Abstract
Sperm DNA damage is prevalent among infertile men and is known to influence natural reproduction. However, the impact of sperm DNA damage on assisted reproduction outcomes remains controversial. Here, we conducted a meta-analysis of studies on sperm DNA damage (assessed by SCSA, TUNEL, SCD, or Comet assay) and clinical pregnancy after IVF and/or ICSI treatment from MEDLINE, EMBASE, and PUBMED database searches for this analysis. We identified 41 articles (with a total of 56 studies) including 16 IVF studies, 24 ICSI studies, and 16 mixed (IVF + ICSI) studies. These studies measured DNA damage (by one of four assays: 23 SCSA, 18 TUNEL, 8 SCD, and 7 Comet) and included a total of 8068 treatment cycles (3734 IVF, 2282 ICSI, and 2052 mixed IVF + ICSI). The combined OR of 1.68 (95% CI: 1.49–1.89; P < 0.0001) indicates that sperm DNA damage affects clinical pregnancy following IVF and/or ICSI treatment. In addition, the combined OR estimates of IVF (16 estimates, OR = 1.65; 95% CI: 1.34–2.04; P < 0.0001), ICSI (24 estimates, OR = 1.31; 95% CI: 1.08–1.59; P = 0.0068), and mixed IVF + ICSI studies (16 estimates, OR = 2.37; 95% CI: 1.89–2.97; P < 0.0001) were also statistically significant. There is sufficient evidence in the existing literature suggesting that sperm DNA damage has a negative effect on clinical pregnancy following IVF and/or ICSI treatment.
Keywords: assisted reproductive technology outcomes, clinical pregnancy, meta-analysis, sperm DNA damage, systematic review
INTRODUCTION
In recent years, a number of sperm-specific biomarkers have been studied to identify useful diagnostic tests of sperm function1,2,3,4 as the conventional semen parameters are shown to have a limited diagnostic value for male fertility. To date, tests of sperm DNA integrity and sperm nuclear protein have shown potential to discriminate infertile from fertile men.5 The integrity of sperm DNA is considered to be vital for normal fertilization, embryo development, and for successful implantation and pregnancies in both natural and assisted reproduction.6,7,8 Although some studies have found some value in the use of sperm DNA tests in the evaluation of male infertility,9,10,11,12 the true prognostic value of sperm DNA assessment to predict assisted reproductive technology (ART) outcomes remains uncertain.
The current literature on sperm DNA damage and its effect on ART outcome is still controversial. The meta-analysis by Li et al.13 concluded that sperm DNA damage is detrimental to IVF clinical pregnancy rates but not with ICSI pregnancy. Another meta-analysis14 concluded that assessment of sperm DNA damage is not strong enough to provide any clinical advantage of these assays to evaluate infertile men. The Practice Committee of the American Society for Reproductive Medicine15 concluded that the existing data do not support a detrimental effect of sperm DNA damage on ART outcomes. In contrast, the meta-analysis by Zini et al.16 shows the negative effect of sperm DNA damage on ART outcomes and provides a clinical indication for the evaluation of sperm DNA damage before IVF or ICSI, and a rationale for further investigating the association between sperm DNA damage and pregnancy loss. A recent meta-analysis17 strongly suggests that assays detecting sperm DNA damage should be recommended to those suffering from recurrent failure to achieve pregnancy.
The lack of agreement in the literature is partially due to the diversity of sperm DNA test methods, lack of standardized protocols, inter-laboratory variations, the use of wide ranges of threshold values, and to some extent, the limited understanding of what each of the sperm DNA assays actually measures.7,8,18 To date, there are four widely used methods to access sperm DNA damage: the Comet assay, terminal deoxyuridine nick end labeling (TUNEL) assay, Sperm Chromatin Structure Assay (SCSA), and Sperm Chromatin Dispersion (SCD) assay.9,19,20,21 The Comet and TUNEL assays detect DNA strand breaks while SCSA and SCD measure chromatin integrity and the susceptibility of DNA to denaturation.7
Each assay is known to measure different aspects of sperm DNA damage.22,23 The ability of these assays to accurately measure the level of DNA damage depends on the technical and biological aspects of each test.24 The alkaline Comet assay may be used to study single- or double-strand DNA breaks and measures the migration of the DNA fragments in the electric field. The intensity of the comet tail represents the amount of fragmented DNA.25 The TUNEL assay quantifies the level of labeled nucleotide incorporated at single- and double-strand DNA breaks in a reaction catalyzed by the template-independent enzyme deoxynucleotidyl transferase.26 The SCD assay is based on the principle that sperm with fragmented DNA fail to produce the characteristic halo following acid denaturation and removal of nuclear proteins.27 With the SCSA, the extent of DNA damage is determined by measuring the metachromatic shift from green fluorescence to red fluorescence following acid denaturation and acridine orange staining.28 Despite differences in the principle and methodology of these assays, the levels of DNA damage measured by these assays show some degree of correlation.29
Tests of sperm DNA damage appear to have some clinical utility in the evaluation of male infertility (discriminate infertile from fertile men) and correlate with conventional sperm parameters030,31 while their ability to predict ART success remains limited.7,13,14 Comparative analysis of these sperm DNA tests shows that some assays may be better predictors of ART outcomes than others.32,33 To reach a more definitive conclusion regarding the predictive value of these assays in the context of ARTs and to further examine why there are discrepancies between the various studies, we have performed a systematic review and meta-analysis with separate subanalyses, evaluating the value of each sperm DNA test in predicting ART outcomes. Furthermore, we conducted additional subanalyses to examine the relationship between these sperm DNA tests and reproductive outcomes after different ARTs (IVF, ICSI, or mixed IVF + ICSI).
METHODS
Literature search strategy and selection criteria
We searched the following electronic databases: MEDLINE, EMBASE, and PUBMED. We did not apply any restriction on date, type of publication, or language. A computerized search was performed in April 2014 using the search strategy by combinations of search terms related to “sperm DNA damage,” “sperm DNA fragmentation,” “sperm DNA integrity,” or “sperm DNA” along with “ART,” “IVF,” “ICSI,” “outcome,” “fertilization,” “embryo,” or “pregnancy,” and in combinations with “Comet,” “TUNEL,” “SCSA,” “Acridine orange,” “Halo,” or “SCD.” Reference lists of previous meta-analyses, relevant articles, and reviews were cross-searched for additional articles. In this way, data from studies that were missed by our search criteria were identified for inclusion. Two authors (L.S. and A.Z.) independently reviewed the abstracts and papers for eligibility and discrepancies were resolved by group discussion. When it was certain from the abstract that the paper was not relevant, the paper was excluded. Authors were contacted whenever possible if full manuscript, translations, or two-by-two data table were not available. We also considered inclusion of studies that collected relevant data but were excluded from the previous meta-analysis due to the inability to extract two-by-two tables.
Inclusion and exclusion criteria
Studies analyzing the relationship between sperm DNA damage and IVF or ICSI clinical pregnancy outcome were considered for inclusion in the meta-analysis if they satisfied the following criteria: (1) clinical study in human; (2) sperm DNA damage detected by the Comet, SCSA, SCD, or TUNEL assays; (3) IVF, ICSI, or mixed (IVF + ICSI) treatment methods; and (4) studies with sufficient data to construct the two-by-two table. Studies were excluded using the following criteria: (1) overlapping data or no original data; (2) conference abstracts; (3) extremely low sample size (n < 10); (4) testing of processed or washed sperm samples (to reduce heterogeneity of the meta-analysis); and (5) studies using slide-based acridine orange staining method as this method is deemed unreliable.29
Data extraction
The primary outcome measures included in the systematic review were clinical pregnancy following IVF, ICSI, and mixed IVF + ICSI treatment methods. The following information was extracted from the articles to perform the systematic analysis: author names, publication year, DNA damage assay, type of treatment, study design, sample size in each group, exclusion of important female factors (e.g., advanced age), and control of female factors (e.g., age).
For studies to be eligible for inclusion in the meta-analysis, we had to be able to construct two-by-two tables from the reported data (with pregnancy rate above and below DNA damage cutoff). The following outcomes were prerequisites for inclusion: clinical pregnancy (presence of a fetal heartbeat confirmed by ultrasound). If necessary, study authors were contacted to clarify the data. We recorded author names, publication year, patient selection, female inclusion/exclusion criteria, the treatment type (e.g., IVF, ICSI), sperm DNA assay type, cutoff point, number of cycles or patients, and number of pregnancies relative to abnormal or normal test results. From the two-by-two tables of test results, the following test properties were calculated for each study: sensitivity, specificity, positive predictive value, negative predictive value, proportion of abnormal tests, and diagnostic odds ratio (OR). In those studies using the SCSA where data with multiple cutoffs were reported, we selected the cutoff closest to the most frequently reported thresholds (e.g., %DFI at 27% or 30%).
Statistical analysis
The measure of treatment effect was the combined odds ratio of clinical pregnancy in the group with high levels of sperm DNA damage compared with the group with low levels of sperm DNA damage. The study-by-study comparisons were synthesized by a standard meta-analytic approach applied to the odds ratios (ORs) of the individual two-by-two tables.34,35 We attributed the value 0.5 to empty cells of the two-by-two tables.34 We tested study homogeneity depending on whether homogeneity was accepted or rejected; we used the fixed or the random effect models for meta-analysis to calculate an overall OR and its 95% CI. Q statistics was used to test between study homogeneity: homogeneity was rejected when the Q statistic P < 0.10. The meta-analysis was conducted using the STATA software (StataCorp LP, College Station, TX, USA).
RESULTS
Eligible studies
The extensive literature search yielded 1279 citations. Of these, 1116 were excluded from the study based on their titles and abstracts. Full texts of 163 articles were obtained as they addressed the study question, but 67 articles were excluded because they were not original research papers (Figure 1). Following a careful review of the 96 articles, we excluded 29 articles for the reasons shown in Table 1.
Figure 1.
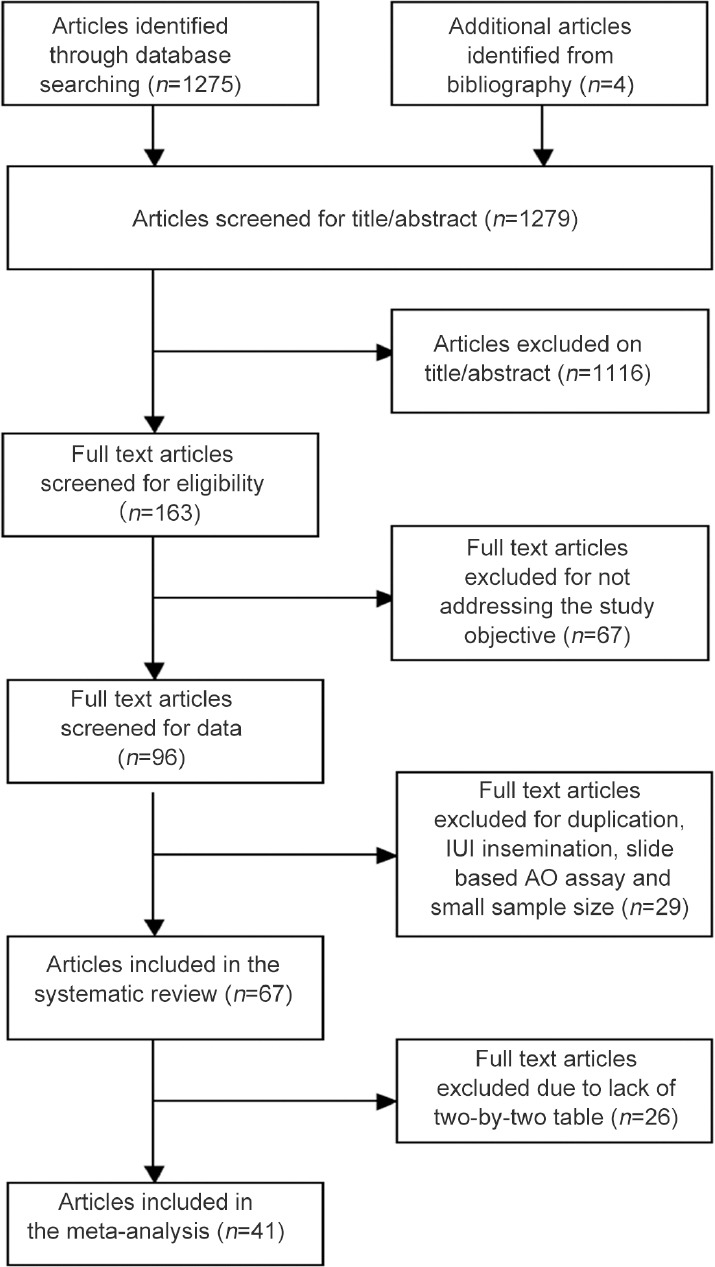
Flowchart for systematic review and meta-analysis.
Table 1.
List of studies excluded from the meta-analysis (n=55)

Study characteristics
There were 67 eligible papers for our analysis and 41 of the 67 papers were used for the meta-analysis (in these 41 papers, a two-by-two tables could be constructed from the data). The 41 articles (with a total of 56 studies) included 16 IVF studies, 24 ICSI studies, and 16 mixed (IVF + ICSI) studies. The studies were segregated into SCSA (23 studies), TUNEL (18 studies), Comet (7 studies), and SCD (8 studies) based on the sperm DNA damage assays. The estimated odds ratio with confidence intervals and weight of the 56 studies using random-effect and fixed-effect models is presented in Table 2.
Table 2.
Selected clinical and statistical characteristics of the individual studies
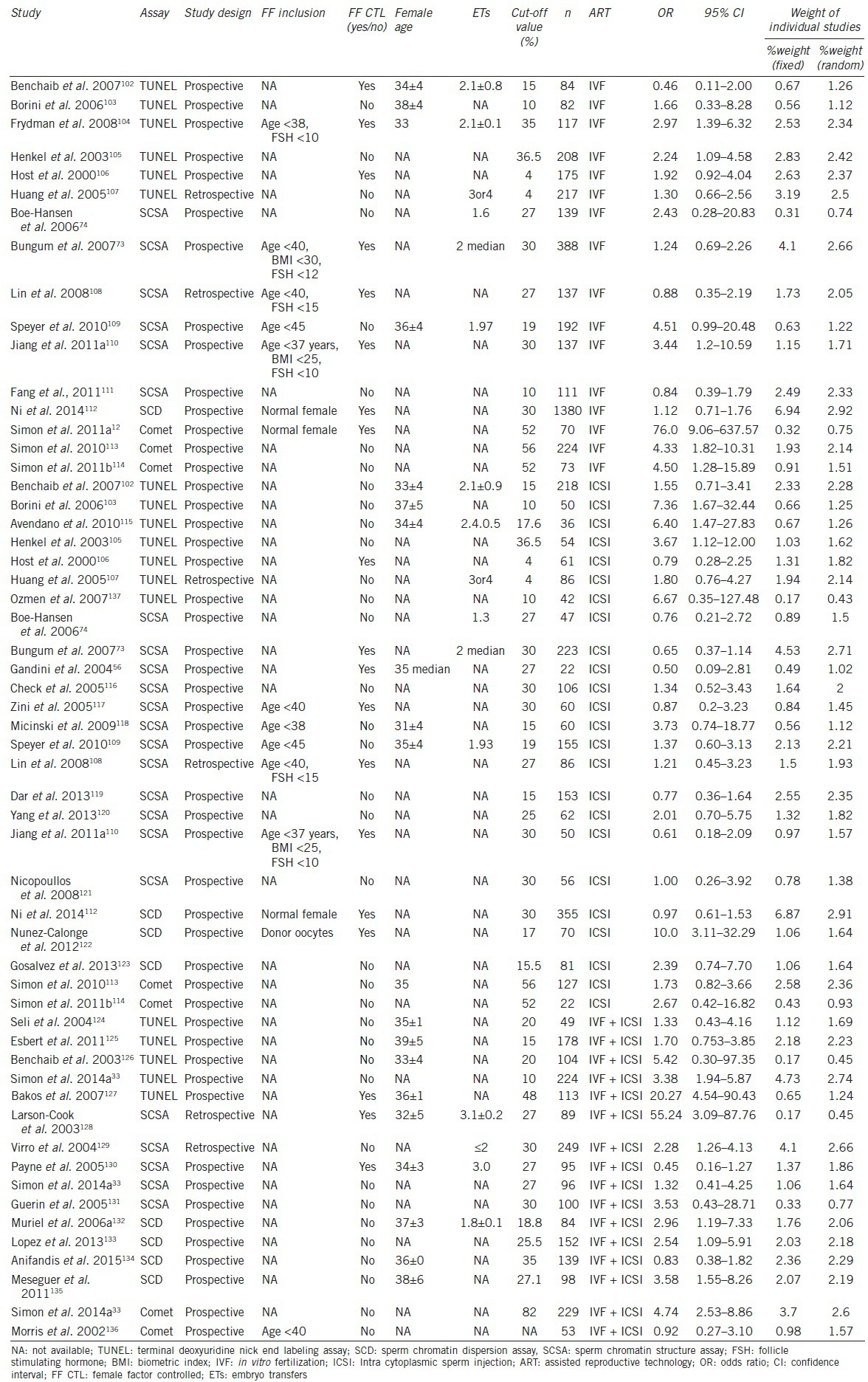
Heterogeneity of the studies included for the meta-analysis
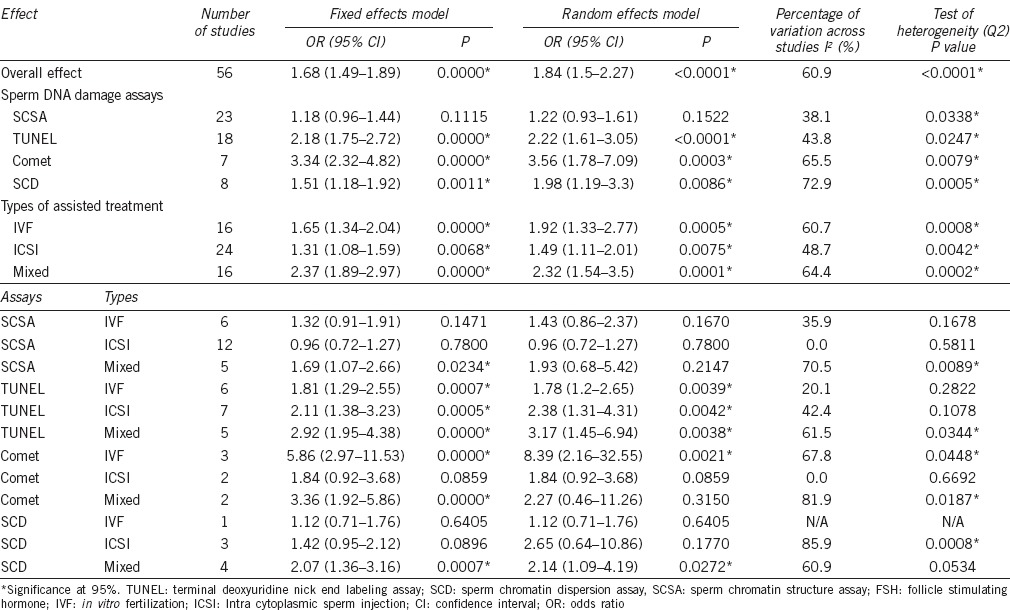
The overall and subgroup combined odds ratios of clinical pregnancy are shown in Table 3. Assessment of the overall consistency of effects across the evaluated studies was low (I2 = 61%). When the studies were segregated based on the type of DNA damage assays, the datasets of SCSA and TUNEL assays were of moderate heterogeneity (I2 = 38% and 44%, respectively) and the datasets for Comet and SCD assays had a high degree of heterogeneity (I2 = 66% and 73%, respectively). Among these studies, there were differences in study design, selection of subjects, and definition of threshold values for DNA damage (for a given assay).
Table 3.
Meta-analysis summary: Overall and subgroup odds ratios of studies on sperm DNA damage and pregnancy
Meta-analysis: relationship between sperm DNA damage and clinical pregnancy after IVF and/or ICSI
In this meta-analysis, 56 studies (including 8068 ART cycles) were combined to determine the overall relationship between sperm DNA damage and clinical pregnancy outcome (Table 4). The diagnostic odds ratios (ORs) ranged from 0.45 to 76 (Table 2), and in 18 of the 56 estimates, the ORs revealed a strong detrimental effect of sperm DNA damage on clinical pregnancy outcome (Table 2 and Figure 2a–2c). The Q statistic P < 0.0001 with an I2 = 60.9% indicates a moderate to high degree of study heterogeneity (Table 3). The negative effect of sperm DNA damage on clinical pregnancy was observed using the fixed effects model where the combined OR estimates of all studies was statistically significant (56 estimates, OR = 1.68; 95% CI: 1.49–1.89, P < 0.0001) (Table 3). Similarly, the negative effect of sperm DNA damage on clinical pregnancy was also observed using the random effects model where the combined OR estimates of all studies were also statistically significant (56 estimates, OR = 1.84; 95% CI: 1.5–2.27, P < 0.0001) (Table 3). Overall, a strong negative association between sperm DNA damage and clinical pregnancy was observed after assisted treatments.
Table 4.
Selected diagnostic properties of studies on sperm DNA damage and clinical pregnancy after assisted reproduction
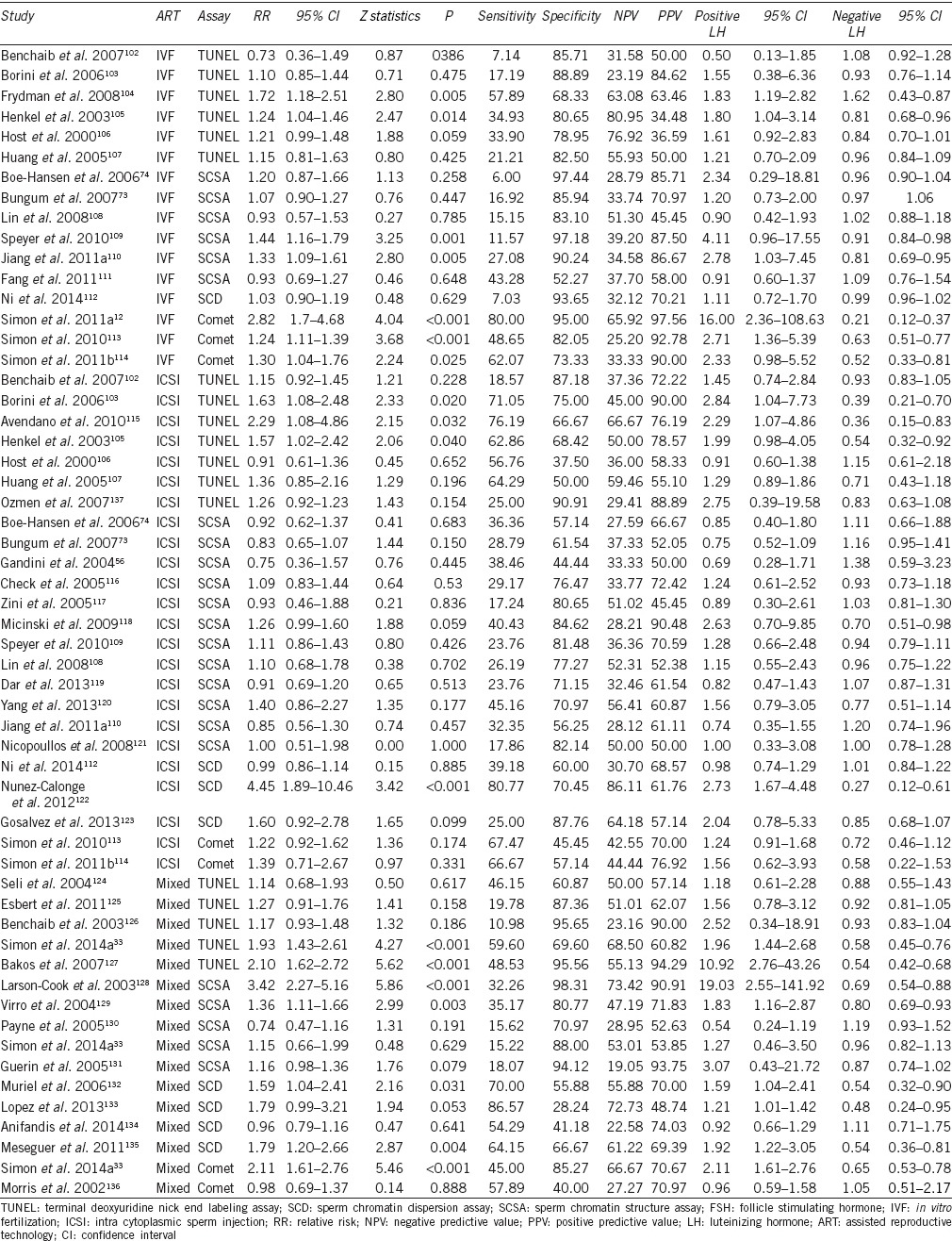
Figure 2.
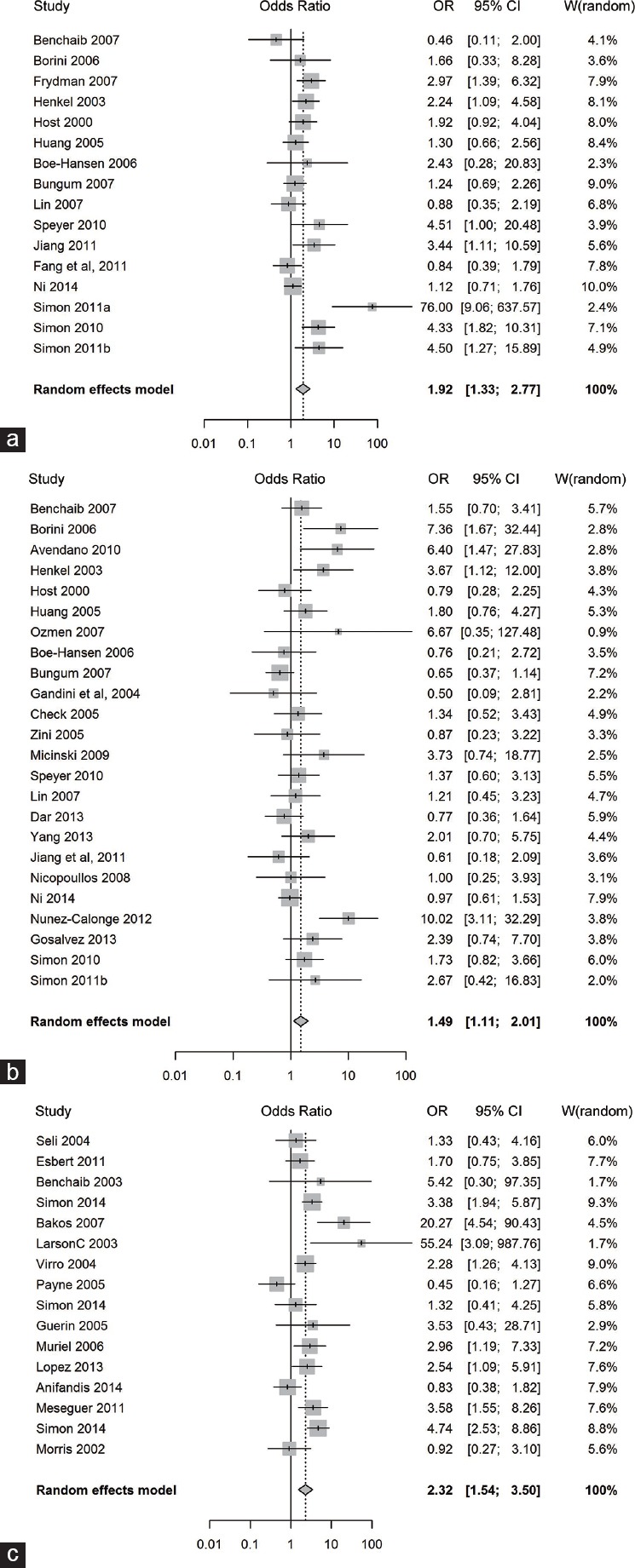
Forest plot of odds ratio to determine the negative effect of sperm DNA damage on clinical pregnancy outcome. (a) following “IVF” type of assisted reproduction, (b) following “ICSI” type of assisted reproduction, (c) following “Mixed” type of assisted reproduction.
Relationship between sperm DNA damage and clinical pregnancy: subgroup analysis by type of assisted reproduction
The relationship between sperm DNA damage (assessed by one of four different DNA damage tests: TUNEL, SCSA, SCD, and Comet assay) and clinical pregnancy was analyzed in 3734 IVF treatment cycles (16 studies), 2282 ICSI treatment cycles (24 studies), and 2052 mixed IVF + ICSI treatment cycles (16 studies) (Table 4). The heterogeneity (I2 value, Q statistic P value) was moderate to high in the IVF and mixed IVF + ICSI studies (60.7%, P = 0.0008 and 64.4%, P = 0.0002, respectively) and moderate in the ICSI studies (48.7%, P = 0.0042) (Table 3). The negative effect of sperm DNA damage on clinical pregnancy was observed with the fixed effects model where the combined OR estimates of IVF (16 estimates, OR = 1.65; 95% CI: 1.34–2.04; P < 0.0001), ICSI (24 estimates, OR = 1.31; 95% CI: 1.08–1.59; P = 0.0068), and mixed IVF + ICSI studies (16 estimates, OR = 2.37; 95% CI: 1.89–2.97; P < 0.0001) were all statistically significant. Similarly, the negative effect of sperm DNA damage on clinical pregnancy was observed using the random effects model where the combined OR estimates of IVF (16 estimates, OR = 1.92; 95% CI: 1.33–2.77; P = 0.0005), ICSI (24 estimates, OR = 1.49; 95% CI: 1.11–2.01; P = 0.0075), and mixed IVF + ICSI studies (16 estimates, OR = 2.32; 95% CI: 1.54–3.5; P = 0.0001) were all statistically significant. The forest plots depicting the individual ORs and random effects model combined OR estimate (with 95% CI) for the IVF, ICSI, and mixed IVF + ICSI studies are shown in Figure 2a–2c, respectively. Overall, a strong negative association between sperm DNA damage and clinical pregnancy was observed after IVF and/or ICSI treatments.
Relationship between sperm DNA damage and clinical pregnancy: subgroup analysis by type of sperm DNA damage assay
In this meta-analysis, data from studies using one of four commonly used sperm DNA damage measurement assays were analyzed separately using the fixed and random effect models. Of the total treatment cycles (n = 8068), sperm DNA damage was measured by SCSA in 2813 cycles (34.9%), SCD in 2359 cycles (29.2%), TUNEL in 2098 cycles (26.0%), and Comet in 798 cycles (9.9%) (Table 4). The heterogeneity (I2 value, Q statistic P value) was moderate to high in studies using the SCD (72.9%, P = 0.0005) and Comet assays (65.5%, P = 0.0079) and low to moderate in studies using the SCSA (38.1%, P = 0.0338) and TUNEL assays (43.8%, P = 0.0247) (Table 3). The negative effect of sperm DNA damage on clinical pregnancy was observed using the random effects model where the combined OR estimates of TUNEL (18 estimates, OR = 2.22; 95% CI: 1.61–3.05; P < 0.0001), Comet (7 estimates, OR = 3.56; 95% CI: 1.78–7.09; P = 0.0003), and SCD studies (8 estimates, OR = 1.98; 95% CI: 1.19–3.3; P = 0.0086) were all statistically significant. In contrast, the random effects model, combined OR estimate of SCSA studies (23 estimates, OR = 1.22; 95% CI: 0.93–1.61; P = 0.1522), was not statistically significant. In summary, the combined ORs for TUNEL, Comet, and SCD studies but not SCSA studies demonstrated a negative effect of sperm DNA damage on clinical pregnancy outcome after ART.
Relationship between sperm DNA damage and clinical pregnancy: subgroup analysis by type of assisted reproduction and type of sperm DNA damage assay
In a further subgroup analysis, we examined the relationship between sperm DNA damage and clinical pregnancy by type of assisted reproduction and type of sperm DNA damage assay using the random effects model (Table 3). The combined OR estimates for TUNEL studies were statistically significant for all types of assisted reproduction (IVF, ICSI, and mixed IVF + ICSI studies) by the random effects model, demonstrating a negative effect of sperm DNA damage on clinical pregnancy outcome. The combined OR estimates for Comet studies were statistically significant (P = 0.0021) for IVF treatment type only and combined OR estimates for SCD studies were statistically significant (P = 0.0272) for mixed IVF + ICSI treatment type only. In contrast, the combined OR estimates for SCSA studies were not statistically significant when subgrouped according to the type of assisted reproduction. Taken together, the data show that a strong negative association between sperm DNA damage and clinical pregnancy (with a statistically significant combined OR estimate) was more consistently demonstrated in studies utilizing assays that measure sperm DNA damage directly (TUNEL and Comet assays) than in studies that measure sperm DNA damage indirectly (SCSA and SCD assay).
DISCUSSION
We conducted a systematic review and meta-analysis of studies on sperm DNA damage (measured by SCSA, TUNEL, SCD, or Comet assay) and reproductive outcome after IVF and/or ICSI. We identified 67 pertinent articles in this systematic review. In 41 of these 67 papers, there were sufficient data to construct two-by-two tables and perform a meta-analysis of studies on the relationship between sperm DNA damage and clinical pregnancy after IVF and/or ICSI. From the 41 articles, we identified 56 studies involving 8068 treatment cycles (IVF and/or ICSI), which include 16 IVF studies (3734 treatment cycles), 24 ICSI studies (2282 treatment cycles), and 16 mixed IVF + ICSI studies (2052 treatment cycles). Of the total treatment cycles (n = 8068), sperm DNA damage was measured by SCSA in 2813 cycles (34.9%), SCD in 2359 cycles (29.2%), TUNEL in 2098 cycles (26.0%), and Comet in 798 cycles (9.9%).
In this study, we identified an overall detrimental effect of sperm DNA damage on clinical pregnancy rate after IVF and/or ICSI (56 IVF, ICSI or mixed IVF + ICSI studies) with a combined odds ratio of 1.68 (95% CI: 1.49–1.89). This is in contrast to prior meta-analyses13,14 and the report from the Practice Committee of the American Society for Reproductive Medicine15 where a negative effect of sperm DNA damage on clinical pregnancy outcome was not established. A recent meta-analysis showed a negative association between sperm DNA damage and IVF pregnancy but not with ICSI outcomes.17 Moreover, in our study, the random effects model combined OR estimates of IVF (16 estimates, OR = 1.92; 95% CI: 1.33–2.77; P = 0.0005), ICSI (24 estimates, OR = 1.49; 95% CI: 1.11–2.01; P = 0.0075), and mixed IVF + ICSI studies (16 estimates, OR = 2.32; 95% CI: 1.54–3.5; P = 0.0001) were all statistically significant, suggesting a detrimental effect of sperm DNA damage on ART outcome. Unlike the previous meta-analyses, where the majority of the studies evaluated sperm DNA damage by SCSA and TUNEL assays, we included more recent studies with several evaluating sperm DNA damage by SCD and Comet assay.13,14 When we segregated our dataset according to the type of DNA damage assay, all but the SCSA studies showed a detrimental effect of sperm DNA damage on clinical pregnancy (after IVF and/or ICSI). The large dataset of SCSA studies (23 studies including 2813 ART cycles) did not show a statistically significant negative association between sperm DNA damage and clinical pregnancy. This is unlike a previous meta-analysis36 where sperm DNA damage assessed by SCSA was positively associated with in vivo, IUI, and routine IVF pregnancy. We observed that studies using the SCSA and SCD assays showed a detrimental effect of sperm DNA damage on clinical pregnancy with the mixed treatment group only (mixed IVF + ICSI studies). In contrast, an analysis of studies using the TUNEL assay demonstrated the negative effect of sperm DNA damage on clinical pregnancy with all the three treatment groups (IVF, ICSI, and mixed IVF + ICSI studies), suggesting that a direct method of DNA damage measurement may be a better predictor of pregnancy outcome.37
Our meta-analysis has a number of strengths. We performed a comprehensive literature search using three databases (MEDLINE, EMBASE, and PUBMED) and a reference search from the bibliography of the articles, which resulted in the identification of 56 studies. Two independent investigators approved the studies to be included or excluded from the meta-analysis. This study had sufficient published data to perform a meta-analysis on various types of treatment (IVF, ICSI, and mixed) as well as different type of DNA damage assays (SCSA, TUNEL, Comet, and SCD assays). We obtained a dataset with consistent protocols by excluding four studies with overlapping data38,39,40,41 and 18 studies with sperm DNA tests that were not one of the four inclusion tests (SCSA, TUNEL, SCD, or alkaline Comet assay): two studies using neutral Comet assay,42,43 two studies using in situ nick translation assay,44,45 and 14 studies using slide-based acridine orange staining method.
Our meta-analysis also has several weaknesses. One of the most important weaknesses of our study is the variable and poorly controlled clinical parameters of the evaluable studies (i.e., female factors, female age, number of embryos transferred, and assay cutoff/threshold). Moreover, the meta-analysis is also weakened by virtue of the different assisted treatment types and sperm DNA damage assays. Another important factor is the high study heterogeneity (61%; P < 0.001). This degree of study heterogeneity together with above factors (clinical parameters, sperm DNA assays) reduces our confidence in the combined ORs.
In the past two decades, sperm DNA damage has been one of the most extensively studied sperm parameters in the hope that this test may have clinical value.46 Conventional semen parameters are shown to diagnose male fertility to some extent, but their associations to ART outcomes are limited; therefore, a need for newer tests has been emphasized.47 A clinically useful sperm function test should have predictive value for natural and/or ART pregnancy outcomes and provide some added value in clinical decision-making.46,48 Although sperm DNA damage has the potential to become a useful clinical biomarker,49,50 the predictive value of this test in the context of IVF and or ICSI remains to be defined.51,52 Assuming that we are confident in the combined ORs derived from our meta-analysis, our data suggest that tests of sperm DNA damage may provide some predictive value in the context of IVF, ICSI, and mixed IVF + ICSI studies. An analysis of the 16 IVF studies (with a median pregnancy rate of 32%) revealed a median PPV of 79% and median NPV of 35%. Thus, in populations with an overall IVF pregnancy rate of 32%, sperm DNA damage assessment can discriminate between IVF pregnancy rates of 21% (positive test) and 35% (negative test), a notable difference in pregnancy rate of important clinical value. An analysis of the 24 ICSI studies (with a median pregnancy rate of 36%) revealed a median PPV of 64% and median NPV of 40%. Thus, in populations with an overall ICSI pregnancy rate of 36%, sperm DNA damage assessment can discriminate between ICSI pregnancy rates of 36% (positive test) and 40% (negative test), a small difference of modest clinical value. An analysis of the 16 mixed (IVF + ICSI) studies (with a median pregnancy rate of 44%) revealed a median PPV of 70% and median NPV of 50%. Thus, in populations with an overall mixed (IVF + ICSI) pregnancy rate of 44%, sperm DNA damage assessment can discriminate between mixed (IVF + ICSI) pregnancy rates of 30% (positive test) and 50% (negative test), a notable difference in pregnancy rate of important clinical value. However, it is important to exercise caution when estimating the predictive value of sperm DNA tests in the context of IVF and/or ICSI because these estimates are derived from relatively small studies (100–200 cycles), the study characteristics are heterogeneous (e.g., different assay types, sperm DNA threshold levels, and clinical parameters) and the precision of the various sperm DNA assays remains to be validated.
In spite of the large number of studies examining the relationship between sperm chromatin and DNA damage with pregnancy rate, the wide acceptance of sperm chromatin tests as part of the assessment of a man's fertility potential has met resistance. This stems from various factors, but the main factor is the lack of standardized protocols shown to provide reproducible results across a range of laboratories (i.e., unknown precision regarding reproducibility and repeatability of the various assays) and the fact that the thresholds for many of these tests have not been validated.15 Moreover, our limited understanding of the underlying nature of sperm DNA damage has also limited the wide acceptance of these assays.53 For example, how can we explain that when sperm DNA damage is measured in a given population using the Comet assay, a threshold value of 82% is obtained, while using the TUNEL assay the threshold value is 10%,33,54 yet both threshold values are associated with clinical pregnancy rates.
To date, several reports have noted that there are insufficient data to demonstrate a negative association between sperm DNA damage and reproductive outcomes after IVF and/or ICSI. In this updated systematic review and meta-analysis, we have found a modest but statistically significant detrimental effect of sperm DNA damage on clinical pregnancy rate after IVF and/or ICSI (IVF, ICSI, and mixed IVF + ICSI studies). Although the adverse effect of sperm DNA damage on clinical pregnancies was observed in all three treatment groups (IVF, ICSI, and mixed IVF + ICSI studies), this effect appears to vary according to the type of assay used to measure sperm DNA damage.
AUTHOR CONTRIBUTIONS
LS is a postdoctoral fellow and was responsible for performing collection of data, and writing the manuscript. AZ was responsible for collection of data, compelling the studies for meta-analysis and writing the manuscript. AD and AC were responsible for statistical analysis and generating the figures. DTC is the principle investigator for this study and is responsible for the design of the study and writing the manuscript. DTC is also the head of ART unit and the corresponding author for this manuscript.
COMPETING INTEREST
DTC has received no personal financial support for this work. AZ has no conflicts of interest. LS has no conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank Dr. Lihua Liu for translating the Chinese abstracts and manuscripts into English.
REFERENCES
- 1.Aitken RJ. Sperm function tests and fertility. Int J Androl. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 2.Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pregl Breznik B, Kovacic B, Vlaisavljevic V. Are sperm DNA fragmentation, hyperactivation and hyaluronan-binding ability predictive for fertilization and embryo development in in vitro fertilization and intracytoplasmic sperm injection? Fertil Steril. 2013;99:1233–41. doi: 10.1016/j.fertnstert.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Ruvolo G, Fattouh RR, Bosco L, Brucculeri AM, Cittadini E. New molecular markers for the evaluation of gamete quality. J Assist Reprod Genet. 2013;30:207–12. doi: 10.1007/s10815-013-9943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacey AA. Assessment of male factor. Best Pract Res Clin Obstet Gynaecol. 2012;26:739–46. doi: 10.1016/j.bpobgyn.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ, de Iuliis GN. Origins and consequences of DNA damage in male germ cells. Reprod Biomed Online. 2007;14:727–33. doi: 10.1016/s1472-6483(10)60676-1. [DOI] [PubMed] [Google Scholar]
- 7.Zini A, Sigman M. Are tests of sperm DNA damage clinically useful? Pros and cons. J Androl. 2009;30:219–29. doi: 10.2164/jandrol.108.006908. [DOI] [PubMed] [Google Scholar]
- 8.Barratt CL, Aitken RJ, Bjorndahl L, Carrell DT, de Boer P, et al. Sperm DNA: organization, protection and vulnerability: from basic science to clinical applications-a position report. Hum Reprod. 2010;25:824–38. doi: 10.1093/humrep/dep465. [DOI] [PubMed] [Google Scholar]
- 9.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien J, Zini A. Sperm DNA integrity and male infertility. Urology. 2005;65:16–22. doi: 10.1016/j.urology.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez JL, Velez de la Calle JF, Tamayo M, Cajigal D, Agarwal A, et al. Sperm DNA integrity and male infertility: current perspectives. Arch Med Sci. 2009;5:S55–62. [Google Scholar]
- 12.Simon L, Lutton D, McManus J, Lewis SE. Sperm DNA damage measured by the alkaline Comet assay as an independent predictor of male infertility and IVF success. Fertil Steril. 2011;95:652–7. doi: 10.1016/j.fertnstert.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Wang L, Cai J, Huang H. Correlation of sperm DNA damage with IVF and ICSI outcomes: a systematic review and meta-analysis. J Assist Reprod Genet. 2006;23:367–76. doi: 10.1007/s10815-006-9066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins JA, Barnhart KT, Schlegel PN. Do sperm DNA integrity tests predict pregnancy with in vitro fertilization? Fertil Steril. 2008;89:823–31. doi: 10.1016/j.fertnstert.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 15.The Practice Committee of the American Society for Reproductive Medicine. The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, Boman JM, Belzile E, Ciampi A. Sperm DNA damage is associated with an increased risk of pregnancy loss after IVF and ICSI: systematic review and meta-analysis. Hum Reprod. 2008;23:2663–8. doi: 10.1093/humrep/den321. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Barratt CL, De Jonge CJ. Clinical relevance of sperm DNA assessment: an update. Fertil Steril. 2010;94:1958–9. doi: 10.1016/j.fertnstert.2010.07.1050. [DOI] [PubMed] [Google Scholar]
- 19.Gorczyca W, Gong J, Drazynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assay. Cancer Res. 1993;53:1945–51. [PubMed] [Google Scholar]
- 20.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod. 1996;2:613–9. doi: 10.1093/molehr/2.8.613. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosálvez J, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833–42. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 22.Henkel R, Hoogendijk CF, Bouic PJ, Kruger TF. TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia. 2010;42:305–13. doi: 10.1111/j.1439-0272.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 23.Schulte RT, Ohl DA, Sigman M, Smith GD. Sperm DNA damage in male infertility: etiologies, assays, and outcomes. J Assist Reprod Genet. 2010;27:3–12. doi: 10.1007/s10815-009-9359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavukcuoglu IS, Al-Azawi T, Khaki AA, Khaki A, Khalil A, et al. Clinical value of DNA fragmentation evaluation tests under ART treatments. J Turk Ger Gynecol Assoc. 2012;13:270–4. doi: 10.5152/jtgga.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostling O, Johanson KJ. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophy Res Commun. 1984;123:291–8. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 26.Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–5. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez JL, Mouriel L, Rivero MT, Goyanes V, Vasquez R, et al. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 28.Drazynkiewicz Z, Kapuscinski J. Melamed MR, Lindmo T, Mendelsohn ML. Flow Cytometry and Sorting. 2nd ed. New York: Wiley-Liss; 1976. Acridine orange: A versatile probe of nucleic acids and other cell constituents; pp. 291–314. [Google Scholar]
- 29.Chohan KR, Griffin JT, Lafromboise M, DeJonge CJ, Carrell DT. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J Androl. 2006;27:53–9. doi: 10.2164/jandrol.05068. [DOI] [PubMed] [Google Scholar]
- 30.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85:629–34. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Natali A, Turek PJ. An assessment of new sperm tests for male infertility. Urology. 2011;77:1027–34. doi: 10.1016/j.urology.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Ribas-Maynou J, Garcra-Peiro A, Fernandez-Encinas A, Abad C, Amengual MJ, et al. Comprehensive analysis of sperm DNA fragmentation by five different assays: TUNEL assay, SCSA, SCD test and alkaline and neutral comet assay. Andrology. 2013;1:715–22. doi: 10.1111/j.2047-2927.2013.00111.x. [DOI] [PubMed] [Google Scholar]
- 33.Simon L, Liu L, Murphy K, Ge S, Hotaling J, et al. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum Reprod. 2014;29:904–17. doi: 10.1093/humrep/deu040. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-analysis in Context. London: BMJ Publishing; 2001. p. 506. [Google Scholar]
- 35.Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12:466–72. doi: 10.1016/s1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 37.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–6. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Larson KL, de Jonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predicted of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–22. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 39.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, et al. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19:1401–8. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 40.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81:965–72. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 41.Simon L, Proutski I, Stevenson M, Jennings D, McManus J, et al. Sperm DNA damage has negative association with live birth rates after IVF. Reprod Biomed Online. 2012;26:68–78. doi: 10.1016/j.rbmo.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Chi HJ, Chung DY, Choi SY, Kim JH, Kim GY, et al. Integrity of human sperm DNA assessed by the neutral comet assay and its relationship to semen parameters and clinical outcomes for the IVF-ET program. Clin Exp Reprod Med. 2011;38:10–7. doi: 10.5653/cerm.2011.38.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu-Hassan D, Koester F, Shoepper B, Schultze-Mosgau A, Asimakopoulos B, et al. Comet assay of cumulus cells and spermatozoa DNA status, and the relationship to oocyte fertilization and embryo quality following ICSI. Reprod Biomed Online. 2006;12:447–52. doi: 10.1016/s1472-6483(10)61997-9. [DOI] [PubMed] [Google Scholar]
- 44.Sakkas D, Urner F, Bianchi P, Bizzaro D, Wagner I, et al. Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum Reprod. 1996;11:837–43. doi: 10.1093/oxfordjournals.humrep.a019263. [DOI] [PubMed] [Google Scholar]
- 45.Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, et al. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16:2160–5. doi: 10.1093/humrep/16.10.2160. [DOI] [PubMed] [Google Scholar]
- 46.Oehninger S, Franken DR, Ombelet W. Sperm functional tests. Fertil Steril. 2014;102:1528–33. doi: 10.1016/j.fertnstert.2014.09.044. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–7. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sigman M. Refining the measurement of sperm DNA fragmentation. Fertil Steril. 2012;98:1123. doi: 10.1016/j.fertnstert.2012.07.1140. [DOI] [PubMed] [Google Scholar]
- 49.Bungum M. Sperm DNA integrity assessment: a new tool in diagnosis and treatment of fertility. Obstet Gynecol Int 2012. 2012:531042. doi: 10.1155/2012/531042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palermo GD, Neri QV, Cozzubbo T, Rosenwaks Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil Steril. 2014;102:1508–17. doi: 10.1016/j.fertnstert.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 51.Tarlatzis BC, Goulis DG. Sperm DNA fragmentation assessment: is it really helpful? Gynecol Endocrinol. 2010;26:315–6. doi: 10.3109/09513590903507370. [DOI] [PubMed] [Google Scholar]
- 52.Beshay VE, Bukulmez O. Sperm DNA damage: how relevant is it clinically? Curr Opin Obstet Gynecol. 2012;24:172–9. doi: 10.1097/GCO.0b013e32835211b5. [DOI] [PubMed] [Google Scholar]
- 53.Ramalho-Santos J. Sins of the fathers: sperm DNA damage in the context of assisted reproduction. Hum Reprod. 2014;29:2356–8. doi: 10.1093/humrep/deu229. [DOI] [PubMed] [Google Scholar]
- 54.Simon L, Murphy K, Shamsi MB, Liu L, Emery B, et al. Paternal influence of sperm DNA integrity on early embryonic development. Hum Reprod. 2014;29:2402–12. doi: 10.1093/humrep/deu228. [DOI] [PubMed] [Google Scholar]
- 55.Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, et al. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79:1597–605. doi: 10.1016/s0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 56.Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–17. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 57.Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, et al. Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol. 2010;183:270–4. doi: 10.1016/j.juro.2009.08.161. [DOI] [PubMed] [Google Scholar]
- 58.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 59.Bungum M, Spano M, Humaidan P, Eleuteri P, Rescia M, et al. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod. 2008;23:4–10. doi: 10.1093/humrep/dem353. [DOI] [PubMed] [Google Scholar]
- 60.Claassens OE, Menkveld R, Franken V, Pretorius E, Swart Y, et al. The acridine orange test: determining the relationship between sperm morphology and fertilization in vitro. Hum Reprod. 1992;7:242–7. doi: 10.1093/oxfordjournals.humrep.a137625. [DOI] [PubMed] [Google Scholar]
- 61.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–9. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 62.Angelopoulos T, Moshel YA, Lu L, Macanas E, Grifo JA, et al. Simultaneous assessment of sperm chromatin condensation and morphology before and after separation procedures: effect on the clinical outcome after in vitro fertilization. Fertil Steril. 1998;69:740–7. doi: 10.1016/s0015-0282(98)00016-8. [DOI] [PubMed] [Google Scholar]
- 63.Hammadeh ME, Al-Hassani S, Gaub C, Rosenbaum P, Georg T, et al. Prediction value of chromatin decondensation in vitro on fertilization rate after intracytoplasmic sperm injection (ICSI) Int J Androl. 2001;24:311–6. doi: 10.1046/j.1365-2605.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- 64.Hammadeh ME, Al Hasani S, Rosenbaum P, Schmidt W, Hammadeh CF. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch Gynecol Obstet. 2008;277:515–26. doi: 10.1007/s00404-007-0507-1. [DOI] [PubMed] [Google Scholar]
- 65.Virant-Klun I, Tomazevic T, Meden-Vrtovec H. Sperm single-stranded DNA, detected by acridine orange staining, reduces fertilization and quality of ICSI-derived embryos. J Assist Reprod Genet. 2002;19:319–28. doi: 10.1023/A:1016006509036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duran EH, Gurgan T, Gunalp S, Enginsu ME, Yarali H, et al. A logistic regression model including DNA status and morphology of spermatozoa for prediction of fertilization in vitro. Hum Reprod. 1998;13:1235–9. doi: 10.1093/humrep/13.5.1235. [DOI] [PubMed] [Google Scholar]
- 67.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–8. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 68.Katayose H, Yanagida K, Hashimoto S, Yamada H, Sato A. Use of diamide-acridine orange fluorescence staining to detect aberrant protamination of human-ejaculated sperm nuclei. Fertil Steril. 2003;79:670–6. doi: 10.1016/s0015-0282(02)04815-x. [DOI] [PubMed] [Google Scholar]
- 69.Cebesoy FB, Aydos K, Unlu C. Effect of sperm chromatin damage on fertilization ratio and embryo quality post-ICSI. Arch Androl. 2006;52:397–402. doi: 10.1080/01485010600666953. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Wang H, Wang L, Zhou Z, Sha J, et al. The clinical significance of sperm DNA damage detection combined with routine semen testing in assisted reproduction. Mol Med Report. 2008;1:617–24. doi: 10.3892/mmr_00000002. [DOI] [PubMed] [Google Scholar]
- 71.Jiang H, He RB, Wang CL, Zhu J. The relationship of sperm DNA fragmentation index with the outcomes of in-vitro fertilisation-embryo transfer and intracytoplasmic sperm injection. J Obstet Gynaecol. 2011;31:636–9. doi: 10.3109/01443615.2011.590910. [DOI] [PubMed] [Google Scholar]
- 72.Wang WB, Zhao LW, Xiang ZQ, Zhou XZ, Gui YL, et al. Correlation analysis of the results of double fluorescence (AO/PI) staining and clinical outcomes. J Reprod Contracept. 2012;23:111–8. [Google Scholar]
- 73.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–9. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 74.Boe-Hansen GB, Fedder J, Ersboll AK, Christensen P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum Reprod. 2006;21:1576–82. doi: 10.1093/humrep/del019. [DOI] [PubMed] [Google Scholar]
- 75.Muriel L, Meseguer M, Fernández JL, Alvarez J, Remohí J, et al. Value of the sperm chromatin dispersion test in predicting pregnancy outcome in intrauterine insemination: a blind prospective study. Hum Reprod. 2006b;21:738–44. doi: 10.1093/humrep/dei403. [DOI] [PubMed] [Google Scholar]
- 76.Thomson LK, Zieschang JA, Clark AM. Oxidative deoxyribonucleic acid damage in sperm has a negative impact on clinical pregnancy rate in intrauterine insemination but not intracytoplasmic sperm injection cycles. Fertil Steril. 2011;96:843–7. doi: 10.1016/j.fertnstert.2011.07.356. [DOI] [PubMed] [Google Scholar]
- 77.Yang XY, Zhang Y, Sun XP, Cui YQ, Qian XQ, et al. Sperm chromatin structure assay predicts the outcome of intrauterine insemination. Natl J Androl. 2011;17:977–83. [PubMed] [Google Scholar]
- 78.Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30:1519–24. doi: 10.1007/s10815-013-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun JG, Jurisicova A, Casper RF. Detection of deoxyribonucleic acid fragmentation in human sperm: correlation with fertilization in vitro. Biol Reprod. 1997;56:602–7. doi: 10.1095/biolreprod56.3.602. [DOI] [PubMed] [Google Scholar]
- 80.Lopes S, Sun JG, Jurisicova A, Meriano J, Casper RF. Sperm deoxyribonucleic acid fragmentation is increased in poor quality semen samples and correlates with failed fertilization in intracytoplasmic sperm injection. Fertil Steril. 1998;69:528–32. doi: 10.1016/s0015-0282(97)00536-0. [DOI] [PubMed] [Google Scholar]
- 81.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Study of mitochondrial membrane potential, reactive oxygen species, DNA fragmentation and cell viability by flow cytometry in human sperm. Hum Reprod. 2002;17:1257–65. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 82.Tomsu M, Sharma V, Miller D. Embryo quality and IVF treatment outcome may correlate with different sperm comet parameters. Hum Reprod. 2002;17:1856–62. doi: 10.1093/humrep/17.7.1856. [DOI] [PubMed] [Google Scholar]
- 83.Lewis SEM, O’onnell M, Stevenson M, Thompson-Cree L, McClure N. An algorithm to predict pregnancy in assisted reproduction. Hum Reprod. 2004;19:1385–94. doi: 10.1093/humrep/deh227. [DOI] [PubMed] [Google Scholar]
- 84.Nasr-Esfahani MH, Salehi M, Razavi S, Anjlmshoa M, Rozahani S, et al. Effect of sperm DNA damage and sperm protamine deficiency on fertilization and embryo development post-ICSI. Reprod Biomed Online. 2005;11:198–205. doi: 10.1016/s1472-6483(10)60959-5. [DOI] [PubMed] [Google Scholar]
- 85.Hammadeh ME, Radwan M, Al-Hasani S, Micu R, Rosenbaum P, et al. Comparison of reactive oxygen species concentration in seminal plasma and semen parameters in partners of pregnant and nonpregnant patients after IVF/ICSI. Reprod Biomed Online. 2006;13:696–706. doi: 10.1016/s1472-6483(10)60661-x. [DOI] [PubMed] [Google Scholar]
- 86.Caglar GS, Koster F, Schopper B, Asimakopoulos B, Nehls B, et al. Semen DNA fragmentation index, evaluated with both TUNEL and comet assay, and the ICSI outcome. In Vivo. 2007;21:1075–80. [PubMed] [Google Scholar]
- 87.Stevanato J, Bertolla RP, Barradas V, Spaine DM, Cedenho AP, et al. Semen processing by density gradient centrifugation does not improve sperm apoptotic deoxyribonucleic acid fragmentation rates. Fertil Steril. 2008;90:889–90. doi: 10.1016/j.fertnstert.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 88.Velez de la Calle JF, Muller A, Walschaerts M, Clavere JL, Jimenez C, et al. Sperm deoxyribonucleic acid fragmentation as assessed by the sperm chromatin dispersion test in assisted reproductive technology programs: results of a large prospective multicenter study. Fertil Steril. 2008;90:1792–9. doi: 10.1016/j.fertnstert.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 89.Gu LJ, Chen ZW, Chen ZJ, Xu JF, Li M. Sperm chromatin anomalies have an adverse effect on the outcome of conventional in vitro fertilization: a study with strictly controlled external factors. Fertil Steril. 2009;92:1344–6. doi: 10.1016/j.fertnstert.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 90.Gu LJ, Lu WH, Chen ZW. Effects of fertilization and pregnancy after embryo transfer - Sperm DNA damage in vitro fertilization. Chin J Birth Health Hered. 2011;19:111–2. [In Chinese] [Google Scholar]
- 91.Nijs M, Creemers E, Cox A, Franssen K, Janssen M, et al. Chromomycin A3 staining, sperm chromatin structure assay and hyaluronic acid binding assay as predictors for assisted reproductive outcome. Reprod Biomed Online. 2009;19:671–84. doi: 10.1016/j.rbmo.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Nijs M, De Jonge C, Cox A, Janssen M, Bosmans E, et al. Correlation between male age, WHO sperm parameters, DNA fragmentation, chromatin packaging and outcome in assisted reproduction technology. Andrologia. 2011;43:174–9. doi: 10.1111/j.1439-0272.2010.01040.x. [DOI] [PubMed] [Google Scholar]
- 93.Tarozzi N, Nadalini M, Stronati A, Prato LD, Coticchio G, et al. Anomalies in sperm chromatin packing: impactions for assisted reproductive treatment. Reprod Biomed Online. 2009;18:486–95. doi: 10.1016/s1472-6483(10)60124-1. [DOI] [PubMed] [Google Scholar]
- 94.Tavalaee M, Razavi S, Nasr-Esfahani MH. Influence of sperm chromatin anomalies on assisted reproductive technology outcome. Fertil Steril. 2009;91:1119–26. doi: 10.1016/j.fertnstert.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 95.Daris B, Goropevnek A, Hojnik N, Vlaisavljevi V. Sperm morphological abnormalities as indicators of DNA fragmentation and fertilization in ICSI. Arch Gynecol Obstet. 2010;281:363–7. doi: 10.1007/s00404-009-1140-y. [DOI] [PubMed] [Google Scholar]
- 96.Kennedy C, Ahlering P, Rodriguez H, Levy S, Sutovsky P. Sperm chromatin structure correlates with spontaneous abortion and multiple pregnancy rates in assisted reproduction. Reprod Biomed Online. 2011;22:272–6. doi: 10.1016/j.rbmo.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 97.Na L, Li L. Effect of sperm DNA damage on the outcome of in vitro fertilization-embryo transfer. Guangxi Med J. 2011;33:257–60. [Google Scholar]
- 98.Rama Raju GA, Prakash GJ, Krishna KM, Madan K, Narayana TS, et al. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012;44:490–8. doi: 10.1111/j.1439-0272.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 99.Sharbatoghli M, Valojerdi MR, Amanlou M, Khosravi F, Jafar-Abadi MA. Relationship of sperm DNA fragmentation, apoptosis and dysfunction of mitochondrial membrane potential with semen parameters and ART outcome after intracytoplasmic sperm injection. Arch Gynecol Obstet. 2012;286:1315–22. doi: 10.1007/s00404-012-2440-1. [DOI] [PubMed] [Google Scholar]
- 100.Lazaros L, Vartholomatos G, Pamporaki C, Kosmas I, Takenaka A, et al. Sperm flow cytometric parameters are associated with ICSI outcome. Reprod Biomed Online. 2013;26:611–8. doi: 10.1016/j.rbmo.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Martin P, Sanchez-Martín F, Gonzalez-Martinez M, Gosalvez J. Increased pregnancy after reduced male abstinence. Syst Biol Reprod Med. 2013;59:256–60. doi: 10.3109/19396368.2013.790919. [DOI] [PubMed] [Google Scholar]
- 102.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, et al. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–101. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 103.Borini A, Tarozzi N, Bizzaro D, Bonu MA, Fava L, et al. Sperm DNA fragmentation: paternal effect on early post-implantation embryo development in ART. Hum Reprod. 2006;21:2876–81. doi: 10.1093/humrep/del251. [DOI] [PubMed] [Google Scholar]
- 104.Frydman N, Prisant N, Hesters L, Frydman R, Tachdjian G, et al. Adequate ovarian follicular status does not prevent the decrease in pregnancy rates associated with high sperm DNA fragmentation. Fertil Steril. 2008;89:93–8. doi: 10.1016/j.fertnstert.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 105.Henkel R, Kierspel E, Hajimohammad M, Stalf T, Hoogendijk C, et al. DNA fragmentation of spermatozoa and assisted reproduction technology. Reprod Biomed Online. 2003;7:477–84. doi: 10.1016/s1472-6483(10)61893-7. [DOI] [PubMed] [Google Scholar]
- 106.Host E, Lindenberg S, Smidt-Jensen S. The role of DNA strand breaks in human spermatozoa used for IVF and ICSI. Acta Obstet Gynecol Scand. 2000;79:559–63. [PubMed] [Google Scholar]
- 107.Huang CC, Lin DP, Tsao HM, Cheng TC, Liu CH, et al. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil Steril. 2005;84:130–40. doi: 10.1016/j.fertnstert.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 108.Lin HH, Lee RK, Li SH, Lu CH, Sun FJ, et al. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril. 2008;90:352–9. doi: 10.1016/j.fertnstert.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 109.Speyer BE, Pizzey AR, Ranieri M, Joshi R, Delhanty JD, et al. Fall in implantation rates following ICSI with sperm with high DNA fragmentation. Hum Reprod. 2010;25:1609–18. doi: 10.1093/humrep/deq116. [DOI] [PubMed] [Google Scholar]
- 110.Jiang HH, He XJ, Song B, Cao YX. Sperm chromatin integrity test for predicting the outcomes of IVF and ICSI. Natl J Androl. 2011;17:1083–6. [PubMed] [Google Scholar]
- 111.Fang L, Lou LJ, Ye YH, Jin F, Zhou J. A study on correlation between sperm DNA fragmentation index and age of male various parameters of sperm and in vitro fertilization outcome. Chin J Med Genet. 2011;28:432–5. doi: 10.3760/cma.j.issn.1003-9406.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 112.Ni W, Xiao S, Qiu X, Jin J, Pan C, et al. Effect of sperm DNA fragmentation on clinical outcome of frozen-thawed embryo transfer and on blastocyst formation. PLoS One. 2014;9:e94956. doi: 10.1371/journal.pone.0094956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simon L, Brunborg G, Stevenson M, Lutton D, McManus J, et al. Clinical significance of sperm DNA damage in assisted reproductive outcome. Hum Reprod. 2010;25:1594–608. doi: 10.1093/humrep/deq103. [DOI] [PubMed] [Google Scholar]
- 114.Simon L, Castillo J, Oliva R, Lewis S. The relationship between human sperm protamines, DNA damage and assisted reproductive outcomes. Reprod Biomed Online. 2011;23:724–34. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Avendano C, Franchi A, Duran H, Oehninger S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil Steril. 2010;94:549–57. doi: 10.1016/j.fertnstert.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 116.Check JH, Graziano V, Cohen R, Krotec J, Check ML. Effect of an abnormal sperm chromatin structural assay (SCSA) on pregnancy outcome following (IVF) with ICSI in previous IVF failures. Arch Androl. 2005;51:121–4. doi: 10.1080/014850190518125. [DOI] [PubMed] [Google Scholar]
- 117.Zini A, Meriano J, Kader K, Jarvi K, Laskin CA, et al. Potential adverse effect of sperm DNA damage on embryo quality after ICSI. Hum Reprod. 2005;20:3476–80. doi: 10.1093/humrep/dei266. [DOI] [PubMed] [Google Scholar]
- 118.Micinski P, Pawlicki K, Wielgus E, Bochenek M, TworkowskaI I. The sperm chromatin structure assay (SCSA) as prognostic factor in IVF/ICSI program. Reprod Biol. 2009;9:65–70. doi: 10.1016/s1642-431x(12)60095-3. [DOI] [PubMed] [Google Scholar]
- 119.Dar S, Grover SA, Moskovtsev SI, Swanson S, Baratz A, et al. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50%) Fertil Steril. 2013;100:75–80. doi: 10.1016/j.fertnstert.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 120.Yang XY, Wang LL, Chen P, Zhang Y, Zhang W, et al. Impact of sperm DNA fragmentation index and sperm malformation rate on the clinical outcome of ICSI. Natl J Androl. 2013;19:1082–7. [PubMed] [Google Scholar]
- 121.Nicopoullos JD, Gilling-Smith C, Almeida PA, Homa S, Norman-Taylor JQ, et al. Sperm DNA fragmentation in subfertile men: the effect on the outcome of intracytoplasmic sperm injection and correlation with sperm variables. Br J Urol Int. 2008;101:1553–60. doi: 10.1111/j.1464-410X.2008.07518.x. [DOI] [PubMed] [Google Scholar]
- 122.Nunez-Calonge R, Caballero P, Lopez-Fernandez C, Guijarro JA, Fernandez JL, et al. An improved experimental model for understanding the impact of sperm DNA fragmentation on human pregnancy following ICSI. Reprod Sci. 2012;19:1163–8. doi: 10.1177/1933719112459238. [DOI] [PubMed] [Google Scholar]
- 123.Gosalvez J, Caballero P, Lopez-Fernandez C, Ortega L, Guijarro JA, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15:812–8. doi: 10.1038/aja.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82:378–83. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 125.Esbert M, Pacheco A, Vidal F, Florensa M, Riqueros M, et al. Impact of sperm DNA fragmentation on the outcome of IVF with own or donated oocytes. Reprod Biomed Online. 2011;23:704–10. doi: 10.1016/j.rbmo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 126.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–8. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 127.Bakos HW, Thompson JG, Feil D, Lane M. Sperm DNA damage is associated with assisted reproductive technology pregnancy. Int J Androl. 2007;31:518–26. doi: 10.1111/j.1365-2605.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 128.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, et al. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80:895–902. doi: 10.1016/s0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 129.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–95. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 130.Payne JF, Raburn DJ, Couchman GM, Price TM, Jamison MG, et al. Redefining the relationship between sperm deoxyribonucleic acid fragmentation as measured by the sperm chromatin structure assay and outcomes of assisted reproductive techniques. Fertil Steril. 2005;84:356–64. doi: 10.1016/j.fertnstert.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 131.Guerin P, Matillon C, Bleau G, Levy R, Menezo Y. Impact of sperm DNA fragmentation on ART outcome. Gynecol Obstet Fertil. 2005;33:665–8. doi: 10.1016/j.gyobfe.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 132.Muriel L, Garrido N, Fernández JL, Remohí J, Pellicer A, et al. Value of the sperm DNA fragmentation level, measured by the sperm chromatin dispersion (SCD) test, in the IVF and ICSI outcome. Fertil Steril. 2006;85:371–83. doi: 10.1016/j.fertnstert.2005.07.1327. [DOI] [PubMed] [Google Scholar]
- 133.Lopez G, Lafuente R, Checa MA, Carreras R, Brassesco M. Diagnostic value of sperm DNA fragmentation and sperm high-magnification for predicting outcome of assisted reproduction treatment. Asian J Androl. 2013;15:790–4. doi: 10.1038/aja.2013.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anifandis G, Bounartzi T, Messini CI, Dafopoulos K, Markandona R, et al. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia. 2015;47:295–302. doi: 10.1111/and.12259. [DOI] [PubMed] [Google Scholar]
- 135.Meseguer M, Santiso R, Garrido N, García-Herrero S, Remohí J, et al. Effect of sperm DNA fragmentation on pregnancy outcome depends on oocyte quality. Fertil Steril. 2011;95:124–8. doi: 10.1016/j.fertnstert.2010.05.055. [DOI] [PubMed] [Google Scholar]
- 136.Morris ID, Iiott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (Comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–8. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 137.Ozmen B, Caglar GS, Koster F, Schopper B, Diedrich K, et al. Relationship between sperm DNA damage, induced acrosome reaction and viability in ICSI patients. Reprod Biomed Online. 2007;15:208–14. doi: 10.1016/s1472-6483(10)60710-9. [DOI] [PubMed] [Google Scholar]


