Abstract
We aimed to determine short-term patient-reported outcomes in men having general anesthetic transperineal (TP) prostate biopsies. A prospective cohort study was performed in men having a diagnostic TP biopsy. This was done using a validated and adapted questionnaire immediately post-biopsy and at follow-up of between 7 and 14 days across three tertiary referral hospitals with a response rate of 51.6%. Immediately after biopsy 43/201 (21.4%) of men felt light-headed, syncopal, or suffered syncope. Fifty-three percent of men felt discomfort after biopsy (with 95% scoring <5 in a 0–10 scale). Twelve out of 196 men (6.1%) felt pain immediately after the procedure. Despite a high incidence of symptoms (e.g., up to 75% had some hematuria, 47% suffered some pain), it was not a moderate or serious problem for most, apart from hemoejaculate which 31 men suffered. Eleven men needed catheterization (5.5%). There were no inpatient admissions due to complications (hematuria, sepsis). On repeat questioning at a later time point, only 25/199 (12.6%) of men said repeat biopsy would be a significant problem despite a significant and marked reduction in erectile function after the procedure. From this study, we conclude that TP biopsy is well tolerated with similar side effect profiles and attitudes of men to repeat biopsy to men having TRUS biopsies. These data allow informed counseling of men prior to TP biopsy and a benchmark for tolerability with local anesthetic TP biopsies being developed for clinical use.
Keywords: attitude to rebiopsy, patient-reported outcome measures, patient satisfaction, prostate cancer diagnosis, transperineal biopsies, transrectal biopsies
INTRODUCTION
Prostate cancer is a significant health problem and a leading cause of death in men throughout the developed world.1 Diagnostic approaches have evolved from finger guided prostatic biopsies to 12 core transrectal-ultrasound-guided (TRUS) biopsies. Despite this, TRUS biopsy misses up to one-third of cancers and frequently characterizes tumors incorrectly in terms of grade, size, and position.2,3 TRUS can be associated with significant pain and distress and Rosario et al.4 reported fever in 17.5% of men after TRUS biopsy. Other studies have found a hospital admission rate of 5%–6.9% due to infectious complications after TRUS biopsy.5,6,7 In the era of increasing anti-microbial resistance of intestinal microorganisms, the transrectal biopsy approach may be hampered by rising rates of prostatitis and sepsis.8
Given some of the limitations of TRUS biopsy, there has been renewed interest in the role of transperineal (TP) prostate biopsy in prostate cancer diagnostics.9 TP biopsies might have a higher rate of cancer detection,10 particularly at the apex and anteriorly, with significantly lower rates of sepsis,11 but usually require a general anesthetic (GA).12,13 With the increasing use of multiparametric magnetic resonance imaging (MRI), integrated MRI-TRUS fusion biopsies are able to improve detection of significant prostate cancer whether by the transrectal14 or TP route.15
Rosario et al.4 recently presented the ProBE study: a multicenter, prospective questionnaire study on short-term patient-reported outcomes after TRUS biopsy on 1147 men. Using a bespoke questionnaire, data relating to pain, infection, and bleeding was collected at 7 days and 35 days postprocedure, as well as attitude to rebiopsy and healthcare use. They found that despite pain reported in 43.6%, hematuria in 65.8%, hemoejaculate in 92.6% of men, prostate biopsy was tolerated well by most men, with a few troublesome side effects in a minority.4 Approximately 20% of men would consider further biopsy a moderate or major problem, and that this negative attitude to rebiopsy was associated with an unfavorable experience at first biopsy.4
The aim of this study was to use the validated patient-reported outcome questionnaire from the ProBE study to document the occurrence and effect of adverse events, early attitudes to repeat biopsy and healthcare resource use in the TP biopsy setting. We hypothesized that patient-reported outcomes would be similar between TP biopsy and the previously reported TRUS biopsy from Rosario et al.4 Here, we present the first prospective evaluation of short-term patient-reported outcomes after TP biopsy across centers in the UK and Germany, using a validated questionnaire.
MATERIALS AND METHODS
A standardized prospective questionnaire-based cohort study was conducted across three centers (Addenbrookes Hospital, Cambridge UK; University Hospital Heidelberg, Heidelberg Germany; and Kings College Hospital, London), using an adapted version of the previously validated ProBE questionnaire.4 The study was registered as a service evaluation audit in all centers with the Local Ethics Committees. Specifically these were Cambridge University Hospitals Ethics Committee, Addenbrookes Hospital, UK; The University Hospital Heidelberg Ethics Review Board and Kings College Hospital, London Ethics Committee.
Population
All men undergoing TP biopsy (diagnostic primary, second, or active surveillance rebiopsy) between February and November 2013 were asked to self-complete the questionnaire immediately after biopsy (baseline) and at follow-up between 7 and 14 days after the biopsy. Three hundred eighty-six men were included in the study divided between primary and secondary TP biopsy.
Patients were given written information on the study as well as instructions on completing the questionnaire. Baseline data including age, Hospital Anxiety and Depression Scale (HADS score), urinary, bowel and sexual symptoms were assessed using the validated International Continence Society - male, International Consultation on Incontinence Modular Questionnaire-urinary incontinence and University of California, Los Angeles Prostate Cancer index questionnaires, International Prostate Symptom Score (IPSS), and International Index of Erectile Function 5 (IIEF-5) shortened questionnaire.
Biopsy
TP biopsies were carried out as a day case surgical procedure.9,16 Under general anesthetic and in the lithotomy position, with antibiotic prophylaxis, standardized “Ginsburg protocol”8 cores were sampled by the transperineal route. Briefly, this involves taking 4 cores from each of the anterior, mid, and posterior prostate sectors targeting the peripheral zone, avoiding the urethra. In addition, further cores are targeted to cancer-suspicious MRI visible lesions if present. Multiparametric MRIs were read by experienced specialist uroradiologists and any “target” lesions identified, with prostate and lesion reporting occurring according to the European Society of Uro-Radiology standards shortly before biopsy.17 One center used the MRI cognitively/visually and two centers with the support of MRI-ultrasound fusion software (BiopSee™, Darmstadt, Germany). In total, 24–40 cores were taken. After recovery, men were observed until they had voided after which they were discharged on the same day. All men were provided with written information regarding postbiopsy instructions.
Outcome measures
The ProBE questionnaire is well described in the original publication.4 A bespoke questionnaire was created and validated to measure the frequency and effect of symptoms related to pain, infection, and bleeding; patient attitude to repeat biopsy and participant healthcare resource use was also evaluated.
For use in patients undergoing TP biopsies, the appropriate questions were altered to allow for the use of GA. The questionnaire had been validated in English-speaking patients only by the ProBE team. For the use in German patients, we followed a formal translation and retranslation process to ensure accurate interpretation. The questionnaire was translated by a German with good knowledge of the English language from English into German and retranslated back into English by another German urologist resident in the UK.
Symptoms
Using a Likert-type scale, men were asked to described pain and discomfort immediately after the procedure and at follow-up describing it as “none,” “mild,” “moderate,” or “severe.” Complications such as hematuria, hematochezia, hemoejaculate, fever, shivers, and pain were self-reported as absent or present and then graded from “none,” “minor,” “moderate,” or “major” to assess the scale of the problem. Alongside, urinary, bowel, and sexual symptoms were assessed using the validated International Continence Society - male, International Consultation on Incontinence Modular Questionnaire-urinary incontinence and University of California, Los Angeles Prostate Cancer index questionnaires,18,19,20 International Prostate Symptom Score,21 and International Index of Erectile Function22 at baseline and follow-up.
Attitude to rebiopsy
To assess attitudes to future biopsies, men were asked “how much of a problem would you find having another biopsy in the future?” and asked to rate it on a four-point Likert-type scale (not a problem, minor problem, moderate problem, major problem) and “how you would describe the procedure to a friend?” (Minor, moderate, or major).
Use of healthcare services
Men were asked in the questionnaire about contact with healthcare, whether they had been prescribed any analgesics or antibiotics or were catheterized. For all the questions enquiry was made as to the severity of the problem (none, minor, moderate, or major).
Classifying adverse events
At follow-up review, adverse events were recorded and classified by the clinician by the Clavien-Dindo classification.23
Data analysis
SPSS (IBM Corporation Released 2012 IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY, USA) was used for data analysis. The proportion of men experiencing each outcome was presented with 95% confidence intervals (CIs) calculated using Wilson's method. A P value of 0.05 or less was considered statistically significant. The effect of risk factors on binary outcome measures was estimated as odds ratios (ORs) using logistic regression adjusted for center. OR with CIs and P values were calculated.
RESULTS
Of 389 men who were included in the study, 201 men answered both questionnaires (response rate = 51.6%). Baseline demographic measures are shown in Table 1. The nonresponders were chased up by telephone calls and contact to referring physicians to ensure that they were not lost to follow-up, or admitted with a serious complication. The main reasons for noncompletion of the study were either forgetting to fill in questionnaires, not filling in follow-up questionnaires, or returning documents unfilled.
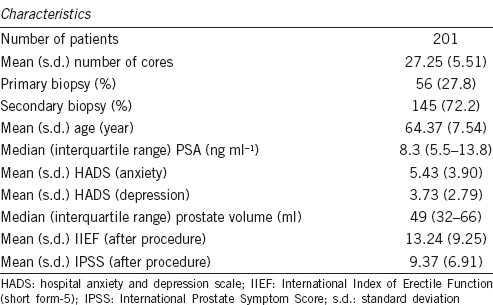
Table 1.
Summary statistics for baseline demographic measures
Symptoms and significance
Immediately after biopsy 43/201 (21.4%) felt men felt light-headed, syncopal, or suffered syncope. Fifty-three percent of men felt discomfort after biopsy (with 95% scoring <5 in a 0–10 scale). Twelve out of 196 (6.1%) men felt pain immediately after the procedure.
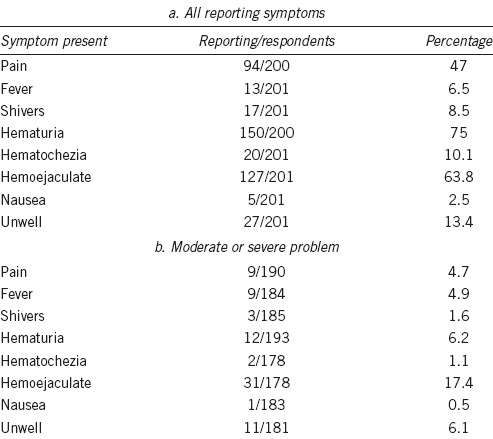
Table 2 highlights the number of men at follow-up questioning who had suffered pain, fever, shivers, hematuria, hematochezia, hemoejaculate, nausea or felt unwell. Table 2 shows in whom this reported symptom was a moderate or serious problem, in those that answered this part of the question (not all men gave the severity of the problem). Over one-third of men reported no sexual activity after biopsy (n = 70/187). Despite the high incidence of symptoms, it was not a moderate or serious problem for most, apart from hemoejaculate which 31 men suffered. There were no inpatient admissions due to complications (hematuria, sepsis, or catheterization).
Table 2.
Overall symptoms and significance
Sexual and urinary matters
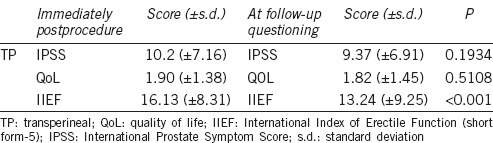
Table 3 shows the IPSS, quality of life (QoL), and IIEF scores immediately after and at follow-up. In general, most men fell into the “moderate” symptom group with regards to IPSS at both timepoints with no urinary symptom deterioration. This was accompanied by a QoL score, which was “mostly pleased” at both intervals. There was a significant and marked reduction in erectile function on repeat questioning at a later timepoint (IIEF score 16.1 vs. 13.2; P < 0.001; Table 3).
Table 3.
Urinary and sexual function scores (IPSS, QoL, and IIEF scores immediately after biopsy and at follow-up questioning)
Attitude to rebiopsy
Just after the procedure, 14 out of 196 men (7.1%) said a repeat biopsy would be a significant problem. On repeat questioning, 25 out of 199 men (12.6%) said repeat biopsy would be a significant problem.
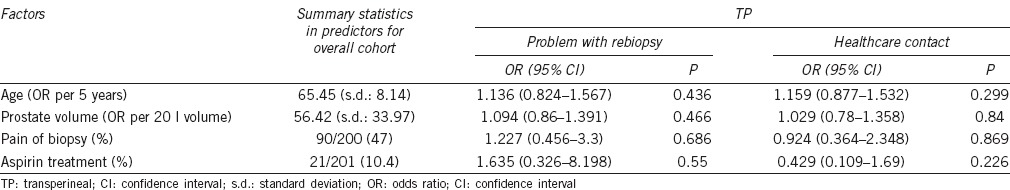
When comparing attitude to biopsy at follow-up with age, prostate volume, pain or aspirin use, using a multiple logistic regression model, there was no effect overall (OR = 1.2; 95% CI: 0.456–3.3) (Table 4).
Table 4.
Measuring the effect of age, prostate volume, biopsy pain, and aspirin use on tolerability of biopsy or healthcare contact
Contact with healthcare
In all, 11 out of 198 (5.5%) men suffered urinary retention requiring catheterization. There was no correlation in healthcare contact with age, prostate volume, pain on biopsy, or aspirin use in either group (Table 4). There were no deaths in the follow-up period.
As per Clavien-Dindo classification, Grade I complications (antiemetic, fluids, etc.) occurred in 147 men (73.1%), Grade II (antibiotics, blood transfusions, etc.) in 54 men (26.9%) and there were no Grades III–IV complications (surgery, intensive care input or death). This was not different across centers (P = 0.35). Overall, there was no difference in overall outcomes between patients having a first or second biopsy (data not shown).
DISCUSSION
This study adds to the literature on patient-reported outcomes following transperineal prostate biopsy. Merrick et al.24 had previously demonstrated a higher urinary retention rate but comparable outcome to TRUS in terms of urinary, bowel and erectile function. Our study had a limited response rate of only 51.6%, and findings are based on patient-reported outcomes alone. Some men rated post-biopsy pain (4.7%) and infective (fever in 4.9%) or hemorrhagic symptoms (hematuria in 6.2%) as a major/moderate problem. Overall, attitude to rebiopsy was favorable. Although there was a significant increase in men in whom rebiopsy would be a major problem on repeat questioning, approximately 7 out of 8 men still had a positive attitude to rebiopsy. Bokhorst et al.25 have previously shown that compliance with repeat biopsy dramatically decreases with time on active surveillance from 81% compliance at year 1 down to 33% in year 10.
Despite being well tolerated on the whole, there were some significant side effects. Of those men who suffered one or more complication, the majority classified as Clavien Grade 1 (73.1%). A high proportion of men suffered from hemoejaculate (63.8%), which was a moderate/serious problem for 17.4%. Despite the high rate of Grade 1 or greater complications, few men contacted healthcare providers (7.6%). Interestingly, the rate of urinary retention after TP saturation biopsy was only 5.5%. There were no hospital admissions and no septic episodes recorded. Similarly, there were no fatalities in our study. This is not surprising given the demographics of the men. We did not dissect the rates of adverse events between those with a cancer diagnosis and those without, but studies have shown this not to be the case.4
Published literature strongly supports our findings. Vyas et al.11 showed that TP biopsies had a good cancer detection rate (54%) with urinary retention in 11 out of 634 men (1.7%) and no cases of urosepsis. Negligible rate of urosepsis after TP biopsy has also been reported recently with rising rates of post-TRUS sepsis and increased incidence of multiresistant bacteria found in rectal flora.26
The results for side effects of our TP biopsy cohort are similar to the original ProBE study in patients undergoing TRUS biopsy with reference to specific symptoms.4 This is surprising given the men having TRUS biopsy in the ProBE study had on average 2 weeks longer to recover from the procedure, and we may assume that men after TP biopsy possibly have better QoL parameters. Similar to the original study, we found that very few men suffered no symptoms (2.1% Grade 0 in ProBE),4 and in those who did, the vast majority were Grade 1 (64.6% in ProBE).4 Again we found that hemoejaculate was a moderate/serious problem in a larger than expected proportion of men, but less than the 20%–26% reported originally.4 After TP biopsy under anesthesia, the attitude to rebiopsy was less negative than in ProBE; we found this to be up to 12% compared to the nearly 20% in the original publication.4 Some of these differences may be due to the later timepoint in the original study; while we collected data immediately after biopsy and at between 7 and 14 days, the ProBE study collected data at 7 and 35 days after biopsy.4 Another reason for the change in attitudes or perceived problem with hemoejaculate may be that the men are better counseled on side effects and rationale for biopsy/rebiopsy in this current cohort who are often having a second biopsy (72.2% in our study).
There are several limitations to this work. Firstly, the response rate was only 51.6% and follow-up relatively short. There are several reasons for this; the main reason for noncompletion of the study was either forgetting to fill in questionnaires, not filling in follow-up questionnaire or returning documents unfilled. However, the nonresponse rate did not seem to bias the data with regards to death rate or hospital admissions. A further criticism is the lack of true “baseline” data reflecting the period before biopsy – this represents a shortcoming of the study. Therefore while we can draw conclusions about outcomes for urinary and sexual matters between the two questionnaires, we cannot truly ascertain the true changes from baseline.
Recall bias is a potential problem, but this was limited by the relatively short follow-up interval (between 7 and 14 days). The presence of nonspecific symptoms, such as fevers/shivers, may not necessarily relate to the biopsy but may influence, for example, attitude to repeat biopsy. There was a mixed population of men having first or secondary biopsy, which reflects current clinical practice.
Several studies have investigated the effect of TP or TRUS biopsies on erectile function and the relationship of prostate biopsies and associated anxiety, with the conclusion that prostate biopsies cause anxiety and temporary erectile dysfunction,27 which may be worse after multiple biopsy episodes.16,28,29 Finally, we did not account for co-morbidity, but given this is a relatively young patient cohort, suitable for treatment with curative intent, we would expect these not to be a significant confounder.
Despite these limitations, we are adding to the literature on patient-reported outcomes after TP biopsy using attitude to biopsy as a surrogate marker for tolerability and reporting healthcare contact in men undergoing TP biopsies under general anesthetic. Local anesthetic strategies for TP biopsy are emerging30 and it will be interesting to see how anesthesia, lower core numbers, and targeted biopsies only may change practice. Prospective studies will be useful to determine patient-reported outcome measures after local anesthetic TP biopsy, particularly in the era of MRI targeting.
CONCLUSION
The current practice of performing TP biopsy under general anesthetic is well tolerated with a good side effect profile and attitude of men to repeat biopsy as a marker of tolerability. We can now quantify complications and inform patients better of what to expect before they embark on the process of general anesthetic transperineal prostate biopsy. However, with the possibility of performing local/regional anesthesia for TP, patient tolerability in this setting is yet to be determined. The current study and modified questionnaire could act as a benchmark for future work in evaluating patient-reported outcomes after local anesthetic TP biopsy.
AUTHOR CONTRIBUTIONS
BH, CK, AD, and VG conceived the study. LCE, TK, and JF designed the questionnaires and carried out the study. LCE, TK, GG, ES, JF, ID, JS, KW PA, and GM collected and formatted the data. LCE, ES, and DP performed the analysis including statistics. All the patients were under the care of PA, GM, AD, VG, and BH and CK, KW, TK, PA, BH, CK, AD, and VJG wrote and edited the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare that they have no competing interests.
ACKNOWLEDGMENTS
Boris Hadaschik received funding from the German Research Foundation and the European Foundation for Urology. Karan Wadhwa is sponsored by a Medical Research Council Research Training Fellowship. No other funding was received for this work.
REFERENCES
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Shaw GL, Thomas BC, Dawson SN, Srivastava G, Vowler SL, et al. Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br J Cancer. 2014;110:2405–11. doi: 10.1038/bjc.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haas GP, Delongchamps B, Jones RF, Chandan V, Serio A, et al. Needle biopsies on autopsy prostates: sensitivity of cancer detection based on true prevalence. J Natl Cancer Inst. 2007;99:1484–9. doi: 10.1093/jnci/djm153. [DOI] [PubMed] [Google Scholar]
- 4.Rosario DJ, Lane JA, Metcalfe C, Donavan JL, Doble A, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ. 2012;344:7894. doi: 10.1136/bmj.d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loeb S, van den Heuval S, Zhu X, Bangma CH, Schroder FH, et al. Infectious complications and hospital admissions after prostate biopsy in a European randomized trial. Eur Urol. 2012;61:1110–4. doi: 10.1016/j.eururo.2011.12.058. [DOI] [PubMed] [Google Scholar]
- 6.Lundstrom KJ, Drevin L, Carlsson S, Garmo H, Loeb S, et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014;192:1116–22. doi: 10.1016/j.juro.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 7.Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92. doi: 10.1016/j.eururo.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Nam RK, Saskin R, Lee Y, Liu Y, Law C, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2010;183:963–8. doi: 10.1016/j.juro.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 9.Kuru TH, Wadhwa K, Chang RT, Carmona-Echevveria L, Roethke M, et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int. 2013;112:568–77. doi: 10.1111/bju.12132. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka A, Hara R, Ishimura T, Fujii T, Jo Y, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008;11:134–8. doi: 10.1038/sj.pcan.4500985. [DOI] [PubMed] [Google Scholar]
- 11.Vyas L, Acher P, Kinsella J, Challacombe B, Chang RT, et al. Indications, results and safety profile of transperineal sector biopsies (TPSB) of the prostate: a single centre experience of 634 cases. BJU Int. 2014;114:32–7. doi: 10.1111/bju.12282. [DOI] [PubMed] [Google Scholar]
- 12.Saredi G, Sighinolfi MC, Francesco F, De Stefani S, Micale S, et al. Does needle calibre affect pain and complication rates in patients undergoing transperineal prostate biopsy? A prospective, randomized trial. Asian J Androl. 2009;11:678–82. doi: 10.1038/aja.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int. 2014;114:e43–9. doi: 10.1111/bju.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan J K, Rais-Bahrami S, Turkbey B, Gomella A, Amalou H, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int. 2014;114:641–52. doi: 10.1111/bju.12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuru TH, Roethke MC, Seidenader J, Simpfendorfer T, Boxler S, et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol. 2013;190:1380–6. doi: 10.1016/j.juro.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Gaziev G, Wadhwa K, Barrett T, Koo B, Gallagher FA, et al. Defining the learning curve for multi-parametric MRI of the prostate using MRI-TRUS fusion guided transperineal prostate biopsies as a validation tool. BJU Int. 2016;117:80–6. doi: 10.1111/bju.12892. [DOI] [PubMed] [Google Scholar]
- 18.Donovan JL, Peters TJ, Abrams P, Brookes ST, De La Rosette JJ, et al. Scoring the short form ICSmaleSF questionnaire. International continence society. J Urol. 2000;164:1948–55. [PubMed] [Google Scholar]
- 19.Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, et al. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23:322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 20.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, et al. The UCLA prostate cancer index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Batista-Miranda JE, Regalado Pareja R, Huguet Perez J, Montlleo Sanchez M, Arano Bertran P. [The use of the IPSS questionnaire in surgical patients. International prostatic symptom score] Actas Urol Esp. 1995;19:227–33. [PubMed] [Google Scholar]
- 22.Rhoden EL, Teloken C, Sogari PR, Vargas Souto CA. The use of the simplified international index of erectile function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int J Impot Res. 2002;14:245–50. doi: 10.1038/sj.ijir.3900859. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merrick GS, Taubenslag W, Andreini H, Brammer S, Butler WM, et al. The morbidity of transperineal template-guided prostate mapping biopsy. BJU Int. 2008;101:1524–9. doi: 10.1111/j.1464-410X.2008.07542.x. [DOI] [PubMed] [Google Scholar]
- 25.Bokhorst LP, Alberts AR, Rannikko A, Valdagni R, Pickles T, et al. Compliance rates with the prostate cancer research international active surveillance (PRIAS) protocol and disease reclassification in noncompliers. Eur Urol. 2015;68:814–21. doi: 10.1016/j.eururo.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114:384–8. doi: 10.1111/bju.12536. [DOI] [PubMed] [Google Scholar]
- 27.Glaser AP, Novakovic K, Helfand BT. The impact of prostate biopsy on urinary symptoms, erectile function, and anxiety. Curr Urol Rep. 2012;13:447–54. doi: 10.1007/s11934-012-0277-6. [DOI] [PubMed] [Google Scholar]
- 28.Murray KS, Bailey J, Zuk K, Lopez-Corona E, Thrasher JB. A prospective study of erectile function after transrectal ultrasonography-guided prostate biopsy. BJU Int. 2015;116:190–5. doi: 10.1111/bju.13002. [DOI] [PubMed] [Google Scholar]
- 29.Braun K, Ahallal Y, Sjoberg DD, Ghoneim T, Dominguez Esteban M, et al. Effect of repeated prostate biopsies on erectile function in men on active surveillance for prostate cancer. J Urol. 2014;191:744–9. doi: 10.1016/j.juro.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 30.Cerruto MA, Vianello F, D’Elia C, Artibani W, Novella G. Transrectal versus transperineal 14-core prostate biopsy in detection of prostate cancer: a comparative evaluation at the same institution. Arch Ital Urol Androl. 2014;86:284–7. doi: 10.4081/aiua.2014.4.284. [DOI] [PubMed] [Google Scholar]


