’-monophosphate and induction of the acrosome reaction in guinea pig spermatozoa. Proc Natl Acad Sci U S A. 1979;76:5699–703. doi: 10.1073/pnas.76.11.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 25.Skålhegg BS, Huang Y, Su T, Idzerda RL, McKnight GS, et al. Mutation of the Cα subunit of PKA leads to growth retardation and sperm dysfunction. Mol Endocrinol. 2002;16:630–9. doi: 10.1210/mend.16.3.0793. [DOI] [PubMed] [Google Scholar]
- 26.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, et al. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci U S A. 2004;101:13483–8. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strünker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–6. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 28.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci U S A. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- 30.Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–10. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet. 2010;18:1178–84. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson L. The regulation of protein phosphorylation. Biochem Soc Trans. 2009;37:627–41. doi: 10.1042/BST0370627. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher J, Ramljak S, Asif AR, Schaffrath M, Zischler H, et al. Evolutionary conservation of mammalian sperm proteins associates with overall, not tyrosine, phosphorylation in human spermatozoa. J Proteome Res. 2013;12:5370–82. doi: 10.1021/pr400228c. [DOI] [PubMed] [Google Scholar]
- 34.Kwon WS, Rahman MS, Pang MG. Diagnosis and prognosis of male infertility in mammal: the focusing of tyrosine phosphorylation and phosphotyrosine proteins. J Proteome Res. 2014;13:4505–17. doi: 10.1021/pr500524p. [DOI] [PubMed] [Google Scholar]
- 35.Visconti PE, Kopf GS. Regulation of protein phosphorylation during sperm capacitation. Biol Reprod. 1998;59:1–6. doi: 10.1095/biolreprod59.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Naz RK. Involvement of protein tyrosine phosphorylation of human sperm in capacitation/acrosome reaction and zona pellucida binding. Front Biosci. 1996;1:d206–13. doi: 10.2741/a126. [DOI] [PubMed] [Google Scholar]
- 37.Busso D, Oñate-Alvarado MJ, Balboa E, Castro J, Lizama C, et al. Spermatozoa from mice deficient in Niemann-Pick disease type C2 (NPC2) protein have defective cholesterol content and reduced in vitro fertilising ability. Reprod Fertil Dev. 2014;26:609–21. doi: 10.1071/RD12059. [DOI] [PubMed] [Google Scholar]
- 38.Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, et al. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1) Dev Biol. 2008;320:12–8. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leclerc P, de Lamirande E, Gagnon C. Cyclic adenosine 3’,5’-monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol Reprod. 1996;55:684–92. doi: 10.1095/biolreprod55.3.684. [DOI] [PubMed] [Google Scholar]
- 40.Ficarro S, Chertihin O, Westbrook VA, White F, Jayes F, et al. Phosphoproteome analysis of capacitated human sperm evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J Biol Chem. 2003;278:11579–89. doi: 10.1074/jbc.M202325200. [DOI] [PubMed] [Google Scholar]
- 41.Mandal A, Naaby-Hansen S, Wolkowicz MJ, Klotz K, Shetty J, et al. FSP95, A testis-specific 95-Kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol Reprod. 1999;61:1184–97. doi: 10.1095/biolreprod61.5.1184. [DOI] [PubMed] [Google Scholar]
- 42.Naaby-Hansen S, Mandal A, Wolkowicz MJ, Sen B, Westbrook VA, et al. CABYR, a novel calcium-binding tyrosine phosphorylation-regulated fibrous sheath protein involved in capacitation. Dev Biol. 2002;242:236–54. doi: 10.1006/dbio.2001.0527. [DOI] [PubMed] [Google Scholar]
- 43.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 44.Buffone MG, Doncel GF, Briggiler CI, Vazquez-Levin MH, Calamera JC. Human sperm subpopulations: relationship between functional quality and protein tyrosine phosphorylation. Hum Reprod. 2004;19:139–46. doi: 10.1093/humrep/deh040. [DOI] [PubMed] [Google Scholar]
- 45.Buffone MG, Calamera JC, Verstraeten SV, Doncel GF. Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction. 2005;129:697–705. doi: 10.1530/rep.1.00584. [DOI] [PubMed] [Google Scholar]
- 46.Yunes R, Doncel GF, Acosta AA. Incidence of sperm-tail tyrosine phosphorylation and hyperactivated motility in normozoospermic and asthenozoospermic human sperm samples. Biocell. 2003;27:29–36. [PubMed] [Google Scholar]
- 47.Leclerc P, De Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22:643–56. doi: 10.1016/s0891-5849(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 48.Bajpai M, Doncel GF. Involvement of tyrosine kinase and cAMP-dependent kinase cross-talk in the regulation of human sperm motility. Reproduction. 2003;126:183–95. doi: 10.1530/rep.0.1260183. [DOI] [PubMed] [Google Scholar]
- 49.Luconi M, Carloni V, Marra F, Ferruzzi P, Forti G, et al. Increased phosphorylation of AKAP by inhibition of phosphatidylinositol 3-kinase enhances human sperm motility through tail recruitment of protein kinase A. J Cell Sci. 2004;117:1235–46. doi: 10.1242/jcs.00931. [DOI] [PubMed] [Google Scholar]
- 50.Luconi M, Cantini G, Baldi E, Forti G. Role of a-kinase anchoring proteins (AKAPs) in reproduction. Front Biosci. 2011;16:1315–30. doi: 10.2741/3791. [DOI] [PubMed] [Google Scholar]
- 51.Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 2003;79:829–43. doi: 10.1016/s0015-0282(02)04948-8. [DOI] [PubMed] [Google Scholar]
- 52.Ford WC. Regulation of sperm function by reactive oxygen species. Hum Reprod Update. 2004;10:387–99. doi: 10.1093/humupd/dmh034. [DOI] [PubMed] [Google Scholar]
- 53.De Lamirande E, Gagnon C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic Biol Med. 1993;14:157–66. doi: 10.1016/0891-5849(93)90006-g. [DOI] [PubMed] [Google Scholar]
- 54.De Lamirande E, Gagnon C. Capacitation-associated production of superoxide anion by human spermatozoa. Free Radic Biol Med. 1995;8:487–95. doi: 10.1016/0891-5849(94)00169-k. [DOI] [PubMed] [Google Scholar]
- 55.Zini A, Lamirande E, Gagnon C. Low levels of nitric oxide promote human sperm capacitation in vitro. J Androl. 1995;16:424–31. [PubMed] [Google Scholar]
- 56.Herrero MB, de Lamirande E, Gagnon C. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol Reprod. 1999;61:575–81. doi: 10.1095/biolreprod61.3.575. [DOI] [PubMed] [Google Scholar]
- 57.O’Flaherty CM, Beorlegui NB, Beconi MT. Reactive oxygen species requirements for bovine sperm capacitation and acrosome reaction. Theriogenology. 1999;52:289–301. doi: 10.1016/S0093-691X(99)00129-6. [DOI] [PubMed] [Google Scholar]
- 58.Du Plessis SS, McAllister DA, Luu A, Savia J, Agarwal A, et al. Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia. 2010;42:206–10. doi: 10.1111/j.1439-0272.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 59.De Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta (BBA)-Proteins Proteomics. 2008;1784:106–15. doi: 10.1016/j.bbapap.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8:338–48. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 61.Aitken RJ, Clarkson JS, Fishel S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989;41:183–97. doi: 10.1095/biolreprod41.1.183. [DOI] [PubMed] [Google Scholar]
- 62.Aitken RJ, Fisher HM, Fulton N, Gomez E, Knox W, et al. Reactive oxygen species generation by human spermatozoa is induced by exogenous NADPH and inhibited by the flavoprotein inhibitors diphenylene iodonium and quinacrine. Mol Reprod Dev. 1997;47:468–82. doi: 10.1002/(SICI)1098-2795(199708)47:4<468::AID-MRD14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 63.Fisher HM, Aitken RJ. Comparative analysis of the ability of precursor germ cells and epididymal spermatozoa to generate reactive oxygen metabolites. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 64.Amaral A, Lourenço B, Marques M, Ramalho-Santos J. Mitochondria functionality and sperm quality. Reproduction. 2013;146:R163–74. doi: 10.1530/REP-13-0178. [DOI] [PubMed] [Google Scholar]
- 65.Paoli D, Gallo M, Rizzo F, Baldi E, Francavilla S, et al. Mitochondrial membrane potential profile and its correlation with increasing sperm motility. Fertil Steril. 2011;95:2315–9. doi: 10.1016/j.fertnstert.2011.03.059. [DOI] [PubMed] [Google Scholar]
- 66.Aitken RJ, West K, Buckingham D. Leukocytic infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J Androl. 1994;15:343–52. [PubMed] [Google Scholar]
- 67.Tremellen K. Oxidative stress and male infertility - A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 68.Gomez E, Buckingham DW, Brindle J, Lanzafame F, Irvine DS, et al. Development of an image analysis system to monitor the retention of residual cytoplasm by human spermatozoa: correlation with biochemical markers of the cytoplasmic space, oxidative stress, and sperm function. J Androl. 1996;17:276–87. [PubMed] [Google Scholar]
- 69.Saleh RA, Agarwal A, Kandirali E, Sharma RK, Thomas AJ, Jr, et al. Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil Steril. 2002;78:1215–24. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 70.Henkel R, Kierspel E, Stalf T, Mehnert C, Menkveld R, et al. Effect of reactive oxygen species produced by spermatozoa and leukocytes on sperm functions in non-leukocytospermic patients. Fertil Steril. 2005;83:635–42. doi: 10.1016/j.fertnstert.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Ochsendorf FR. Infections in the male genital tract and reactive oxygen species. Hum Reprod Update. 1999;5:399–420. doi: 10.1093/humupd/5.5.399. [DOI] [PubMed] [Google Scholar]
- 72.Pasqualotto FF, Sharma RK, Potts JM, Nelson DR, Thomas AJ, Jr, et al. A. Seminal oxidative stress in patients with chronic prostatitis. Urology. 2000;55:881–5. doi: 10.1016/s0090-4295(99)00613-5. [DOI] [PubMed] [Google Scholar]
- 73.Pasqualotto FF, Sundaram A, Sharma RK, Borges E, Jr, Pasqualotto EB, et al. Semen quality and oxidative stress scores in fertile and infertile patients with varicocele. Fertil Steril. 2008;89:602–7. doi: 10.1016/j.fertnstert.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 74.Saleh RA, Agarwal A, Sharma RK, Nelson DR, Thomas AJ., Jr Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: a prospective study. Fertil Steril. 2002;78:491–9. doi: 10.1016/s0015-0282(02)03294-6. [DOI] [PubMed] [Google Scholar]
- 75.Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, et al. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20:1006–12. doi: 10.1093/humrep/deh725. [DOI] [PubMed] [Google Scholar]
- 76.Maneesh M, Dutta S, Chakrabarti A, Vasudevan DM. Alcohol abuse-duration dependent decrease in plasma testosterone and antioxidants in males. Indian J Physiol Pharmacol. 2006;50:291–6. [PubMed] [Google Scholar]
- 77.Eskiocak S, Gozen AS, Taskiran A, Kilic AS, Eskiocak M, et al. Effect of psychological stress on the L-arginine-nitric oxide pathway and semen quality. Bras J Med Biol Res. 2006;39:581–8. doi: 10.1590/s0100-879x2006000500003. [DOI] [PubMed] [Google Scholar]
- 78.Naha N, Bhar RB, Mukherjee A, Chowdhury AR. Structural alteration of spermatozoa in the persons employed in lead acid battery factory. Indian J Physiol Pharmacol. 2005;49:153–62. [PubMed] [Google Scholar]
- 79.Junqueira VB, Barros S, Chan SS, Rodrigues L, Giavarotti L, et al. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Kao SH, Chao HT, Chen HW, Hwang TI, Liao TL, et al. Increase of oxidative stress in human sperm with lower motility. Fertil Steril. 2008;89:1183–90. doi: 10.1016/j.fertnstert.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 81.Urata K, Narahara H, Tanaka Y, Egashira T, Takayama F, et al. Effect of endotoxin-induced reactive oxygen species on sperm motility. Fertil Steril. 2001;76:163–6. doi: 10.1016/s0015-0282(01)01850-7. [DOI] [PubMed] [Google Scholar]
- 82.Griveau JF, Dumont E, Renard P, Callegari JP, Le Lannou D. Reactive oxygen species, lipid peroxidation and enzymatic defence systems in human spermatozoa. J Reprod Fertil. 1995;103:17–26. doi: 10.1530/jrf.0.1030017. [DOI] [PubMed] [Google Scholar]
- 83.De Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995;10:15–21. doi: 10.1093/humrep/10.suppl_1.15. [DOI] [PubMed] [Google Scholar]
- 84.Gomez E, Irvine D, Aitken R. Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: relationships with semen quality and sperm function. Int J Androl. 1998;21:81–94. doi: 10.1046/j.1365-2605.1998.00106.x. [DOI] [PubMed] [Google Scholar]
- 85.Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 1998;13:1429–36. doi: 10.1093/humrep/13.6.1429. [DOI] [PubMed] [Google Scholar]
- 86.Cooper TG, Yeung C. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61:28–38. doi: 10.1002/jemt.10314. [DOI] [PubMed] [Google Scholar]
- 87.Yeung CH, Anapolski M, Sipila P, Wagenfeld A, Poutanen M, et al. Sperm volume regulation: maturational changes in fertile and infertile transgenic mice and association with kinematics and tail angulation. Biol Reprod. 2002;67:269–75. doi: 10.1095/biolreprod67.1.269. [DOI] [PubMed] [Google Scholar]
- 88.Yeung CH, Barfield JP, Cooper TG. Physiological volume regulation by spermatozoa. Mol Cell Endocrinol. 2006;250:98–105. doi: 10.1016/j.mce.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 89.Rengan AK, Agarwal A, van der Linde M, du Plessis SS. An investigation of excess residual cytoplasm in human spermatozoa and its distinction from the cytoplasmic droplet. Reprod Biol Endocrinol. 2012;10:1–8. doi: 10.1186/1477-7827-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu H, Yuan SQ, Zheng ZH, Yan W. The cytoplasmic droplet may be indicative of sperm motility and normal spermiogenesis. Asian J Androl. 2013;15:799–805. doi: 10.1038/aja.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yeung CH, Anapolski M, Depenbusch M, Zitzmann M, Cooper TG. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum Reprod. 2003;18:1029–36. doi: 10.1093/humrep/deg204. [DOI] [PubMed] [Google Scholar]
- 92.Yeung CH, Barfield JP, Cooper TG. Chloride channels in physiological volume regulation of human spermatozoa. Biol Reprod. 2005;73:1057–63. doi: 10.1095/biolreprod.105.044123. [DOI] [PubMed] [Google Scholar]
- 93.Barfield JP, Yeung CH, Cooper TG. Characterization of potassium channels involved in volume regulation of human spermatozoa. Mol Hum Reprod. 2005;11:891–7. doi: 10.1093/molehr/gah208. [DOI] [PubMed] [Google Scholar]
- 94.Yeung C, Barfield JP, Cooper TG. The role of anion channels and Ca2+ in addition to K+ channels in the physiological volume regulation of murine spermatozoa. Mol Reprod Dev. 2005;71:368–79. doi: 10.1002/mrd.20261. [DOI] [PubMed] [Google Scholar]
- 95.Pasantes-Morales H, Lezama RA, Ramos-Mandujano G, Tuz KL. Mechanisms of cell volume regulation in hypo-osmolality. Am J Med. 2006;119:S4–11. doi: 10.1016/j.amjmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Rossato M, Balercia G, Lucarelli G, Foresta C, Mantero F. Role of seminal osmolarity in the regulation of human sperm motility. Int J Androl. 2002;25:230–5. doi: 10.1046/j.1365-2605.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- 97.Caballero I, Parrilla I, Almiñana C, del Olmo D, Roca J, et al. Seminal plasma proteins as modulators of the sperm function and their application in sperm biotechnologies. Reprod Domest Anim. 2012;47:12–21. doi: 10.1111/j.1439-0531.2012.02028.x. [DOI] [PubMed] [Google Scholar]
- 98.Alves M, Sá R, Jesus T, Sousa M, Oliveira P. CFTR regulation of Aquaporin-mediated water transport: a target in male fertility. Curr Drug Targets. 2015;16:993–1006. doi: 10.2174/1573399811666150615144108. In press. [DOI] [PubMed] [Google Scholar]
- 99.Chen Q, Peng H, Lei L, Zhang Y, Kuang H, et al. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011;21:922–33. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saito K, Kageyama Y, Okada Y, Kawakami S, Kihara K, et al. Localization of aquaporin-7 in human testis and ejaculated sperm: possible involvement in maintenance of sperm quality. J Urol. 2004;172:2073–6. doi: 10.1097/01.ju.0000141499.08650.ab. [DOI] [PubMed] [Google Scholar]
- 101.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, et al. Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med. 2001;345:1388–93. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 102.Maettner R, Sterzik K, Isachenko V, Strehler E, Rahimi G, et al. Quality of human spermatozoa: relationship between high-magnification sperm morphology and DNA integrity. Andrologia. 2014;46:547–55. doi: 10.1111/and.12114. [DOI] [PubMed] [Google Scholar]
- 103.Hotaling JM, Smith JF, Rosen M, Muller CH, Walsh TJ. The relationship between isolated teratozoospermia and clinical pregnancy after in vitro fertilization with or without intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2011;95:1141–5. doi: 10.1016/j.fertnstert.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 104.Menkveld R, Wong WY, Lombard CJ, Wetzels AM, Thomas CM, et al. Semen parameters, including WHO and strict criteria morphology, in a fertile and subfertile population: an effort towards standardization of in-vivo thresholds. Hum Reprod. 2001;16:1165–71. doi: 10.1093/humrep/16.6.1165. [DOI] [PubMed] [Google Scholar]
- 105.Chemes HE. Phenotypes of sperm pathology: genetic and acquired forms in infertile men. J Androl. 2000;21:799–808. [PubMed] [Google Scholar]
- 106.Chemes HE, Rawe VY. The making of abnormal spermatozoa: cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010;341:349–57. doi: 10.1007/s00441-010-1007-3. [DOI] [PubMed] [Google Scholar]
- 107.Sousa M, Oliveira E, Alves A, Gouveia M, Figueiredo H, et al. Ultrastructural analysis of five patients with total sperm immotility. Zygote. 2015;23:900–7. doi: 10.1017/S0967199414000616. In press doi: 10.1017/S0967199414000616. [DOI] [PubMed] [Google Scholar]
- 108.Chemes HE, Rawe VY. Sperm pathology: a step beyond descriptive morphology. Origin, characterization and fertility potential of abnormal sperm phenotypes in infertile men. Hum Reprod Update. 2003;9:405–28. doi: 10.1093/humupd/dmg034. [DOI] [PubMed] [Google Scholar]
- 109.Baccetti B, Capitani S, Collodel G, Di Cairano G, Gambera L, et al. Genetic sperm defects and consanguinity. Hum Reprod. 2001;16:1365–71. doi: 10.1093/humrep/16.7.1365. [DOI] [PubMed] [Google Scholar]
- 110.Pereira R, Oliveira J, Ferraz L, Barros A, Santos R, et al. Mutation analysis in patients with total sperm immotility. J Assist Reprod Genet. 2015;32:893–902. doi: 10.1007/s10815-015-0474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turner RM, Musse MP, Mandal A, Klotz KE, Friederike C, et al. Molecular genetic analysis of two human sperm fibrous. J Androl. 2001;22:302–15. [PubMed] [Google Scholar]
- 112.Moretti E, Scapigliati G, Pascarelli NA, Baccetti B, Collodel G. Localization of AKAP4 and tubulin proteins in sperm with reduced motility. Asian J Androl. 2007;9:641–9. doi: 10.1111/j.1745-7262.2007.00267.x. [DOI] [PubMed] [Google Scholar]
- 113.Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–22. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pereira R, Oliveira J, Sousa M. A molecular approach to sperm immotility in humans: a review. Med Reprod Embriol Clí. 2014;1:15–25. [Google Scholar]
- 115.Tamowski S, Aston KI, Carrell DT. The use of transgenic mouse models in the study of male infertility. Syst Biol Reprod Med. 2010;56:260–73. doi: 10.3109/19396368.2010.485244. [DOI] [PubMed] [Google Scholar]
- 116.Inaba K. Molecular architecture of the sperm flagella: molecules for motility and molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci. 2003;20:1043–56. doi: 10.2108/zsj.20.1043. [DOI] [PubMed] [Google Scholar]
- 117.Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components unicellular algae Chlamydomonas. Mol Hum Reprod. 2011;17:524–38. doi: 10.1093/molehr/gar034. [DOI] [PubMed] [Google Scholar]
- 118.King SM. The dynein microtubule motor. Biochim Biophys Acta-Mol Cell Res. 2000;1496:60–75. doi: 10.1016/s0167-4889(00)00009-4. [DOI] [PubMed] [Google Scholar]
- 119.Burgess SA, Knight PJ. Is the dynein motor a winch? Curr Opin Struct Biol. 2004;14:138–46. doi: 10.1016/j.sbi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 120.Yagi T. Bioinformatic approaches to dynein heavy chain classification. Methods Cell Biol. 2009;92:1–9. doi: 10.1016/S0091-679X(08)92001-X. [DOI] [PubMed] [Google Scholar]
- 121.Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–33. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gardner LC, O’Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–25. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125:1109–17. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stannard W, Rutman A, Wallis C, O’allaghan C. Central microtubular agenesis causing primary ciliary dyskinesia. Am J Respir Crit Care Med. 2004;169:634–7. doi: 10.1164/rccm.200306-782OC. [DOI] [PubMed] [Google Scholar]
- 125.Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, et al. Mutations in radial spoke head protein genes RSPH 9 and RSPH 4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84:197–209. doi: 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith EF, Lefebvre PA. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 128.Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, et al. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10:1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Onoufriadis A, Shoemark A, Schmidts M, Patel M, Jimenez G, et al. Targeted NGS gene panel identifies mutations in RSPH 1 causing primary ciliary dyskinesia and a common mechanism for ciliary central-pair agenesis due to radial spoke defects. Hum Mol Genet. 2014;23:3362–74. doi: 10.1093/hmg/ddu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kott E, Legendre M, Copin B, Papon JF, Dastot-Le Moal F, et al. Loss-of-function mutations in RSPH 1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93:561–70. doi: 10.1016/j.ajhg.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Antony D, Becker-Heck A, Zariwala MA, Schmidts M, Onoufriadis A, et al. Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat. 2013;34:462–72. doi: 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pelliccione F, Micillo A, Cordeschi G, D’Angeli A, Necozione S, et al. Altered ultrastructure of mitochondrial membranes is strongly associated with unexplained asthenozoospermia. Fertil Steril. 2011;95:641–6. doi: 10.1016/j.fertnstert.2010.07.1086. [DOI] [PubMed] [Google Scholar]
- 133.Spiropoulos J, Turnbull DM, Chinnery PF. Can mitochondrial DNA mutations cause sperm dysfunction? Mol Hum Reprod. 2002;8:719–21. doi: 10.1093/molehr/8.8.719. [DOI] [PubMed] [Google Scholar]
- 134.Baklouti-Gargouri S, Ghorbel M, Mahmoud AB, Mkaouar-Rebai E, Cherif M, et al. Identification of a novel m.9588G>A missense mutation in the mitochondrial COIII gene in asthenozoospermic Tunisian infertile men. J Assist Reprod Genet. 2014;31:595–600. doi: 10.1007/s10815-014-0187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abasalt HC, Gholamali JS, Maryam GC. Lipid peroxidation and large-scale deletions of mitochondrial DNA in asthenoteratozoospermic patients. Indian J Biochem Biophys. 2013;50:492–9. [PubMed] [Google Scholar]
- 136.Song GJ, Lewis V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil Steril. 2008;90:2238–44. doi: 10.1016/j.fertnstert.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 137.Kao SH, Chao HT, Liu HW, Liao TL, Wei YH. Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil Steril. 2004;82:66–73. doi: 10.1016/j.fertnstert.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 138.Holyoake AJ, McHugh P, Wu M, O’arroll S, Benny P, et al. High incidence of single nucleotide substitutions in the mitochondrial genome is associated with poor semen parameters in men. Int J Androl. 2001;24:175–82. doi: 10.1046/j.1365-2605.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 139.Thangaraj K, Joshi MB, Reddy AG, Rasalkar AA, Singh L. Sperm mitochondrial mutations as a cause of low sperm motility. J Androl. 2003;24:388–92. doi: 10.1002/j.1939-4640.2003.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 140.Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–52. doi: 10.1016/j.devcel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Steels JD, Estey MP, Froese CD, Reynaud D, Pace-Asciak C, et al. Sept12 is a component of the mammalian sperm tail annulus. Cell Motil Cytoskeleton. 2007;64:794–807. doi: 10.1002/cm.20224. [DOI] [PubMed] [Google Scholar]
- 142.Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, et al. The Sept4 Septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–64. doi: 10.1016/j.devcel.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 143.Chao HC, Lin YH, Kuo YC, Shen CJ, Pan HA, et al. The expression pattern of SEPT7 correlates with sperm morphology. J Assist Reprod Genet. 2010;27:299–307. doi: 10.1007/s10815-010-9409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sugino Y, Ichioka K, Soda T, Ihara M, Kinoshita M, et al. Septins as diagnostic markers for a subset of human asthenozoospermia. J Urol. 2008;180:2706–9. doi: 10.1016/j.juro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 145.Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metabol. 2005;90:984–91. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- 146.Oliveira H, Spanò M, Santos C, de Lourdes Pereira M. Lead chloride affects sperm motility and acrosome reaction in mice. Cell Biol Toxicol. 2009;25:341–53. doi: 10.1007/s10565-008-9088-4. [DOI] [PubMed] [Google Scholar]
- 147.Donnelly P, McClure N, Kennedy S, Lewis M. Direct effect of alcohol on the motility and morphology of human spermatozoa. Andrologia. 1999;31:43–7. [PubMed] [Google Scholar]
- 148.Kumar S, Kumari A, Murarka S, Kumar M. Lifestyle factors in deteriorating male reproductive health. Indian J Exp Biol. 2009;47:615–24. [PubMed] [Google Scholar]
- 149.Avendano C, Mata A, Sarmiento S, Doncel F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil Steril. 2012;97:39–45. doi: 10.1016/j.fertnstert.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 150.Erogul O, Oztas E, Yildirim I, Kir T, Aydur E, et al. Effects of electromagnetic radiation from a cellular phone on human sperm motility: an in vitro study. Arch Med Res. 2006;37:840–3. doi: 10.1016/j.arcmed.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 151.Ohl DA, Naz RK. Infertility due to antisperm antibodies. Urology. 1995;46:591–602. doi: 10.1016/S0090-4295(99)80282-9. [DOI] [PubMed] [Google Scholar]
- 152.Lombardo F, Gandini L, Dondero F, Lenzi A. Immunology and immunopathology of the male genital tract: antisperm immunity in natural and assisted reproduction. Hum Reprod Update. 2001;7:450–6. doi: 10.1093/humupd/7.5.450. [DOI] [PubMed] [Google Scholar]
- 153.Naz RK. Modalities for treatment of antisperm antibody mediated infertility: novel perspectives. Am J Reprod Immunol. 2004;51:390–7. doi: 10.1111/j.1600-0897.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 154.Weidner W, Krause W, Ludwig M. Relevance of male accessory gland infection for subsequent fertility with special focus on prostatitis. Hum Reprod Update. 1999;5:421–32. doi: 10.1093/humupd/5.5.421. [DOI] [PubMed] [Google Scholar]
- 155.Haidl G. Management strategies for male factor infertility. Drugs. 2002;62:1741–53. doi: 10.2165/00003495-200262120-00004. [DOI] [PubMed] [Google Scholar]
- 156.Menkveld R. Leukocytospermia. Int Congr Ser. 2004;1266:218–24. [Google Scholar]
- 157.Parinaud J, Le Lannou D, Vieitez G, Griveau JF, Milhet P, et al. Enhancement of motility by treating spermatozoa with an antioxidant solution (Sperm-Fit) following ejaculation. Hum Reprod. 1997;12:2434–6. doi: 10.1093/humrep/12.11.2434. [DOI] [PubMed] [Google Scholar]
- 158.Suleiman SA, Ali ME, Zaki ZM, El-Malik EM, Nasr MA. Lipid peroxidation and human sperm motility: protective role of Vitamin E. J Androl. 1996;17:530–7. [PubMed] [Google Scholar]
- 159.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51:1000–13. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Keskes-Ammar L, Feki-Chakroun N, Rebai T, Sahnoun Z, Ghozzi H, et al. Sperm oxidative stress and the effect of an oral Vitamin E and selenium supplement on semen quality in infertile men. Syst Biol Reprod Med. 2003;49:83–94. doi: 10.1080/01485010390129269. [DOI] [PubMed] [Google Scholar]
- 161.Balercia G, Buldreghini E, Vignini A, Tiano L, Paggi F, et al. Coenzyme Q 10 treatment in infertile men with idiopathic asthenozoospermia: a placebo-controlled, double-blind randomized trial. Fertil Steril. 2009;91:1785–92. doi: 10.1016/j.fertnstert.2008.02.119. [DOI] [PubMed] [Google Scholar]
- 162.Kumar R, Gautam G, Gupta NP. Drug therapy for idiopathic male infertility: rationale versus evidence. J Urol. 2006;176:1307–12. doi: 10.1016/j.juro.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 163.Shen MR, Chiang PH, Yang RC, Hong CY, Chen SS. Pentoxifylline stimulates human sperm motility both in vitro and after oral therapy. Br J Clin Pharmacol. 1991;31:711–4. doi: 10.1111/j.1365-2125.1991.tb05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Moein MR, Khalili MA, Davoudi A. The effect of oral administration of Pentoxifylline on sperm motility of asthenozoospermic ejaculates from men with or without testicular varicoceles. Iran J Reprod Med. 2005;3:25–9. [Google Scholar]
- 165.Yunes R, Fernández P, Doncel GF, Acosta AA. Cyclic nucleotide phosphodiesterase inhibition increases tyrosine phosphorylation and hyper motility in normal and pathological human spermatozoa. Biocell. 2005;29:287–93. [PubMed] [Google Scholar]
- 166.Agarwal A, Nallella KP, Allamaneni SS, Said TM. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod Biomed Online. 2004;8:616–27. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 167.Chan HC, Sun X. SLC26 anion exchangers in uterine epithelial cells and spermatozoa: clues from the past and hints to the future. Cell Biol Int. 2014;38:1–7. doi: 10.1002/cbin.10183. [DOI] [PubMed] [Google Scholar]
- 168.Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–72. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod. 2009;80:1092–8. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol. 2009;7:119. doi: 10.1186/1477-7827-7-119. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–7. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 172.Aitken RJ, Harkiss D, Knox W, Paterson M, Irvine D. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J Cell Sci. 1998;111:645–56. doi: 10.1242/jcs.111.5.645. [DOI] [PubMed] [Google Scholar]
- 173.Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’onnor KT, et al. Targeted ablation of plasma membrane Ca2+ -ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742–50. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 174.Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell. 2006;24:383–95. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 175.Carrera A, Moos J, Ning XP, Gerton GL, Tesarik J, et al. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180:284–96. doi: 10.1006/dbio.1996.0301. [DOI] [PubMed] [Google Scholar]
- 176.Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod. 1999;61:240–6. doi: 10.1095/biolreprod61.1.240. [DOI] [PubMed] [Google Scholar]
- 177.Petrunkina AM, Harrison RA, Ekhlasi-Hundrieser M, Töpfer-Petersen E. Role of volume-stimulated osmolyte and anion channels in volume regulation by mammalian sperm. Mol Hum Reprod. 2004;10:815–23. doi: 10.1093/molehr/gah106. [DOI] [PubMed] [Google Scholar]
- 178.Petrunkina AM, Harrison RA, Tsolova M, Jebe E, Töpfer-Petersen E. Signalling pathways involved in the control of sperm cell volume. Reproduction. 2007;133:61–73. doi: 10.1530/rep.1.01137. [DOI] [PubMed] [Google Scholar]