Abstract
Our previous studies have demonstrated that erectile function was preserved in aged transgenic rats (TGR) harboring the human tissue kallikrein 1 (hKLK1), while the molecular level of hKLK1 on corporal fibrosis to inhibit age-related erectile dysfunction (ED) is poorly understood. Male wild-type Sprague-Dawley rats (WTR) and TGR harboring the hKLK1 gene were fed to 4- or 18-month-old and divided into three groups: young WTR (yWTR) as the control, aged WTR (aWTR), and aged TGR (aTGR). Erectile function of all rats was assessed by cavernous nerve electrostimulation method. Masson's trichrome staining was used to evaluate corporal fibrosis in the corpus cavernosum. We found that the erectile function of rats in the aWTR group was significantly lower than that of other two groups. Masson's trichrome staining revealed that compared with those of the yWTR and aTGR groups, the ratio of smooth muscle cell (SMC)/collagen (C) was significantly lower in the aWTR group. Immunohistochemistry and Western blotting analysis were performed, and results demonstrated that expression of α-SMA was lower, while expressions of transforming growth factor-β 1 (TGF-β1), RhoA, ROCK1, p-MYPT1, p-LIMK2, and p-cofilin were higher in the aWTR group compared with those in other two groups. However, LIMK2 and cofilin expressions did not differ among three groups. Taken together, these results indicated that the RhoA/ROCK1/LIMK/cofilin pathway may be involved in the corporal fibrosis caused by advanced age, and hKLK1 may reduce this corporal fibrosis by inhibiting the activation of this pathway to ameliorate age-related ED.
Keywords: aged, corporal fibrosis, erectile dysfunction, gene therapy, hKLK1
INTRODUCTION
Erectile dysfunction (ED) is a common disease that affects a male individual's quality of life. According to the International Consultation Committee, the prevalence of ED in men aged <40 years is 1%–10%,1 whereas in the men aged 60–69 years, it is 20%–40%, and most men aged older than 70 years experience ED.2 Thus, the prevalence of ED in men increases with the age. Phosphodiesterase 5 inhibitors, the first-line drug treatment of ED, improve ED by inducing cavernous smooth muscle relaxation, with an effective rate of 72% in men aged 18–49 years,3 but <53% in men older than 50 years. Therefore, a new treatment method for ED in older men is urgently needed; however, a detailed understanding of the mechanism for age-related ED is first required.
Age-related ED is characterized histologically by the compliance loss of the corpus cavernosum smooth muscle cells (CCSMCs) owing to the progressive replacement of these cells by collagen fibers.4,5 Transforming growth factor-β (TGF-β) is an important cytokine reported to induce fibrogenesis in the corpus cavernosum and to enhance the change of epithelial to mesenchymal and extracellular matrix production. The increase of TGF-β expression and corporal fibrosis has been noted in an animal model of age-related ED.6,7,8,9 However, the mechanism of TGF-β-induced corporal fibrosis is not fully understood. In a previous study, Cho et al.10 reported that the RhoA/ROCK signaling pathway might play an important role in the corporal fibrosis characterized by loss of smooth muscle through its coordination with TGF-β after cavernous nerve injury. Similarly, Song et al.11 found that the ROCK1/LIMK2/cofilin pathway may be connected with ED related to corporal fibrosis after cavernous nerve injury. Therefore, although one may conclude that TGF-β induces corporal fibrosis after cavernous nerve injury through Rho-kinase/LIM-kinase/cofilin pathway, whether this signaling pathway plays an important role in aged-related ED still needs to be determined.
Tissue kallikrein 1 (KLK1), a member of the serine proteinase superfamily, was first determined as a hypotensive agent in human urine. KLK1 has a role in cleaving low-molecular-weight kininogen (LMWK) into several kinin peptides and derivatives, such as bradykinin (BK).12 BK, as a vasoactive nanopeptide enzymatically generated from KLK1, plays its biological role through activating the kinin receptor. Moreover, kininogens, kallikrein and pharmacologically active kinins from the kallikrein-kinin system (KKS), have been reported by numerous studies to be beneficial to cardiovascular, cerebrovascular, and renal diseases.12 Previous studies have demonstrated that KLK gene delivery reduces renal fibrosis via suppression of oxidative stress and the TGF-β/Smad signaling pathway.13,14,15 In addition, transgenic expression of hKLK1 resists the progression of cardiac fibrosis in a rat model of diabetic cardiomyopathy.16 Considering the association between cardiovascular system and corpus cavernosum, whether hKLK1 can exert its preventive role on corporal fibrosis, and the link between its role and the Rho-kinase/LIM-kinase/cofilin signaling pathway, warrants further study.
MATERIALS AND METHODS
Acquisition of the transgenic rat
We obtained transgenic rats (TGR) harboring the hKLK1 gene from the Max-Delbrück-Center for Molecular Medicine (Berlin, Germany). The transgenic rat was generated by microinjecting a 5.6-kb DNA fragment containing the entire hKLK1 gene under the control of the heavy metal-responsive mouse metallothionein promoter into the oocytes of Sprague-Dawley (SD) rats. The presence of the transgene in genomic DNA was verified by Southern blotting. Offspring with the homozygous hKLK1 gene were selected for further experiments.
Animals and treatment
All procedures were approved by the Committee for Animal Care and Use in Huazhong University of Science and Technology (Wuhan, China). A total of thirty male SD rats were used, twenty of which were wild-type SD rats (WTRs) obtained from the Laboratory Animal Center of Tongji Medical College (Huazhong University of Science and Technology), and the remaining ten rats were the TGRs as described above. The animals were bred by professional breeders under the same conditions until they were 4-month-old (weighing 250–300 g) or 18-month-old (weighing 450–500 g).
Thirty rats were divided into three groups: the young WTR group (yWTR) as the control group (4-month-old, n = 10); the aged WTR group (aWTR) (18-month-old, n = 10); and the aged TGR group (aTGR) (18-month-old, n = 10). All rats were bred by professional breeders.
Verification of TGR
The levels of genome, genomics transcription and translation were used to detect the existence and expression of the hKLK1 gene in corpus cavernosum. The existence of the hKLK1 gene in the genomic DNA obtained from frozen corpus cavernosum samples was detected through conventional PCR and agarose gel electrophoresis. The primer sequences are listed in Table 1. The mRNA and protein expressions were assayed by real-time reverse transcriptase-PCR (RT-PCR) and Western blotting analysis.
Table 1.
Primers used in conventional PCR and real-time RT-PCR
Assessment of erectile function
As in our previous experiments,17,18 the erectile function of all rats was assessed using electrostimulation of cavernous nerves. Briefly, rats were anesthetized by injection of pentobarbital sodium (35 mg kg−1, intraperitoneally). The carotid artery was cannulated (PE-50 tubing) for continuous measurement of mean arterial pressure (MAP). Cavernous nerves were isolated at the posterolateral aspect of the prostate gland. When cavernous nerves were stimulated by stainless-steel bipolar wire electrodes, a 25-gauge needle was inserted into the right crura to continuously monitor intracavernosal pressure. Data were recorded with a data-acquisition system (Powerlab 4SP; ADInstruments, Dunedin, New Zealand). An erectile response was induced by electrical field stimulation at a frequency of 15 Hz for 1 min at 2.5 V and 5.0 V, with a 3 min interval between stimulations. After the functional studies, the middle regions of the skin-denuded penile shaft were maintained overnight in 4% paraformaldehyde and then embedded in paraffin for histologic studies. The remaining penile tissues were harvested and then frozen at −80°C for subsequent experiments.
Histological assessment
Specimens of penile tissue were cut in a transverse direction (thickness, 5 μm). The sections were deparaffinized with xylene and rehydrated in a graded ethanol series to distilled water. All of the sections were stained with Masson's trichrome staining to examine smooth muscle and connective tissue histology.
For immunohistochemical examination, sections were incubated overnight at 4°C with antibodies against: RhoA (1:50 dilution; Proteintech, Wuhan, China), ROCK1 (1:50; Proteintech, Wuhan, China), α-smooth muscle actin (α-SMA; 1:100; Abcam, Cambridge, MA, USA), transforming growth factor-β1 (TGF-β1; 1:100, Abcam, Cambridge, MA, USA), p-LIMK2 (1:100; Abcam, Cambridge, MA, USA), and p-cofilin (1:100; Abcam, Cambridge, MA, USA). After being washed, the sections were incubated with a biotinylated secondary antibody. Finally, antigen-antibody reactions were developed by diaminobenzidine. Semiquantitative analysis was performed to evaluate intensity using Image-Pro plus software (Media Cybernetics, Silver Spring, MD, USA).
Real-time RT-PCR
Total RNA of corpus cavernosum was obtained using a Multisource Total RNA Minipre Kit (AXYGEN, Union City, CA, USA) according to the manufacturer's instructions. As in our previous experiments,17,18 reverse transcription and real-time PCR were conducted with PrimeScript RT Master Mix and SYBR Green PCR Master Mix (TaKaRa, Dalian, China). The relative mRNA expression of the level of the examined genes to that of β-actin was calculated using the 2−ΔCt method. The primer sequences used for hKLK1, rat tissue KLK1 (rKLK1), and β-actin are shown in Table 1.
Western blotting analysis
As in our previous study,17,18 penile tissues from each group were homogenized in RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China) containing a protease inhibitor cocktail and phosphatase inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Concentrations of soluble proteins were measured using the bicinchoninic acid assay (Beyotime Institute of Biotechnology, Haimen, China). Protein samples (40 μg per lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking in Tris buffered saline-Tween (TBST) with 5% bovine serum albumin, membranes were incubated overnight at 4°C with primary antibodies against: hKLK1 (1:5000, Sigma-Aldrich, St. Louis, MO, USA), rKLK1 (1:1000, Sigma-Aldrich, St. Louis, MO, USA), α-SMA (1:1000, Abcam), TGF-β1 (1:1000, Abcam), RhoA (1:1000, Proteintech), ROCK1 (1:1000, Proteintech), p-MYPT1 (1:1000, Millipore, Billerica, MA, USA), LIMK2 (1:1000, Abcam, Cambridge, MA, USA), p-LIMK2 (T505, 1:1000, Abcam), cofilin (1:1000, Proteintech, Wuhan, China), and p-cofilin (1:1000, Abcam). Washed membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:5000) for 1 h at room temperature. Finally, bands were developed with an enhanced chemiluminescence detection system.
Statistical analysis
Data were analyzed using SPSS version 15.0 (IBM, Armon, NY, USA). Continuous variables are the mean ± standard deviation. Analysis of variance was used to analyze differences among the three groups. P <<i> 0.05 was considered statistically significant.
RESULTS
Erectile function
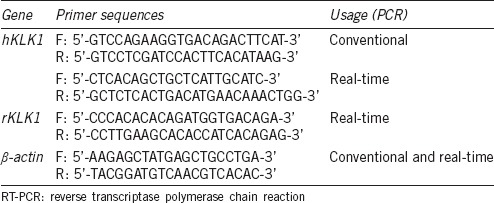
Erectile function of all rats was measured through electric stimulation on the cavernous nerves. Compared with aWTR group, the erectile function of animals in groups yWTR and aTGR were significantly higher (both P < 0.05; Figure 1).
Figure 1.
Erectile function of all rats was measured through electric stimulation on the cavernous nerves. Representative carotid artery pressure and ICP tracing were measured through stimulation of 2.5 V (a) and 5 V (b) setting for 1 min, respectively, in rats of all groups. The max ICP/MAP ratio of different volts were presented through bar graphs: (c) for 2.5V, and (d) for 5V. Data are expressed as mean ± s.d. (n = 10 rats per group). *P < 0.05 when comparing the two groups under each end of the capped line. ICP: intracavernous pressure; MAP: mean arterial pressure; yWTR: young wild-type Sprague-Dawley rats; aWTR: aged wild-type Sprague-Dawley rats; aTGR: aged transgenic rats; s.d.: standard deviation.
Verification of hKLK1 gene in corpus cavernosum
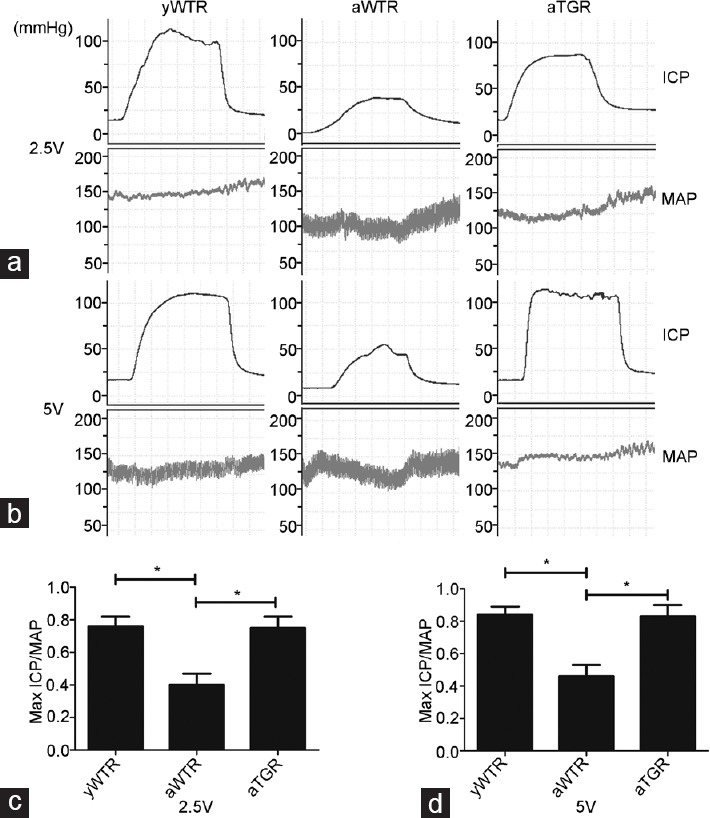
The existence and expression of the hKLK1 gene in penile tissues of rats were verified at the levels of genomic DNA, mRNA, and protein. All rats in the aTGR group contained the hKLK1 DNA fragments, and they were able to transcribe and translate the fragments into hKLK1 mRNA and protein. However, the hKLK1 gene did not exist in both groups of yWTR and aWTR. Meanwhile, the mRNA and protein expression of rKLK1 were lower in the aWTR group than that of the yWTR group (all P < 0.05; Figure 2).
Figure 2.
Verification of expressions of hKLK1 and rKLK1 genes in the corpus cavernosum. (a) Representative hKLK1 genomics DNA bands in rats’ corpus cavernosum by agarose gel electrophoresis followed by conventional PCR. Relative mRNA expressions of rKLK1 (b) and hKLK1 (c) with β-actin as the loading control in the corpus cavernosum of three groups by real-time RT-PCR. (d) Representative Western blotting results of rKLK1, hKLK1, and β-actin in the corpus cavernosum of three groups. Expressions of rKLK1 (e) and hKLK1 (f) with β-actin as the loading control in the corpus cavernosum of three groups were presented through bar graphs. Data are expressed as mean ± s.d. (n = 10 rats per group). *P < 0.05 when comparing the two groups under each end of the capped line. RT-PCR: reverse transcriptase PCR.
Masson's trichrome staining, immunohistochemistry and Western blotting analysis for corporal fibrosis
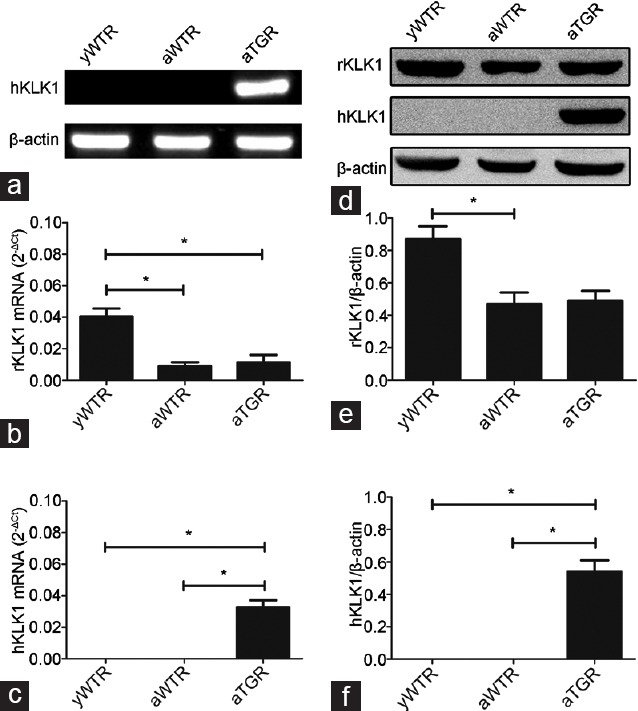
Masson's trichrome staining was conducted to determine the ratio of smooth muscle/collagen. We found that the area of CCSMCs (red stain) was reduced whereas that of collagen (blue stain) was increased in the corpus cavernosum of rats in the aWTR group compared with that of rats in both yWTR and aTGR groups. Immunohistochemistry and Western blotting analysis results showed that expression of α-SMA was reduced while expression of TGF-β1 was increased in the aWTR group compared with those in both yWTR and aTGR groups (all P < 0.05; Figure 3).
Figure 3.
Effects of hKLK1 on histological changes of penile tissues. (a) Masson's trichrome staining (×200) of corpus cavernosum: the area of smooth muscle is represented by red stain and the area of collagen is blue stain. Expressions of α-SMA (b) and TGF-β1 (c) were assessed through immunohistochemistry (×200). (d) Representative Western blotting results of α-SMA, TGF-β1 and β-actin in the corpus cavernosum of three groups. Expressions of α-SMA (e) and TGF-β1 (f) with β-actin as the loading control in the corpus cavernosum of three groups were presented through bar graphs. (g) The ratio of smooth muscle/collagen. Data are expressed as mean ± s.d. (n = 10 rats per group). *P < 0.05 when comparing the two groups under each end of the capped line. yWTR: young wild-type Sprague-Dawley rats; aWTR: aged wild-type Sprague-Dawley rats; aTGR: aged transgenic rats; s.d.: standard deviation
Immunohistochemistry and Western blotting analysis of RhoA/ROCK pathway
Immunohistochemistry and Western blotting analysis results showed that expressions of RhoA, ROCK1, and p-MYPT1 in aWTR group were higher compared with groups of yWTR and aTGR (all P < 0.05), indicating that hKLK1 gene in TGRs blocked the age-related change in the RhoA/ROCK pathway (Figure 4).
Figure 4.
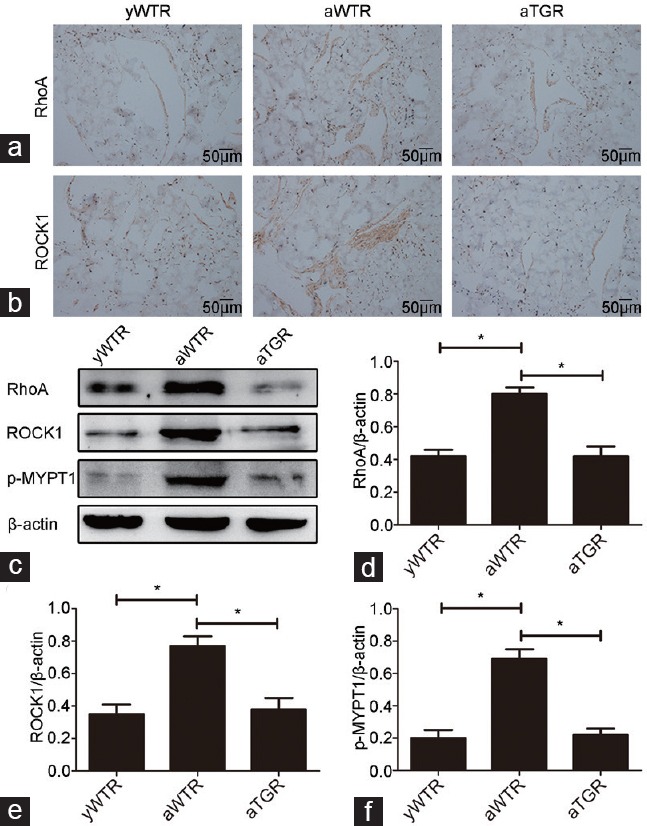
Immunohistochemistry and Western blotting analysis of the activation of RhoA/ROCK pathway. Expressions of RhoA (a) and ROCK1 (b) were assessed through immunohistochemistry (×200). (c) Representative Western blotting results of RhoA, ROCK1, p-MYPT1 and β-actin in the corpus cavernosum of three groups. Expressions of RhoA (d), ROCK1 (e) and p-MYPT1 (f) with β-actin as the loading control in the corpus cavernosum of three groups were presented through bar graphs. Data are expressed as mean ± s.d. (n = 10 rats per group). *P < 0.05 when comparing the two groups under each end of the capped line. yWTR: young wild-type Sprague-Dawley rats; aWTR: aged wild-type Sprague-Dawley rats; aTGR: aged transgenic rats; s.d.: standard deviation.
Immunohistochemistry and Western blotting analysis of LIMK2/cofilin pathway
Immunohistochemistry results showed that expressions of p-LIMK2 and p-cofilin in aWTR group were increased compared with groups of yWTR and aTGR (all P < 0.05), which were consistent with the Western blotting analysis results of p-LIMK2 and p-cofilin (all P < 0.05). Results of Western blotting analysis of LIMK2 and cofilin showed no significant difference in their expressions levels among three groups (Figure 5).
Figure 5.
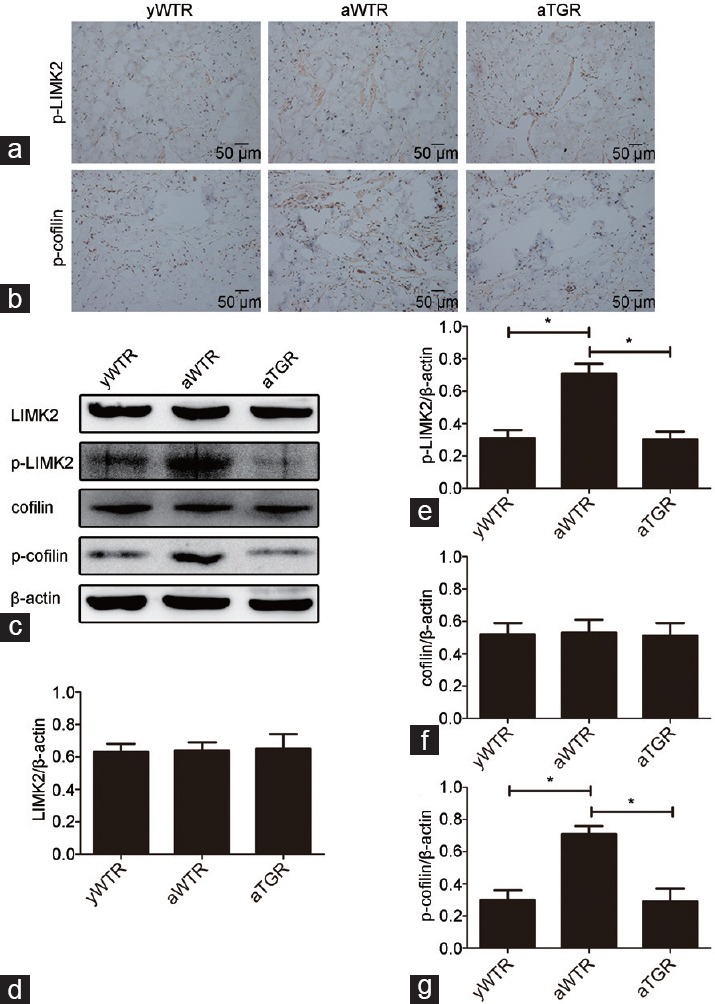
Immunohistochemistry and Western blotting analysis of the activation of LIMK2/cofilin pathway. Expressions of p-LIMK2 (a) and p-cofilin (b) were assessed through immunohistochemistry (×200). (c) Representative Western blotting results of LIMK2, p-LIMK2, cofilin, p-cofilin, and β-actin in the corpus cavernosum of three groups. Expressions of LIMK2 (d), p-LIMK2 (e), cofilin (f), and p-cofilin (g) with β-actin as the loading control in the corpus cavernosum of three groups were presented through bar graphs. Data are expressed as mean ± s.d. (n = 10 rats per group). *P < 0.05 when comparing the two groups under each end of the capped line. s.d.: standard deviation.
DISCUSSION
Here, our report was the first to investigate the mechanism of the inhibitory role of hKLK1 on corporal fibrosis and age-related ED in an aged rat model, showing that this inhibition is functionally regulated by a novel signaling pathway of RhoA/ROCK1/LIMK2/cofilin. Our experiment results suggested that aged rats with ED have decreased tissue KLK1 levels, an over-activated RhoA/ROCK1/LIMK2/cofilin pathway, and subsequent corporal fibrosis. However, stimulating the KKS through overexpression of hKLK1 inhibited the activation of RhoA/ROCK1/LIMK2/cofilin pathway, ameliorated corporal fibrosis, maintained the integrity of CCSMCs, and finally protected erectile function of aged rats.
Although endothelial cells are thought to be associated with the ability to achieve normal penile corporal veno-occlusion, the more important factor is smooth muscle content.19 Both in human and rat, decreased smooth muscle content and a corresponding decreased ratio of smooth muscle/collagen in the corpus cavernosum, are associated with age-related ED.5,20,21 The aging process in the penis leading to defective CCSMC relaxation, which is considered the most important factor in age-related ED, causes veno-occlusive dysfunction and increases the severity of ED.22 Ferrini et al.4 reported that ED in the aging male individual mainly results from corporal fibrosis, which is described as the loss of compliance of the corpus cavernosum smooth muscle owing to the progressive replacement of CCSMCs by collagen fibers. This same group further reported that age-related ED was mainly caused by a reduction in CCSMCs and an increase in collagen fibers, which was assumed to result from an increase in reactive oxygen species as well as many other profibrotic factors.23 Therefore, the key to ameliorate age-related ED is to block the decrease of smooth muscle content and to reduce excessive deposit of collagen for maintaining the integrity of CCSMCs.
TGF-β, an essential profibrotic cytokine, is closely associated with the pathological process of corporal fibrosis. TGF-β exerts its effects in vascular fibrosis through Smad signaling and non-Smad signaling pathways.24,25 Although most researches on vascular fibrosis have focused on the TGF-β/Smad signaling pathway, the role of non-Smad signaling pathway also warrants attention. Of all non-Smad signaling pathways, the RhoA/ROCK pathway is most important in the progression of TGF-β-induced vascular fibrosis.25 Haudek et al.26 reported that the RhoA/ROCK pathway was associated with the development of cardiac fibrosis in the differentiation of monocytes. A previous study by Zhang and his colleague provided direct support for a role of ROCK1 in nonadaptive fibrosis in response to pressure overload and in the regulation of fibrogenic cytokine expressions in cardiomyocytes in response to hypertrophic stimuli, which revealed the potential therapeutic target of ROCK1 in cardiac fibrotic diseases.27 TGF-β can upregulate the activation of RhoA/ROCK pathway to promote cytoskeletal rearrangement in various types of cells, inducing a pathological process that is characterized by fibroblast-to-myofibroblast differentiation.8 Moreover, Cho et al.10 suggested that the RhoA/ROCK signaling pathway may be connected with the corporal fibrosis characterized by the loss of smooth muscle through its coordination with TGF-β after cavernous nerve injury. Therefore, based on the role of RhoA/ROCK pathway in cardiac and corporal fibrosis, activation of RhoA/ROCK pathway may be involved in the corporal fibrosis in the aged rats.
Activation of RhoA/ROCK pathway is followed by activation of the downstream target proteins LIMK2 and cofilin, resulting in a pathologic process marked by the differentiation of fibroblast-to-myofibroblast.8 Members of the LIMK family, including LIMK1 and LIMK2, are serine kinases that exert important effects on the regulation of the actin cytoskeleton through the phosphorylation of cofilin. LIMK2, a downstream factor of RhoA/ROCK signaling pathway,28 regulates actin cytoskeletal reorganization by phosphorylating cofilin, which is also associated with cytoskeletal rearrangements as well as fibroblast-to-myofibroblast differentiation.29 Song et al.11 reported that the RhoA/ROCK/LIMK2/cofilin pathway may be connected with ED related to corporal fibrosis after cavernous nerve injury. However, whether this pathway is associated with corporal fibrosis in aged rats and whether hKLK1 can inhibits this pathological process have not been determined.
Tissue KLK1, being part of a group of serine proteinases, are encoded by tandemly arranged large proteinases genes of multigene families in human and rats.30 Tissue KLK1 processes LMWK to generate vasoactive kinins, which exert biological functions via binding to kinin receptors. For example, BK, as a kind of effector peptide, plays an important role in cardiovascular system through mediating varieties of physiological and pathological functions.31 In previous studies, Silva et al.32 reported that they established a transgenic rat model with an overactive KKS through inserting hKLK1, and this model could be used to study multiple issues such as cardiovascular regulation and other physiological or pathological mechanisms in which kinins might participate. They demonstrated an important role of hKLK1 in the regulation of extracellular matrix. Moreover, numerous studies also demonstrated that KLK gene delivery reduces renal fibrosis through its effect on the TGF-β signaling pathway. Tschöpe et al.16 also revealed that transgenic expression of hKLK1 resisted the progression of cardiac fibrosis in a rat model of diabetic cardiomyopathy. Similarly, Gallagher et al.33 revealed that the KKS could inhibit collagen synthesis in vivo by activating nitric oxide (NO) and the prostaglandin system via the BK receptor. Therefore, hKLK1 may be an ideal point of treatment to inhibit renal and cardiac fibrosis. In addition, Song et al.11 reported that the RhoA/ROCK1/LIMK2/cofilin signaling pathway might be involved in the ED related to corporal fibrosis. Our results also verified that hKLK1 could inhibit corporal fibrosis through attenuating the activation of this signaling pathway.
To further demonstrate the antifibrotic effect of hKLK1 on corporal fibrosis in the aged transgenic rats, we compared aWTR and aTGR. First, we detected the expression of hKLK1 DNA, mRNA, and protein in the corpus cavernosum obtained from TGRs. These results showed that only TGRs contained the hKLK1 gene and expressed mRNA and protein at similar levels in corpus cavernosum of rats, demonstrating the reliability of our TGR model. In addition, a decrease in the expression of rKLK1 in aged rats was shown using RT-PCR and Western blotting analysis.
Using this TGR model, we found that although erectile function of rats in the aWTR group was significantly impaired compared with that in the yWTR group, there was almost no difference in erectile function between rats in the aTGR group and those in the yWTR group, indicating that hKLK1 exerts a protective effect on erectile function in aged rats. According to the following results of Masson's trichrome staining, immunohistochemistry, and Western blotting assays, we can conclude that the activation of RhoA/ROCK pathway and its downstream factors, including LIMK2 and cofilin, were closely associated with corporal fibrosis, and expression of the hKLK1 gene could reduce it in aTGR to protect erectile function through inhibition of RhoA/ROCK1/LIMK2/cofilin pathway.
The main limit of our present study was the absence of cell-based experiments. In addition, our blocking methods including inhibitors of ROCK and LIMK and a siRNA knockdown were not performed to further investigate the role of RhoA/ROCK1 pathway in the corporal fibrosis associated with aging. For the next step, we would try to conduct cell-based experiments to further study the association between corporal fibrosis and the RhoA/ROCK1/LIMK2/cofilin pathway, and investigate the upstream of this pathway to reveal the detail mechanism of corporal fibrosis inhibition by hKLK1.
CONCLUSIONS
Based on our results, we can conclude that the RhoA/ROCK1/LIMK2/cofilin signaling pathway was involved in the corporal fibrosis caused by advanced age, suggesting that these factors might be novel targets in the treatment of age-related corporal fibrosis. In addition, expression of the hKLK1 gene could reduce age-related corporal fibrosis to protect erectile function by inhibiting the activation of RhoA/ROCK1/LIMK2/cofilin pathway. Considering the point of gene therapy, hKLK1 might be a new treatment method for age-related ED in animals and human beings.
AUTHOR CONTRIBUTIONS
KC participated in the design of the trial, conducted the data acquisition, interpreted and analyzed the data, and drafted and revised the manuscript. YL, TW, and LZ designed the study and contributed to the study materials. KR, SGW, and ZQY pointed out deficiencies and ameliorated the manuscript. JHL and DWW guided the experiment directions and drafted the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The TGR (hKLK1) was a generous gift from the Max-Delbrück-Center for Molecular Medicine, Berlin, Germany. This work was supported by the grant from the National Natural Science Foundation of China (NSFC #81270690).
REFERENCES
- 1.Lewis RW, Fugl-Meyer KS, Corona G, Hayes RD, Laumann EO, et al. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 2.Nicolosi A, Glasser DB, Kim SC, Marumo K, Laumann EO, et al. Sexual behaviour and dysfunction and help-seeking patterns in adults aged 40-80 years in the urban population of Asian countries. BJU Int. 2005;95:609–14. doi: 10.1111/j.1464-410X.2005.05348.x. [DOI] [PubMed] [Google Scholar]
- 3.Rendell MS, Rajfer J, Wicker PA, Smith MD. Sildenafil for treatment of erectile dysfunction in men with diabetes: a randomized controlled trial. JAMA. 1999;281:421–6. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 4.Ferrini M, Magee TR, Vernet D, Rajfer J, González-Cadavid NF. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod. 2001;64:974–82. doi: 10.1095/biolreprod64.3.974. [DOI] [PubMed] [Google Scholar]
- 5.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, et al. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–8. [PubMed] [Google Scholar]
- 6.Morland RB. Is there a role of hypoxemia in penile fibrosis: a viewpoint presented to the Society for the Study of Impotence. Int J Impot Res. 1998;10:113–20. doi: 10.1038/sj.ijir.3900328. [DOI] [PubMed] [Google Scholar]
- 7.Massagué J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–20. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 8.Kardassis D, Murphy C, Fotsis T, Moustakas A, Stournaras C. Control of transforming growth factor beta signal transduction by small GTPases. FEBS J. 2009;276:2947–65. doi: 10.1111/j.1742-4658.2009.07031.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F, Li GY, Gao ZZ, Liu J, Liu T, et al. The TGF-β1/Smad/CTGF pathway and corpus cavernosum fibrous-muscular alterations in rats with streptozotocin-induced diabetes. J Androl. 2012;33:651–9. doi: 10.2164/jandrol.111.014456. [DOI] [PubMed] [Google Scholar]
- 10.Cho MC, Park K, Chai JS, Lee SH, Kim SW, et al. Involvement of sphingosine-1-phosphate/RhoA/Rho-kinase signaling pathway in corporal fibrosis following cavernous nerve injury in male rats. J Sex Med. 2011;8:712–21. doi: 10.1111/j.1743-6109.2010.02147.x. [DOI] [PubMed] [Google Scholar]
- 11.Song SH, Park K, Kim SW, Paick JS, Cho MC. Involvement of Rho-kinase/LIM Kinase/Cofilin signaling pathway in corporal fibrosis after cavernous nerve injury in male rats. J Sex Med. 2015;12:1522–32. doi: 10.1111/jsm.12903. [DOI] [PubMed] [Google Scholar]
- 12.Chao J, Shen B, Gao L, Xia CF, Bledsoe G, et al. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–55. doi: 10.1515/BC.2010.042. [DOI] [PubMed] [Google Scholar]
- 13.Bledsoe G, Shen B, Yao Y, Zhang JJ, Chao L, et al. Reversal of renal fibrosis, inflammation, and glomerular hypertrophy by kallikrein gene delivery. Hum Genet Ther. 2006;17:545–55. doi: 10.1089/hum.2006.17.545. [DOI] [PubMed] [Google Scholar]
- 14.Seccia TM, Belloni AS, Guidolin D, Sticchi D, Nussdorfer GG, et al. The renal antifibrotic effects of angiotensin-converting enzyme inhibition involve bradykinin B2 receptor activation in angiotensin II-dependent hypertension. J Hypertens. 2006;24:1419–27. doi: 10.1097/01.hjh.0000234124.94013.ac. [DOI] [PubMed] [Google Scholar]
- 15.Cardenas A, Campos J, Ehrenfeld P, Mezzano S, Ruiz-Ortega M, et al. Up-regulation of the kinin B2 receptor pathway modulates the TGF-beta/Smad signaling cascade to reduce renal fibrosis induced by albumin. Peptides. 2015;73:7–19. doi: 10.1016/j.peptides.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Tschöpe C, Walther T, Koniger J, Spillmann F, Westermann D, et al. Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J. 2004;18:828–35. doi: 10.1096/fj.03-0736com. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Wang T, Guo S, Rao K, Liu J, et al. Expression of oxytocin receptor in diabetic rat penis. Andrologia. 2012;44(Suppl 1):716–20. doi: 10.1111/j.1439-0272.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- 18.Yang J, Wang T, Zhang Y, Li R, Wang S, et al. Altered expression of mitofusin 2 in penile tissues of diabetic rats. Andrologia. 2014;46:522–8. doi: 10.1111/and.12108. [DOI] [PubMed] [Google Scholar]
- 19.Wespes E, Sattar AA, Golzarian J, Wery D, Daoud N, et al. Corporeal veno-occlusive dysfunction: predominantly intracavernous muscular pathology. J Urol. 1997;157:1678–80. doi: 10.1016/s0022-5347(01)64833-x. [DOI] [PubMed] [Google Scholar]
- 20.Melman A, Gingell JC. The epidemiology and pathophysiology of erectile dysfunction. J Urol. 1999;161:5–11. [PubMed] [Google Scholar]
- 21.Melman A. Pathophysiologic basis of erectile dysfunction. What can we learn from animal models? Int J Impot Res. 2001;13:140–2. doi: 10.1038/sj.ijir.3900679. [DOI] [PubMed] [Google Scholar]
- 22.Rogers RS, Graziottin TM, Lin CS, Kan YW, Lue TF. Intracavernosal vascular endothelial growth factor (VEGF) injection and adeno-associated virus-mediated VEGF gene therapy prevent and reverse venogenic erectile dysfunction in rats. Int J Impot Res. 2003;15:26–37. doi: 10.1038/sj.ijir.3900943. [DOI] [PubMed] [Google Scholar]
- 23.Ferrini MG, Davila HH, Valente EG, Gonzalez-Cadavid NF, Rajfer J. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovasc Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Samarakoon R, Higgins PJ. Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb Haemost. 2008;100:976–83. [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Haudek SB, Gupta D, Dewald O, Schwartz RJ, Wei L, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83:511–8. doi: 10.1093/cvr/cvp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J. 2006;20:916–25. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 28.Scott RW, Olson M. LIM kinases: function, regulation and association with human disease. J Mol Med (Berl) 2007;85:555–68. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- 29.Amano T, Tanabe K, Eto T, Narumiya S, Mizuno K. LIM-kinase 2 induces formation of stress fibres, focal adhesions and membrane blebs, dependent on its activation by Rho-associated kinase-catalysed phosphorylation at threonine-505. Biochem J. 2001;354:149–59. doi: 10.1042/0264-6021:3540149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44:1–80. [PubMed] [Google Scholar]
- 31.Tschöpe C, Schultheiss HP, Walther T. Multiple interactions between the renin-angiotensin and the kallikrein-kinin systems: role of ACE inhibition and AT1 receptor blockade. J Cardiovasc Pharmacol. 2002;39:478–87. doi: 10.1097/00005344-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Silva JA, Jr, Araujo RC, Baltatu O, Oliveira SM, Tschöpe C, et al. Reduced cardiac hypertrophy and altered blood pressure control in transgenic rats with the human tissue kallikrein gene. FASEB J. 2000;14:1858–60. doi: 10.1096/fj.99-1010fje. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher AM, Yu H, Printz MP. Bradykinin-induced reductions in collagen gene expression involve prostacyclin. Hypertension. 1998;32:84–8. doi: 10.1161/01.hyp.32.1.84. [DOI] [PubMed] [Google Scholar]


